1. Introduction
Breeding for Fusarium head blight (FHB) resistance is the most effective means of providing useful protection of wheat [
1,
2]. It is well known that resistance to
Fusarium graminearum and
Fusarium culmorum is not race-specific [
3,
4,
5,
6,
7]. Research has shown that in wheat, the resistance to different
Fusaria is also species-non-specific, e.g., the genotypes, being resistant to
F. graminearum, will also show resistance to other
Fusarium spp. tested [
8,
9]. As pathogenicity is a characteristic of the species, the differences in the disease-causing capacity of the individual isolates for non-specialized pathogens, such as
Fusarium, is referred to as aggressiveness [
10,
11,
12]. This relates to the primary symptoms; reactions to other traits (
Fusarium-damaged kernels (FDK) and deoxynivalenol (DON)) might differ. However, their aggressiveness shows a very wide variation [
13,
14]. “Adjusting” is the process in which a given inoculum will be positioned to a given conidium or fungal mass concentration by dilution or concentration. In this study, the only dilution was tested, and only from previous research that used single isolates. However, it became apparent that resistance expression and aggressiveness are interdependent; at lower aggressiveness, the differentiation of the genotypes for resistance is problematic. Variable aggressiveness in different isolates or in different inocula of the same isolate might influence phenotyping [
15,
16,
17]. To the extent that it is possible, this should be considered in resistance screening, quantitative trait loci (QTL) analysis, and any task that needs reliable and exact data.
The regulation of aggressiveness is a longstanding unanswered question. The early findings were summarized by Dill-Macky [
4].
F. graminearum is not a good conidium producer, and most isolates are poor in terms of Potato dextrose agar (PDA) [
18]. Eide [
19] was among the first who performed pathogenicity tests of
F. graminearum isolates, finding significant differences between them. Snijders and Perkowski [
20] (1990) found highly significant isolate aggressiveness differences. However, although the two isolates showed acceptable DI differences, the DON data were inconclusive. A clear differentiation was found in both traits in the case of the IPO-39-01 isolate. Gilbert et al. [
10] found similarly large differences in aggressiveness, but here DON was also measured with variable results. Jardine and Leslie [
11] found for
F. verticillioides many-fold differences in levels of aggressiveness at the same conidium concentration of the same isolates. This earlier study is important because it proves clearly that the same conidium concentration does not secure the same aggressiveness in the same genotypes tested. Wu et al. [
21] compared aggressiveness in seedlings and heads. In nearly all cases they found significant and positive correlations between seedling and FHB visual scores at a mean r value of about r = 0.50. Similar results were published by Mesterhazy [
18]. These results raise the idea that a seedling test could be useful for preselecting of the inocula for field tests.
In considering what should be regulated, in most cases, the conidium concentration is the subject. However, Takegami and Sasai [
22] found that mycelia are as effective as infectious agents as conidia. Sutton [
23] considered all spores and mycelium fragments as valid infecting agents. This was also proven for ascospores and macroconidia were considered as generally equivalent infecting agents [
24,
25]. Grausgruber et al. [
26] found that all propagula could infect, and therefore, they spoke about colony-forming units (CFU). Finally, the possibility of directly regulating aggressiveness has been suggested [
13,
27]. In the dilution test [
27], an isolate was found where 20-fold dilution in the seedling test did not change aggressiveness. However, in another isolate, the aggressiveness was reduced to near zero at the same dilution rate. As there were no field experiments at hand, in the current study, it was decided to investigate this problem in field inoculation tests.
No clear tendency has been found in aggressiveness related to the media used for inoculum production [
4]. The correlation between conidium concentration and aggressiveness has rarely been studied. Stein et al. [
28] tested inoculum concentration treatments, including suspensions of 0, 100, 500, 1000, 5000, 10,000, 50,000, and 100,000 conidia/mL in sterile, deionized water (sdH
2O). For the test, a highly aggressive isolate called Fg4 was used. The correlations between conidium concentration and DON contamination were highly significant for grain (r = 0.93,
p = 0.001) for both greenhouse and field experiments. However, as only one isolate was tested, general conclusions cannot be made. The correlations with incidence and severity were much looser, in agreement with Mesterházy et al. [
16]. Stack and McMullen [
29] also found a close correlation until a maximum was reached, and thereafter, no additional change was seen. Because only one inoculum was tested in different dilutions, the functions should not necessarily be the same for other tests.
In the current study, a number of papers were screened (
Table 1) regarding the applied methodology [
3,
20,
21,
26,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61]. The cited papers, relating mostly to wheat and published during the past several decades, give no information regarding the means of adjustment, whether by dilution or concentration. It is possible that the stock suspension may either need dilution to reach the wanted conidium concentration (such as 5 × 10
5 conidia/mL) or must be concentrated when its density is too low. No details were given in the literature [
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61], with the expression “adjusted” used in most cases. It appears that the researchers did not think that this practice could influence experimental results. Moreover, there is a significant variation in the conidium concentrations used (10
4–10
6/mL), with 5 × 10
5 /mL the most commonly used concentration. In one previous paper, different concentrations were used for spray and point inoculation [
49]; however, no explanation was provided why the given concentrations were used. The conclusion, therefore, is that there is no agreement regarding which the optimal concentration. None of the reviewed papers mentioned that the regulation of the conidium concentration was originally intended to regulate aggressiveness nor presented any data about the aggressiveness of the given inoculum before inoculation. Thus, it appeared that most authors, first, regulated the conidium concentration because of usage, and second, because they did not consider that this could cause a serious problem. As genetic differences in aggressiveness level between isolates exist, this does not support the existence of a generally used conidium concentration for every isolate. However, many more papers reported on the use of a highly pathogenic isolate based on previous experience. This means that aggressiveness was considered an important factor, but was not connected to the conidium concentration problem.
Most previous studies worked with
F. graminearum; in several cases in cooler regions,
F. culmorum was mentioned [
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61]. Aggressiveness was evaluated by the FHB visual head data. Eighteen papers used point inoculation (P) and 24 presented spraying inoculation (S) results. Several papers dealt with the fungal genetics problem when the inoculum was required, but plant infection was not conducted. Two main inoculation methods were used: Spraying inoculation, in which heads were sprayed by a conidium suspension, or point inoculation, in which one floret in the middle of the head was inoculated. Therefore, adjustment of the conidium concentration (or fungal mass concentration) is a general problem for all inoculation methods. The proportion of studies we identified that used point inoculation was higher (19 P
versus 24 S) than its proportion in the review paper in Buerstmayr et al. [
62] who identified more than 60 QTL by P and more than 110 by S inoculation. Of the 44 cases in this previous study, 7 cases reported very high aggressiveness, 14 good, 7 medium, 6 low, and 9 cases were not documented or mentioned. Zwart et al. [
63] spoke first about overall resistance to FHB by combining Type I and Type II resistance together, their inoculum had 1 × 10
5 cinidia/mL. We also detected significant variability in aggressiveness at the same conidium concentration. It should also be considered that not all experiments could be published because the aggressiveness was not high enough to demonstrate convincing genotype differences. Therefore, it is supposed that the problem is larger than the analysis of the published data shows. All reviewed papers [
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61] stress the significance of the toxin problem, but toxin data were published in only 15 cases, and no data were given in 29 cases. From these 15 cases, values in 6 were too low to detect reliable differences in response. Seven papers reported high or very high DON concentrations and two produced medium level DON. Therefore, the question arises, how can genotypes be selected for low toxin contamination, when toxins are not tested? Therefore, resistance expression does not only involve checking for visual symptoms, but also testing for FDK and DON contamination.
In recent decades, important research has been conducted in Szeged, Hungary, using four or eight isolates independently [
16,
17,
64]. In all cases, the response to FDK and DON was regularly tested, providing the motivation to extend these previous studies to consider the dilution problem at different resistance levels using four isolates, and also to investigate the FDK and DON response under different dilution rates. This is an unusually extensive and complex approach to the problem to better understand the processes involved in forming disease response and the significance of the dilution in this process.
The main objectives of this study were to examine: (i) The aggressiveness response of the isolates at different dilution grades; (ii) the relationship of visual symptoms to FDK and DON; (iii) how the expression of resistance may be influenced by the dilution effect for the three traits; and (iv) how a more precise phenotyping system could be developed.
3. Discussion
3.1. Aggressiveness and Dilution Rate
The lesson from the previous papers reviewed [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61] shows clearly that the present practice of regulating conidium concentration has severe problems. It appears that the authors of this previous research fulfilled a formal requirement to apply a given or expected conidium concentration. However, no aggressiveness tests were made to examine the process of inoculum production. Furthermore, previous studies did not indicate that a specific conidium concentration would be required to appropriately regulate aggressiveness [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61]. Some authors mentioned that the isolate that was applied was aggressive based on the experience of past tests. The traits measuring aggressiveness (e.g., symptom severity) were varied: Eleven cases used the disease index, and others examined incidence or severity, typically 21 days after inoculation or AUDPC, when more ratings were made. Evaluation was conducted based on different scales, either in percentage terms or using the traditional 1–9 scale used from breeding practice. All of the previous studies we reviewed described only a part of the FHB resistance. A more significant problem is that a large number of the sources (15) recorded only medium or low infection data, indicating that the formally used adjustment of conidium concentration is not an effective means regulating aggressiveness. In the tests conducted in 24 studies, no FDK and DON data were published, even though the significance of toxin contamination was heavily stressed in each paper. From the 15 papers in which DON was measured, only seven produced acceptable DON levels. This means that only half of the papers were in a suitable position to evaluate genotypes or other differences in the tests—related to the 44 papers, this is only 15%.
The results in the current study show that the relationship between dilution rate and aggressiveness is complicated. Comparing results in a previous study [
13] with those found here, the more aggressive isolates were more stable in both tests. The baseline aggressiveness was very similar for the five isolates tested in 1977. Nonetheless, the most aggressive isolate showed an increase at 20-fold dilution, and the least aggressive caused no damage at a dilution ratio of 1:20. It was also similar in that the response to the dilution cannot be predicted exactly. In the present FHB test, the variation was relatively high. The aggressiveness of the most aggressive isolate was reduced only moderately, but the remaining three isolates, which had significant differences, responded similarly to the dilution. This indicates that a given conidium concentration may lead to different disease severities, as suggested in the cited literature.
The impression given by the earlier research is that aggressiveness is thought to be stable. It is known [
65] that
Fusarium isolates normally lose aggressiveness, implying that a change is necessary at some stage. A passage may help, but in most cases, more aggressive new isolates should be involved in the tests. There might be also other causes. In seedling tests, the aggressiveness of the isolates varies [
66]. Under field conditions, the visual symptoms, FDK, or DON can vary strongly because temperature, humidity, rain, and other conditions are not stable [
5,
7,
64]. Therefore, the differences between years are mostly due to these events. In the case of only one inoculum, the stability cannot be tested, and the variation in response will be understood as a year effect. Following a previous study [
13], the Szeged tests were conducted using four different isolates [
5,
7,
9,
12,
64], in which highly significant isolate × year interactions were found. Another experience [
2] showed that parallel inocula from the same test tube at the same time showed variable aggressiveness. Therefore, the probability is high that a given conidium concentration will result in different aggressiveness levels.
The previous research has identified a number of pathogenicity genes that were expressed very differently in different isolates [
66,
67,
68,
69]. The probability is very low that all of these genes correlate with aggressiveness [
20]. It is more probable that each
Fusarium isolate has a different mix (with unknown numbers and functions) of these genes. Therefore, the conidium concentration is only one of the traits that influence aggressiveness. For this reason, the belief that a given conidium concentration can reach the target to secure the wanted aggressiveness level is a false assumption, which is clearly shown by the cited literature [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61].
The comparison between field and laboratory aggressiveness tests [
18,
70] showed that the same inocula of the same isolate resulted in a good correlation. Since we did not regulate conidium concentration, we directly tested aggressiveness [
13,
27]. Wu et al. [
21] also found medium level correlations between seedling and FHB tests. These make it possible to screen inocula at the seedling stage, which is of benefit in FHB resistance tests.
The experience of several decades shows that the aggressiveness test will not alone resolve problems relating to resistance testing. It is clear that the low- and non-aggressive inocula can be discarded easily. However, one–two isolates have occasionally produced poor field results. This means that, although a previous aggressiveness test may serve better than an arbitrary conidium or fungal biomass concentration, it does not secure high aggressiveness for the experiments.
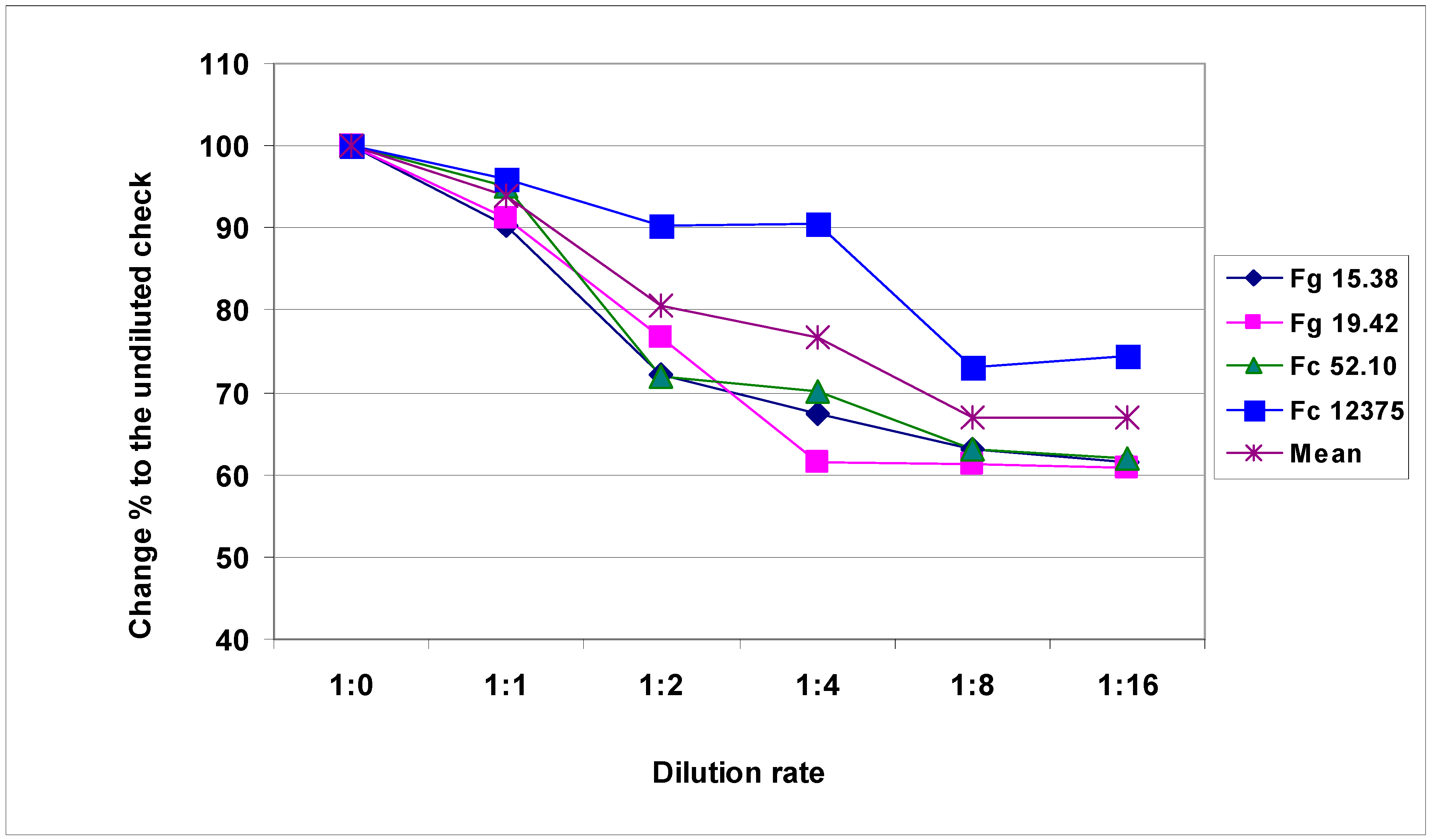
The aggressiveness test (
Figure S1, Table S1) shows the dilutability of the given inoculum. Better field performance is achieved with an inoculum where all ratings are zero, such as Fc 12375, e.g., during the evaluation, no healthy germ could be identified. Among inocula that have all zero at the original concentration and higher numbers at 1:1, 1:2, and 1:4 concentrations, disease severity in the field is lower. To secure high aggressiveness, typically dilution is not used, but when there is a shortage in inoculum, a dilution of 1:0.5 or 1:1 is suitable. The results shown here indicate that this dilution rate is unlikely to result in significant changes in aggressiveness [
71]. This also means that so much inoculum should be produced that under normal conditions would be enough for the whole experiment without changing inoculum.
The data clearly show that forecasting aggressiveness is not possible based on the conidium concentration and that the risk is greater when the level of FDK of DON level should be forecasted. In addition to conidium concentration, quantitative PCR is suitable for the estimation of fungal mass (Gosman et al. [
61]). The correlation between the fungal mass in the grain of different genotypes and DON contamination was r = 0.61. Furthermore, the number of colony-forming units can be used as the basis for regulation [
26]. However, without an aggressiveness test, they provide no advantage compared to the conidium concentration. It is well known that the production of DON is a virulence factor of the fungus. Therefore, selection for high aggressiveness is generally accompanied by a high DON production ability. Similarly, previous experience also has a major significance in choosing the right isolate.
In summary, dilution significantly changes the aggressiveness of the given inoculum, especially at higher rates. As shown in the cited literature, without information about the original concentration, the applied dilution rate cannot be known. Based on the largest and smallest conidium concentrations (104 and 106 /mL, respectively), the possible maximum dilution rate can reach 1:100. Because the dilution rate normally decreases the aggressiveness of the inoculum, it automatically decreases resistance differences, even if high aggressiveness is the focus. It is suggested that a direct aggressiveness test can better serve the targets of the experiment and possibly produce high aggressiveness. This is valid mainly for producing inocula for breeding, selection, and phenotyping purposes. In other tests, the conidium concentration will retain its significance.
3.2. Resistance Expression
To the best of our knowledge, the relationship between resistance expression and dilution has not been analyzed. The resistance level is genetically fixed, but the rate of the disease depends on epidemic severity, ecological, and other conditions that influence the build-up of an epidemic. In this process, both dilution and aggressiveness play an important role. Dilution has a direct influence on resistance expression. As for aggressiveness decreases, the probability of highly significant genotype differences is reduced. We found deviations in the response of different cultivars to different dilution rates. The genotype ranking was often different, and in FDK the maximum–minimum differences at a given dilution rate were wider by about 30%, and genotype ranking differed at different dilution rates. This means that dilution can strongly influence the maximum–minimum rates. Thus, the best and worst genotypes were the same, but the difference varied between them. In contrast, genotypes with smaller differences often changed position. Another problem is that one isolate produces only one aggressiveness level; therefore, the degree of resistance cannot be estimated properly; more parallel isolates provide more reliable results. Because F. graminearum and F. culmorum are highly pathogenic species, the selection of highly aggressive isolates is not a major concern. For less pathogenic species, however, this needs careful work.
The cultivar GK Csillag is worthy of interest. During the past 12 years, it has survived several epidemics of varying magnitudes. The reaction of the heads was comparable to a more susceptible cultivar, but DON contamination was significantly lower. The data clearly show that it was the second-best genotype in terms of DON; but for FDK and DI, it ranked only in fifth place. This finding was valuable, because for some time Csillag was the most popular cultivar in Hungary. Of the present genotype collection, the resistance level of F569/Kő is higher, but among regular winter wheat cultivars, this seldom occurs. The single yearly inoculation with four isolates did not yield a definitive result and only signalized a tendency. Rather than 4, in this test, we had 4 × 6 = 24 epidemic situations, thus proving the usefulness of additional DON resistance is a reality. This complexity and additional DON resistance were also proven in a previous methodical study [
16].
3.3. Breeding and Scientific Aspects
The tests clearly showed that the regulation of the conidium concentration—or, more specifically, the concentration of the CFU (which, in addition to the conidia, also includes mycelium fragments)—is not equivalent to the regulation of the aggressiveness. Through regulation of the aggressiveness by direct laboratory tests, we increased the probability of securing the desired level of aggressiveness [
13,
18]. From Takegami and Sasai [
22], we know that infection traits (such as mycelium) are equivalent to conidia. This is also valid for the conidia and ascospores [
24,
25].
Breeding programs require two types of inoculation methodology. The first is the production of large quantities of inoculum; 1000 L or more of inoculum should be prepared. Because this cannot be produced with ascospore, the conidia and conidium + mycelium-containing inocula should be chosen. Conidium production requires an additional cleaning phase to separate conidia from mycelium. For large quantities, this is a considerable problem. Nevertheless, using inocula with conidia and mycelium can provide a simple solution for this problem. The aggressiveness test provides direct information on the usefulness of the given inoculum to secure the infection pressure needed. Thus, scientific control can help the control of the inoculum production in the necessary quality. Because the single inoculum of the present methodology is not sufficiently reliable, even aggressiveness tests are made, this does not solve the problems coming from the use of single isolates. This is the reason of the suggestion to use parallel more isoltes. The testing of parental lines requires similar care. Because FDK and DON responses often differ from that of the disease index, their control is highly important in advanced stages of breeding. For mass selection, an aggressive isolate or a mixture of isolates is sufficient.