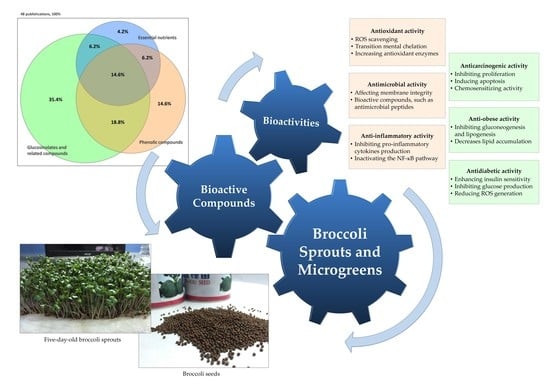
Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective
Abstract
:1. Introduction
2. Bioactive Compounds
2.1. Glucosinolates and Related Compounds
2.2. Phenolic Compounds
2.3. Other Compounds
2.4. Bioavailability of Bioactive Compounds
3. Biological Activities
3.1. Antioxidant Activity
| Study Type | Germination Time | Antioxidant Activity Assays | Sample Treatment | Ref. |
|---|---|---|---|---|
| In vitro | 1–9 days | DPPH | 50% methanol | [80] |
| In vitro | 3–14 days | DPPH, ABTS | 70% methanol | [54] |
| In vitro | 5 days | DPPH, ABTS, FRAP | 70% methanol | [78] |
| In vitro | 9 days | DPPH, FRAP | 90% methanol | [51] |
| In vitro | 4–12 days | DPPH, FRAP | Methanol | [14] |
| In vitro | 3–9 days | DPPH, FRAP, POD, CAT, SOD, GPX | 75% methanol | [48] |
| In vitro | 7 days | TEAC, ORAC | Methanol | [20] |
| In vitro | 2–9 days | T-AOC kit | 50% ethanol | [69] |
| In vitro | 9 days | DPPH, T-AOC Kit | 75% methanol | [44] |
| In vitro | 4 days | T-AOC Kit | 50% methanol | [63] |
| In vitro | 5–7 days | DPPH, ABTS, FRAP | Methanol | [79] |
| In vitro | 7 days | FRAP | 50% ethanol | [57] |
| In vitro | 7 days | FRAP | Boiling water | [58] |
| In vitro | 12 days | DPPH, ABTS | Ethanol | [45] |
| In vitro | 3–7days | DPPH | Dichloromethane | [67] |
| In vitro | 6 days | ABTS, FRAP, CHEL, LPO, LOXI, XOI, CAT | 50% ethanol | [76] |
| In vitro | 3–10 days | DPPH, ABTS, FRAP | 70% ethanol, 70% methanol, boiling water | [24] |
| In vitro | 3 days | DPPH | 80% methanol | [65] |
| In vitro | 5–12 days | DPPH, CHEL, LPO | Boiling water | [81] |
| In vitro | 3–11 days | DPPH | 80% methanol | [22] |
| In vitro | 5–9 days | DPPH | 70% ethanol | [77] |
| In vitro | NS | TEAC | Buffer solution | [46] |
| In vitro | 7 days | DPPH, MA/GC, Carboxylic Acid | Hexane, dichloromethane, acetone | [96] |
| In vitro | 8 days | DPPH, ABTS, FRAP | 60% methanol | [82] |
| In vitro | 6 days | ABTS, FRAP, CHEL, LPO, LOXI, XOI, CAT, SOD | Buffer solution | [83] |
| In vitro | 9 day | ORAC, TEAC | Buffer solution | [73] |
| In vitro | 6 days | LOXI, XOI | 50% methanol | [99] |
| In vivo | 4 days | FRAP, GPX | Male Wistar rats’ plasma | [72] |
| In vivo | NS | NQO1, HO-1 | Female SKH-1 mice’ skin tissue | [100] |
3.2. Anticancer Activity
| Study Type | Germination Time | Subjects | Potential Mechanisms | Ref. |
|---|---|---|---|---|
| In vitro | 3–7 days | HepG2, CT26 cells | Inhibiting cell proliferation | [45] |
| In vitro | 5 days | PC-3 cells | Inducing apoptosis; increasing ROS generation | [67] |
| In vitro | 4 days | HepG2, SW480, BJ cells | Inhibiting cell proliferation; inducing apoptosis | [70] |
| In vitro | 4–12 days | MAT-LyLu, AT-2 cells | Inhibiting cell proliferation and motility | [76] |
| In vitro | 5 days | HepG2, Caco-2, A549, FL83B cells | Inducing cell cycle arrest and apoptosis; decreasing MMP level | [24] |
| In vitro | 5 days | Caco-2, HT-29, HepG2 cells | Inhibiting cell proliferation | [59] |
| In vitro | 8 days | U251, MCF-7, 786-0, NCI-H460, HT-29 cells | Inhibiting cell proliferation | [82] |
| In vitro | 5 days | AGS cells | Inhibiting cell proliferation and motility | [83] |
| In vitro | 7 days | LNCaP, PC-3, DU-145 cells | Decreasing PSA secretion; inducing apoptosis | [71] |
| In vitro | NS | MCF7, SUM159 cells | Inhibiting cell proliferation; inducing apoptosis | [102] |
| In vitro | NS | A549, H460, H446, HCC827, H1975, H1299 cells | Inhibiting cell proliferation; inducing apoptosis | [25] |
| In vitro | NS | Caco-2, CCD18-Co cells | Inducing cell cycle arrest and apoptosis; decreasing MMP level; increasing ROS generation | [73] |
| In vivo | NS | Female NOD/SCID mice | Eliminating breast CSCs in vivo; downregulating Wnt/β-catenin pathway | [102] |
| In vivo | NS | Female nude BALB/c mice | Inhibiting the PI3K-AKT signaling pathway | [25] |
| In vivo | 3 days | Female SKH-1 hairless mice | Stabilizing p53; inducing phase 2 enzyme; inhibiting iNOS upregulation | [106] |
| In vivo | NS | Male TRAMP mice in C57BL/6 background | Decreasing HDAC3 protein expression | [103] |
| In vivo | NS | SV40 and Her2/neu mice | Modulating epigenetic pathways; regulating epigenetic-controlled gene expression | [107] |
| In vivo | NS | Female Her2/neu mice | Regulating tumor- and epigenetic-related gene expression; increasing tumor suppressor gene expression | [104] |
| In vivo | 3 days | Female Sprague-Dawley rats | Inducing GST and NQO1 | [105] |
3.3. Antimicrobial Activity
3.4. Anti-Inflammatory Activity
3.5. Antidiabetic Activity
3.6. Anti-Obesity Activity
3.7. Other Effects
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Godfray, H.C.J.; Garnett, T. Food security and sustainable intensification. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20120273. [Google Scholar] [CrossRef]
- Weber, C.F. Broccoli Microgreens: A Mineral-Rich Crop That Can Diversify Food Systems. Front. Nutr. 2017, 4, 7. [Google Scholar] [CrossRef]
- Aschemann, J.-W.; Peschel, A.O. How circular will you eat? The sustainability challenge in food and consumer reaction to either waste-to-value or yet underused novel ingredients in food. Food Qual. Prefer. 2019, 77, 15–20. [Google Scholar] [CrossRef]
- B Butkutė, B.; Taujenis, L.; Norkevičienė, E. Small-seeded legumes as a novel food source. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds-sprouted seeds-microgreens. Molecules 2019, 24, 133. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Prakash, D.; Gupta, S. Relationships between bioactive food components and their health benefits. In Introduction to Functional Food Science Textbook, 1st ed.; Create Space Independent Publishing Platform: Scotts Valley, CA, USA, 2013; pp. 66–85. [Google Scholar]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Le, T.N.; Sakulsataporn, N.; Chiu, C.-H. Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals 2020, 13, 82. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Nagarajan, J.; Krishnamurthy, N.P.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Ooi, C.W. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: Extraction, characterization and kinetic studies. Food Chem. 2019, 296, 47–55. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; Garcia-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- de la Fuente, B.; Lopez, G.-G.; Manez, V.; Alegria, A.; Barbera, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Rodriguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [Green Version]
- López, J.-C.; Tirado-Noriega, L.G.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; Núñez-Gastélum, J.A. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development. Int. J. Food Sci. Technol. 2013, 48, 2267–2275. [Google Scholar]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. (Phila) 2009, 2, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Luong, H.Q.; Li, H.P.; Chiu, C.H.; Hsieh, P.C. Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods 2019, 8, 532. [Google Scholar]
- Yang, M.; Wang, H.; Zhou, M.; Liu, W.; Kuang, P.; Liang, H.; Yuan, Q. The natural compound sulforaphene, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget 2016, 7, 76656. [Google Scholar] [CrossRef]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Cho, K.; Park, Y.U.; Choi, H.J.; Kim, S.Y. Sulforaphane-Enriched Broccoli Sprouts Pretreated by Pulsed Electric Fields Reduces Neuroinflammation and Ameliorates Scopolamine-Induced Amnesia in Mouse Brain through Its Antioxidant Ability via Nrf2-HO-1 Activation. Oxid. Med. Cell Longev. 2019, 2019, 3549274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods 2012, 4, 837–841. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef]
- Latte, K.P.; Appel, K.E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Alpharetta, GA, USA, 2019; pp. 23–54. [Google Scholar]
- Kovačević, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Galanakis, C.M. Evaluation of microwave-assisted extraction technology for separation of bioactive components of saffron (Crocus sativus L.). Ind. Crops Prod. 2020, 145, 111978. [Google Scholar] [CrossRef]
- Barba, F.J.; Galanakis, C.M.; Esteve, M.J.; Frigola, A.; Vorobiev, E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J. Food Eng. 2015, 167, 38–44. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Perez-Balibrea, S.; Moreno, D.A.; Garcia-Viguera, C. Glucosinolates in broccoli sprouts (Brassica oleracea var. italica) as conditioned by sulphate supply during germination. J. Food Sci. 2010, 75, 673–677. [Google Scholar]
- Guo, L.; Yang, R.; Guo, Q.; Gu, Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J. Funct. Foods 2014, 9, 70–77. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Yang, R.; Hui, Q.; Gu, Z.; Zhou, Y.; Guo, L.; Shen, C.; Zhang, W. Effects of CaCl2 on the metabolism of glucosinolates and the formation of isothiocyanates as well as the antioxidant capacity of broccoli sprouts. J. Funct. Foods 2016, 24, 156–163. [Google Scholar] [CrossRef]
- Kestwal, R.M.; Lin, C.; Bagal, D.; Chiang, B.H. Glucosinolates fortification of cruciferous sprouts by sulphur supplementation during cultivation to enhance anti-cancer activity. Food Chem. 2011, 126, 1164–1171. [Google Scholar] [CrossRef]
- Rychlik, J.; Olejnik, A.; Olkowicz, M.; Kowalska, K.; Juzwa, W.; Myszka, K.; Dembczyński, R.; Moyer, M.P.; Grajek, W. Antioxidant capacity of broccoli sprouts subjected to gastrointestinal digestion. J. Sci. Food Agric. 2015, 95, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Lelario, F.; Bianco, G.; Bufo, S.A.; Cataldi, T.R. Establishing the occurrence of major and minor glucosinolates in Brassicaceae by LC-ESI-hybrid linear ion-trap and Fourier-transform ion cyclotron resonance mass spectrometry. Phytochemistry 2012, 73, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, Y.; Wang, F. Calcium sulfate treatment enhances bioactive compounds and antioxidant capacity in broccoli sprouts during growth and storage. Postharvest Biol. Technol. 2018, 139, 12–19. [Google Scholar] [CrossRef]
- Moreira-Rodriguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [Green Version]
- Vicas, S.I.; Cavalu, S.; Laslo, V.; Tocai, M.; Costea, T.O.; Moldovan, L. Growth, Photosynthetic Pigments, Phenolic, Glucosinolates Content and Antioxidant Capacity of Broccoli Sprouts in Response to Nanoselenium Particles Supply. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 821–828. [Google Scholar] [CrossRef]
- Avila, F.W.; Faquin, V.; Yang, Y.; Ramos, S.J.; Guilherme, L.R.; Thannhauser, T.W.; Li, L. Assessment of the anticancer compounds Se-methylselenocysteine and glucosinolates in Se-biofortified broccoli (Brassica oleracea L. var. italica) sprouts and florets. J. Agric. Food Chem. 2013, 61, 6216–6223. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Lamers, S.; Capuano, E.; Dekker, M.; Verkerk, R. Bioavailability of Isothiocyanates From Broccoli Sprouts in Protein, Lipid, and Fiber Gels. Mol. Nutr. Food Res. 2018, 62, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Genotypic effects on the phytochemical quality of seeds and sprouts from commercial broccoli cultivars. Food Chem. 2011, 125, 348–354. [Google Scholar] [CrossRef]
- Lopez-Chillon, M.T.; Carazo-Diaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villano, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Silván, J.M.; Medina, S.; de Pascual, S.-T.; García, C.-V.; Moreno, D.A. Metabolism and antiproliferative effects of sulforaphane and broccoli sprouts in human intestinal (Caco-2) and hepatic (HepG2) cells. Phytochem. Rev. 2015, 14, 1035–1044. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Brüggemann, N.; Brodehl, A.; Schreiner, M.; Rohn, S.; Kroh, L.W. Characterization of products from the reaction of glucosinolate-derived isothiocyanates with cysteine and lysine derivatives formed in either model systems or broccoli sprouts. J. Agric. Food Chem. 2012, 60, 7735–7745. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and biochemical metabolism of germinating broccoli seeds and sprouts. J. Agric. Food Chem. 2012, 60, 209–213. [Google Scholar] [CrossRef]
- Fahey, J.W.; Holtzclaw, W.D.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Guo, L.; Zhou, Y.; Shen, C.; Gu, Z. Calcium mitigates the stress caused by ZnSO4 as a sulphur fertilizer and enhances the sulforaphane formation of broccoli sprouts. RSC Adv. 2015, 5, 12563–12570. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, A.A.; Saber, W.I.A.; Hassan, H.A. Increasing Antioxidant Content of Broccoli Sprouts Using Essential Oils During Cold Storage. Agriculture (Polnohospodárstvo) 2016, 62, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Guo, L.; Wang, Z.; Zhuang, Y.; Gu, Z. Response surface optimization and identification of isothiocyanates produced from broccoli sprouts. Food Chem. 2013, 141, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Sharma, U.; Vig, A.P.; Singh, B.; Arora, S. Free radical scavenging, antiproliferative activities and profiling of variations in the level of phytochemicals in different parts of broccoli (Brassica oleracea italica). Food Chem. 2014, 148, 373–380. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Lohr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Hu, H.; Liu, Y.; Pan, S. Optimisation of enzymatic production of sulforaphane in broccoli sprouts and their total antioxidant activity at different growth and storage days. J. Food Sci. Technol. 2017, 54, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Pasko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasinska, J.; Zmudzki, P.; Barton, H.; Zagrodzki, P.; Gorinstein, S. Comparative Study of Predominant Phytochemical Compounds and Proapoptotic Potential of Broccoli Sprouts and Florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Abdulah, R.; Faried, A.; Kobayashi, K.; Yamazaki, C.; Suradji, E.W.; Ito, K.; Suzuki, K.; Murakami, M.; Kuwano, H.; Koyama, H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer 2009, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Pasko, P.; Krosniak, M.; Prochownik, E.; Tyszka-Czochara, M.; Folta, M.; Francik, R.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Effect of broccoli sprouts on thyroid function, haematological, biochemical, and immunological parameters in rats with thyroid imbalance. Biomed. Pharmacother. 2018, 97, 82–90. [Google Scholar] [CrossRef]
- Fuente, B.; Lopez-Garcia, G.; Manez, V.; Alegria, A.; Barbera, R.; Cilla, A. Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Patras, A.; Stoleru, V.; Filimon, R.V.; Padureanu, S.; Chelariu, E.L.; Biliaderis, C.G. Influence of Sodium and Maturity Stage on the Antioxidant Properties of Cauliflower and Broccoli Sprouts. Not. Bot. Horti Agrobot. Cluj. Napoca 2017, 45, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Pajak, P.; Socha, R.; Galkowska, D.; Roznowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Chen, L.; Tan, G.J.T.; Pang, X.; Yuan, W.; Lai, S.; Yang, H. Energy Regulated Nutritive and Antioxidant Properties during the Germination and Sprouting of Broccoli Sprouts (Brassica oleracea var. italica). J. Agric. Food Chem. 2018, 66, 6975–6985. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric Characteristics, Polyphenols and Ascorbic Acid Variation in Brassica oleracea L. Novel Foods: Sprouts, Microgreens and Baby Leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Vale, A.P.; Cidade, H.; Pinto, M.; Oliveira, M.B. Effect of sprouting and light cycle on antioxidant activity of Brassica oleracea varieties. Food Chem. 2014, 165, 379–387. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Gawlik-Dziki, U.; Swieca, M.; Dziki, D.; Seczyk, L.; Zlotek, U.; Rozylo, R.; Kaszuba, K.; Ryszawy, D.; Czyz, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Yanaka, A. Daily intake of broccoli sprouts normalizes bowel habits in human healthy subjects. J. Clin. Biochem. Nutr. 2018, 62, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epriliati, I.; Ginjom, I.R. Bioavailability of phytochemicals. In Phytochemicals-a Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 401–428. [Google Scholar]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Panjwani, A.A.; Liu, H.; Cornblatt, G.; Cornblatt, B.S.; Ownby, S.L.; Fuchs, E.; Holtzclaw, W.D. Bioavailability of Sulforaphane Following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients 2019, 11, 1489. [Google Scholar] [CrossRef] [Green Version]
- Moreno, D.A.; Carvajal, M.; Lopez-Berenguer, C.; Garcia-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 193. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Jang, H.W.; Moon, J.K.; Shibamoto, T. Analysis and Antioxidant Activity of Extracts from Broccoli (Brassica oleracea L.) Sprouts. J. Agric. Food. Chem. 2015, 63, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on In Vivo and In Vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D. Enhancement of antioxidant abilities and the lipoxygenase and xanthine oxidase inhibitory activity of broccoli sprouts by biotic elicitors. Acta Sci. Pol. Hortorum Cultus. 2012, 11, 13–25. [Google Scholar]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Wade, K.L.; Jenkins, S.N.; Shapiro, T.A.; Fuchs, E.J.; Kerns, M.L.; Talalay, P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Alimbetov, D.; Askarova, S.; Davis, T.; Kipling, D. Pharmacological Targeting of Cell Cycle, Apoptotic and Cell Adhesion Signaling Pathways Implicated in Chemoresistance of Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1690. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Beaver, L.M.; Löhr, C.V.; Clarke, J.D.; Glasser, S.T.; Watson, G.W.; Wong, C.P.; Zhang, Z.; Williams, D.E.; Dashwood, R.H.; Shannon, J. Broccoli sprouts delay prostate cancer formation and decrease prostate cancer severity with a concurrent decrease in HDAC3 protein expression in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice. Curr. Dev. Nutr. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Wu, H.; Li, Y.; Tollefsbol, T.O. Maternal Epigenetic Regulation Contributes to Prevention of Estrogen Receptor–negative Mammary Cancer with Broccoli Sprout Consumption. Cancer Prev. Res. 2020, 13, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Munday, R.; Mhawech-Fauceglia, P.; Munday, C.M.; Paonessa, J.D.; Tang, L.; Munday, J.S.; Lister, C.; Wilson, P.; Fahey, J.W.; Davis, W. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008, 68, 1593–1600. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Jenkins, S.N.; Fahey, J.W.; Ye, L.; Wehage, S.L.; Liby, K.T.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006, 240, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Buckhaults, P.; Li, S.; Tollefsbol, T. Temporal Efficacy of a Sulforaphane-Based Broccoli Sprout Diet in Prevention of Breast Cancer through Modulation of Epigenetic Mechanisms. Cancer Prev. Res. (Phila) 2018, 11, 451–464. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.P.; Nascimento da Silva, L.C.; Martins da Fonseca, C.S.; de Araujo, J.M.; Correia, M.T.; Cavalcanti Mda, S.; Lima, V.L. Antimicrobial Activity and Phytochemical Analysis of Organic Extracts from Cleome spinosa Jaqc. Front. Microbiol. 2016, 7, 963. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.; Pisciottano, M.N.; Amorim, E.L.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid. Based Complement Alternat. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef]
- Tako, M.; Kerekes, E.B.; Zambrano, C.; Kotogan, A.; Papp, T.; Krisch, J.; Vagvolgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Cano, R.; Salcedo-Hernández, R.; López-Meza, J.; Bideshi, D.; Barboza-Corona, J. Antimicrobial activity of broccoli (Brassica oleracea var. italica) cultivar Avenger against pathogenic bacteria, phytopathogenic filamentous fungi and yeast. J. Appl. Microbiol. 2018, 124, 126–135. [Google Scholar] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Yeganeh, M.Z.; Hosseinpanah, F.; Zojaji, H.; Azizi, F. Complementary and alternative medicinal effects of broccoli sprouts powder on Helicobacter pylori eradication rate in type 2 diabetic patients: A randomized clinical trial. J. Funct. Foods 2014, 7, 390–397. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trost, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Sotokawauchi, A.; Ishibashi, Y.; Matsui, T.; Yamagishi, S.I. Aqueous Extract of Glucoraphanin-Rich Broccoli Sprouts Inhibits Formation of Advanced Glycation End Products and Attenuates Inflammatory Reactions in Endothelial Cells. Evid. Based Complement Alternat. Med. 2018, 2018, 9823141. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Nagata, N.; Ota, T. Glucoraphanin: A broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte 2018, 7, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, A.M.; Armstrong, E.A.; Scott, O.; Juurlink, B.J.H.; Yager, J.Y. Broccoli sprout supplementation during pregnancy prevents brain injury in the newborn rat following placental insufficiency. Behav. Brain. Res. 2015, 291, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Gonzalez-Trujano, M.E.; Guadarrama-Enriquez, O.; Pellicer, F.; Garcia-Viguera, C.; Moreno, D.A. Broccoli sprouts in analgesia—Preclinical in vivo studies. Food Funct. 2017, 8, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Noah, T.L.; Zhang, H.; Zhou, H.; Glista-Baker, E.; Muller, L.; Bauer, R.N.; Meyer, M.; Murphy, P.C.; Jones, S.; Letang, B.; et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: A randomized, double-blind study. PLoS ONE 2014, 9, e98671. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| No. | Compounds | Molecular Formula | Germination Time | Characterization Method | Ref. |
|---|---|---|---|---|---|
| Aliphatic Glucosinolates | |||||
| 1 | Sinigrin | C10H17NO9S2 | 4–12 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS | [14,45,46,47] |
| 2 | Gluconapin | C11H19NO9S2 | 3–12 days | HPLC-UV, HPLC-DAD-MS | [14,45,46,48] |
| 3 | Progoitrin | C11H19NO10S2 | 3–12 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [14,21,45,46,47,49,50,51] |
| 4 | Glucocochlearin | C11H21NO9S2 | NS | HPLC-ESI-MS | [47] |
| 5 | Glucoconringianin | C11H21NO9S2 | NS | HPLC-ESI-MS | [47] |
| 6 | Glucoiberverin | C11H21NO9S3 | 4–12 days | HPLC-DAD | [14,47] |
| 7 | Glucosativin | C11H21NO9S3 | NS | HPLC-ESI-MS | [47] |
| 8 | Glucoiberin | C11H21NO10S3 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS | [14,19,21,41,45,46,47,49,50,51,52,53,54,55] |
| 9 | Glucoraphenin | C12H21NO10S3 | 8 days | HPLC-DAD | [19] |
| 10 | Glucojiaputin | C12H23NO9S2 | NS | HPLC-ESI-MS | [47] |
| 11 | 3-Methylbutyl-GLS | C12H23NO9S2 | NS | HPLC-ESI-MS | [47] |
| 12 | 3-Methylpentyl-GLS | C13H25NO9S2 | NS | HPLC-ESI-MS | [47] |
| 13 | 4-Methylpentyl-GLS | C13H25NO9S2 | NS | HPLC-ESI-MS | [47] |
| 14 | Glucoerucin | C12H23NO9S3 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS, UHPLC-MS/MS | [14,19,21,41,45,46,48,49,51,52,53,54,55,56,57,58,59] |
| 15 | Glucoraphanin | C12H23NO10S3 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS, UHPLC-MS/MS | [14,19,21,41,42,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] |
| 16 | n-Pentyl-GLS | C13H25NO9S3 | 4–12 days | HPLC-DAD | [14] |
| 17 | Glucoalyssin | C13H25NO10S3 | 3–12 days | HPLC-DAD, HPLC-MS/MS, HPLC-ESI-MS | [14,47,50,52,57,58] |
| 18 | Glucohirsutin | C16H31NO10S3 | 12 days | HPLC-UV | [45] |
| 19 | Diglucothiobeinin | C17H31NO14S4 | NS | HPLC-ESI-MS | [47] |
| Aromatic glucosinolates | |||||
| 20 | Glucosinalbin | C14H19NO10S2 | 4–12 days | HPLC-DAD | [14] |
| 21 | Gluconasturtiin | C15H21NO9S2 | 4–12 days | HPLC-DAD | [14] |
| Indolic glucosinolates | |||||
| 22 | n-Hexyl-GLS | C13H25NO9S2 | 4–12 days | HPLC-DAD | [14] |
| 23 | Glucobrassicin | C16H20N2O9S2 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS | [19,21,41,45,46,48,49,50,51,52,54,55,57,58,59] |
| 24 | 4-Hydroxy-GLB | C16H20N2O10S2 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS | [14,19,21,41,46,48,49,50,51,52,54,55,57,58,59] |
| 25 | 4-Methoxy-GLB | C17H22N2O10S2 | 3–12 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS | [14,19,21,41,45,48,49,51,52,55,57,58,59] |
| 26 | Neoglucobrassicin | C17H22N2O10S2 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS, HPLC-MS/MS | [14,19,21,41,45,46,48,50,51,52,54,55,57,58,59] |
| Total glucosinolates | 3–12 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, GOD/PAP kit | [14,19,43,44,55,63,64,65] | ||
| No. | Compounds | Molecular Formula | Germination Time | Characterization Method | Ref. |
|---|---|---|---|---|---|
| 1 | Butyronitrile | C4H7N | 3–7 days | GC–MS | [67] |
| 2 | Allyl isothiocyanate | C4H5NS | 4 days | GC–MS | [66] |
| 3 | 2-Methyl-2-nitropropane | C4H9NO2 | 3–7 days | GC–MS | [67] |
| 4 | 4-(Methylthio)-butanenitrile | C5H9NS | 5 days | GC–MS | [42] |
| 5 | Butyl isothiocyanate | C5H9NS | 4–9 days | GC–MS | [42,44,66,67] |
| 6 | Isobutyl isothiocyanate | C5H9NS | 4 days | GC–MS | [19] |
| 7 | Iberin | C5H9NOS2 | 4–8 days | HPLC-DAD, UHPLC-MS/MS, GC–MS | [19,53,66] |
| 8 | 4-Isothiocyanato-1-butene | C6H9NS2 | 4–9 days | GC–MS | [42,44,66] |
| 9 | 3-Methylbutyl isothiocyanate | C6H11NS | 4 days | GC–MS | [66] |
| 10 | Isoamyl methyl sulfoxide | C6H14OS | 3–7 days | GC–MS | [67] |
| 11 | Erucin | C6H11NS2 | 3–9 days | UHPLC-MS/MS, GC–MS | [19,44,56,61,67] |
| 12 | Sulforaphene | C6H9NOS2 | 8 days | UHPLC-MS/MS | [19] |
| 13 | Sulforaphane | C6H11NOS2 | 2–12 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS, HPLC-MS/MS, UHPLC-MS/MS, GC–MS, GC−FID | [19,22,42,44,46,48,53,56,57,59,60,61,62,63,64,66,67,68,69,70,71,72] |
| 14 | Indole-3-carbinol | C9H9NO | 8 days | UHPLC-MS/MS | [19] |
| 15 | Indole-3-carboxylic acid | C9H7NO2 | NS | HPLC-DAD-MS | [46] |
| 16 | Indole-3-acetic acid | C10H9NO2 | NS | HPLC-DAD-MS | [46] |
| 17 | 1-Methoxyindole-3-carbaldehyde | C10H9NO2 | NS | HPLC-DAD-MS | [46] |
| Total isothiocyanates | 8 days | UV/Vis, UHPLC-MS | [19,20,73] |
| No. | Compounds | Molecular Formula | Germination Time | Characterization Method | Ref. |
|---|---|---|---|---|---|
| Phenolic acids and derivatives | |||||
| 1 | Benzoic acid | C7H6O2 | 6 days | HPLC-UV | [76] |
| 2 | Salicylic acid | C7H6O3 | 6 days | HPLC-UV | [76] |
| 3 | p-Hydroxybenzoic acid | C7H6O3 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 4 | Protocatechuic acid | C7H6O4 | 5 days | HPLC-UV, HPLC-DAD-MS | [46,78] |
| 5 | Gentisic acid | C7H6O4 | 4 days | HPLC-UV | [70,72] |
| 6 | Gallic acid | C7H6O5 | 5–8 days | HPLC-UV, HPLC-DAD-MS, HPLC-ESI-MS | [21,24,46,49,76,78,79,80] |
| 7 | Vanillic acid | C8H8O | 5 days | HPLC-UV | [78] |
| 8 | p-Coumaric acid | C9H8O3 | 4–7 days | HPLC-DAD, HPLC-UV | [70,72,78,79,80] |
| 9 | Esculetin | C9H6O4 | 5 days | HPLC-UV | [24] |
| 10 | Caffeic acid | C9H8O4 | 4–7 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS | [24,43,72,78,79,80] |
| 11 | Ferulic acid | C10H10O4 | 3–14 days | HPLC-UV, HPLC-DAD, HPLC-DAD-MS | [24,54,70,72,76,78,79] |
| 12 | Sinapic acid | C11H12O5 | 3–12 days | HPLC-UV, HPLC-DAD-MS, HPLC-ESI-MS | [14,19,21,43,46,49,54,70,72,76,78,79] |
| 13 | Gallic acid hexoside | C13H16O10 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 14 | Gallic acid 4-O-glucoside | C13H16O10 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 15 | Sinapoyl malate | C15H16O9 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 16 | Isochlorogenic acid | C16H18O9 | 4 days | HPLC-UV | [70] |
| 17 | Chlorogenic acid | C16H18O9 | 3–12 days | HPLC-DAD, HPLC-UV | [14,70,72,76,78,79,80] |
| 18 | Caffeoyl-quinic acid | C16H18O9 | 3–8 days | HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [21,43,49] |
| 19 | 1-O-sinapoyl-β-D-glucose | C17H22O10 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 20 | 5-O-Sinapoylquinic acid | C18H22O10 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 21 | Digalloyl hexoside | C20H20O14 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 22 | 1,2-Diferuloylgentiobiose | C32H38O19 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 23 | 2-Feruloyl-1-sinapoylgentiobiose | C33H40O18 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 24 | 1,2-Disinapoylgentiobiose | C34H42O19 | 7–8 days | HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [46,49] |
| 25 | 1-Sinapoyl-2,2′-diferuloylgentiobiose | C43H46O24 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 26 | 2-Feruloyl-1,2’-disinapoylgentiobiose | C44H50O22 | 7–8 days | HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [21,46,49] |
| 27 | 1,2-Disinapoyl-1′-ferulolylgentiobiose | C44H50O23 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 28 | 1,2,2′-Trisinapoylgentiobiose | C45H52O23 | 7–8 days | HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [21,46,49] |
| 29 | Gallotannic acid | C76H52O46 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| Flavonoids and derivatives | |||||
| 30 | Apigenin | C15H10O5 | 5 days | HPLC-UV | [72,78,80] |
| 31 | Kaempferol | C15H10O6 | 5–12 days | HPLC-UV, HPLC-DAD-MS, HPLC-ESI-MS | [14,46,50,76,78,79,80] |
| 32 | Luteolin | C15H10O6 | 5 days | HPLC-UV | [72,78] |
| 33 | Quercetin | C15H10O7 | 5–12 days | HPLC-UV, HPLC-DAD, HPLC-ESI-MS | [14,24,46,50,72,76,78,79] |
| 34 | Myricetin | C15H10O8 | 5 days | HPLC-UV | [24,72] |
| 35 | Astragalin | C21H20O11 | 7–8 days | HPLC-DAD, HPLC-ESI-MS | [21,49] |
| 36 | Rutin | C27H30O16 | NS | HPLC-DAD-MS | [46] |
| 37 | Robinin | C33H40O19 | 4 days | HPLC-UV | [70,72] |
| Total phenolic compounds | 3–14 days | UV/Vis, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [20,24,48,51,54,57,64,65,70,73,77,78,80,81,82,83] | ||
| Total flavonoid compounds | 3–14 days | UV/Vis, HPLC-DAD, HPLC-DAD-MS, HPLC-ESI-MS | [14,22,24,50,54,64,65,70,77,78,81] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.N.; Chiu, C.-H.; Hsieh, P.-C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. https://doi.org/10.3390/plants9080946
Le TN, Chiu C-H, Hsieh P-C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants. 2020; 9(8):946. https://doi.org/10.3390/plants9080946
Chicago/Turabian StyleLe, Thanh Ninh, Chiu-Hsia Chiu, and Pao-Chuan Hsieh. 2020. "Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective" Plants 9, no. 8: 946. https://doi.org/10.3390/plants9080946
APA StyleLe, T. N., Chiu, C.-H., & Hsieh, P.-C. (2020). Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants, 9(8), 946. https://doi.org/10.3390/plants9080946