Phytochemical Assessment of Native Ecuadorian Peppers (Capsicum spp.) and Correlation Analysis to Fruit Phenomics
Abstract
:1. Introduction
2. Results
2.1. Biochemical Analysis
2.2. Morphological Assessment with Conventional and TA Descriptors
2.3. Differences Among Geographical Regions
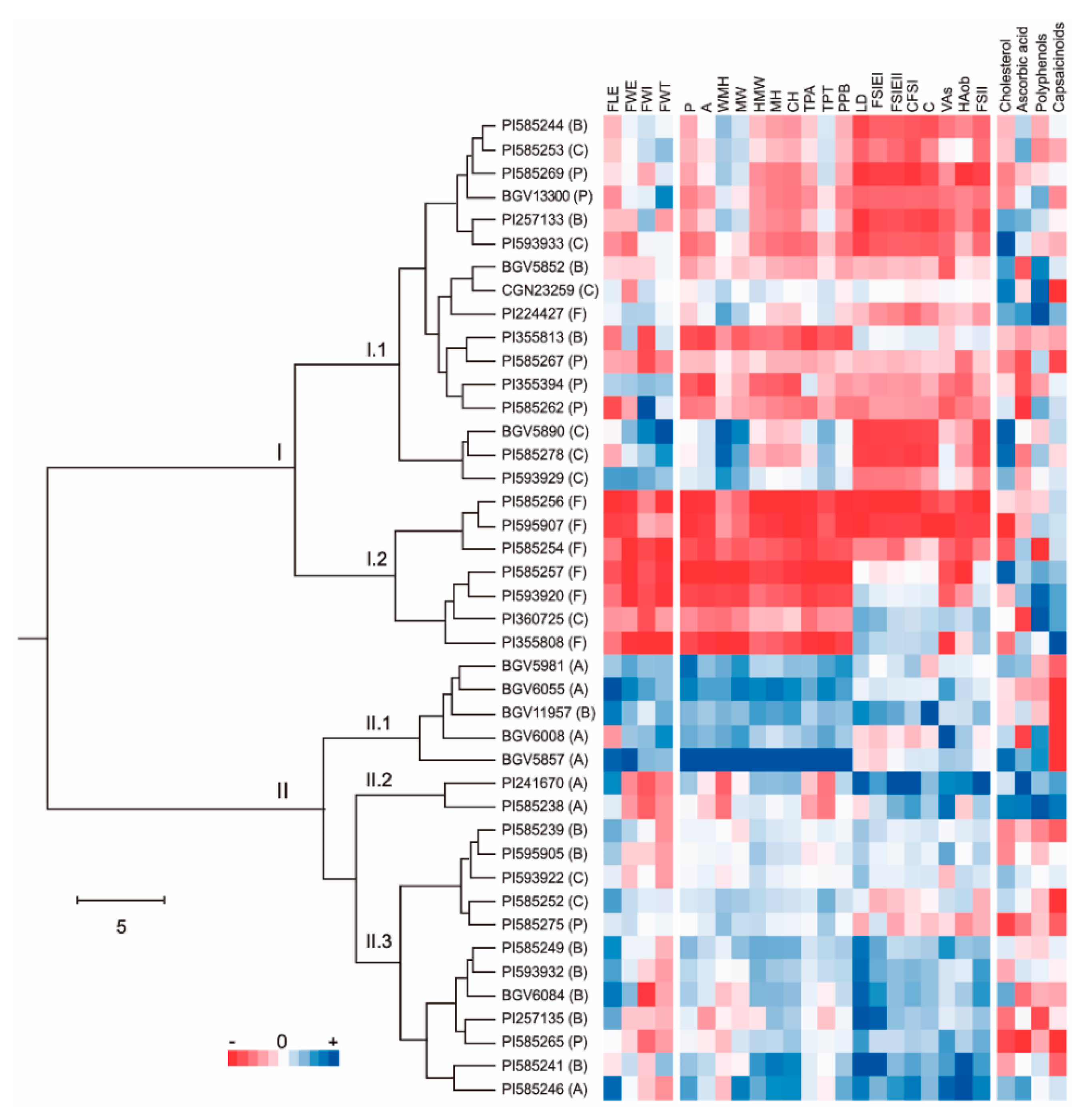
2.4. Correlation between Nutraceutical and Morphological Parameters
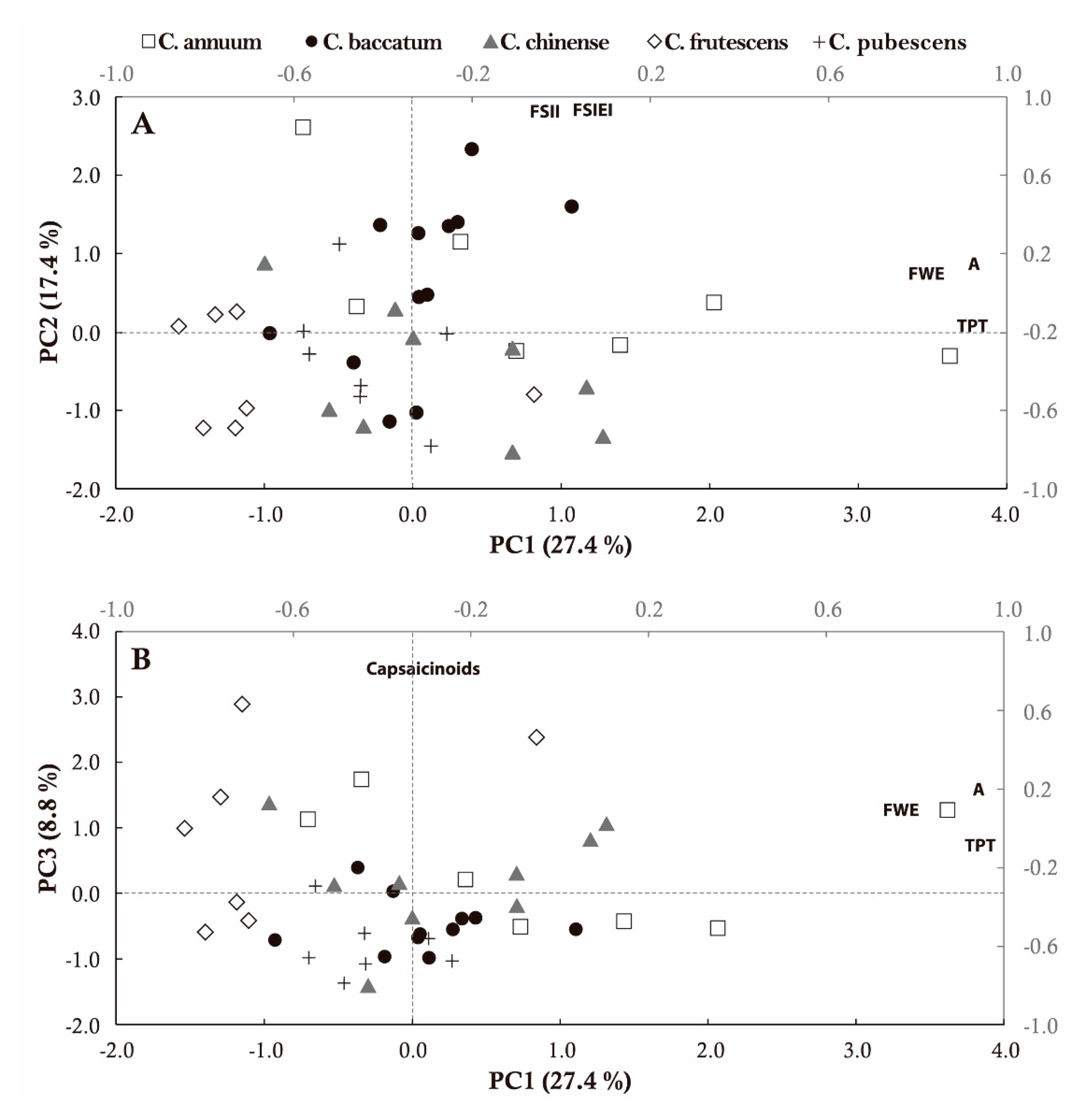
2.5. Multivariate Analysis
3. Discussion
4. Materials and Methods
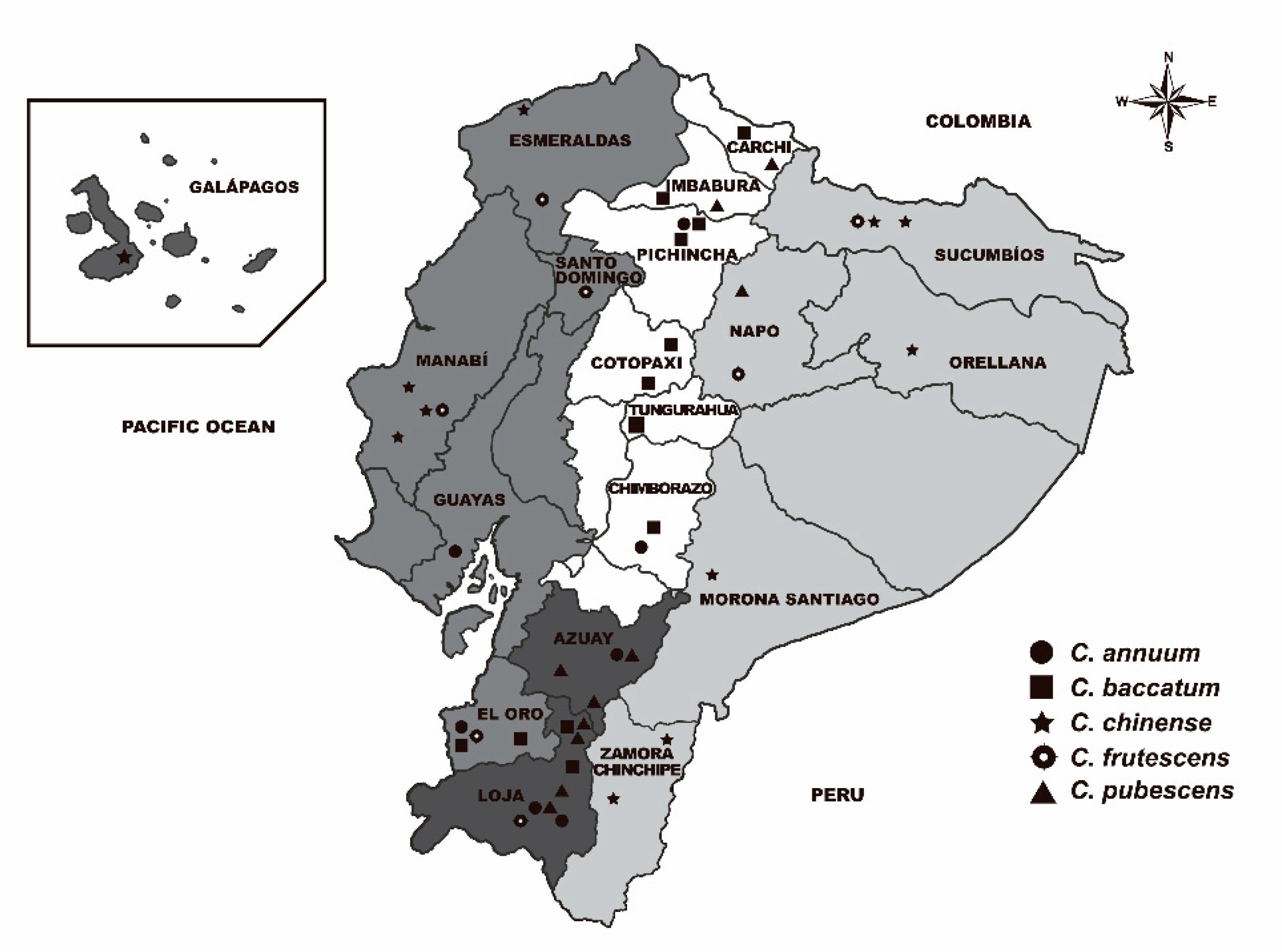
4.1. Plant Material
4.2. Conventional Descriptors Assessment
4.3. Fruit Characterization with Tomato Analyzer
4.4. Analytical Methods
4.4.1. Analysis of Ascorbic Acid
4.4.2. Determination of Total Polyphenols Content
4.4.3. Determination of the Inhibition Capability in Preventing Cholesterol Oxidation during Heating
4.4.4. Extraction and Analysis of Capsaicinoids
4.5. Data Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops and Livestock Products. 2018. Available online: http://faostat.fao.org/site/535/default.aspx#ancor (accessed on 19 April 2020).
- Bosland, P.W.; Votava, E.J. Peppers: Vegetable and Spice Capsicum, 2nd ed.; CABI Publishing: Wallingford, UK, 2012. [Google Scholar]
- Walsh, B.M.; Hoot, S.B. Phylogenetic Relationships of Capsicum (Solanaceae) Using DNA Sequences from Two Noncoding Regions: The Chloroplast atpB-rbcL Spacer Region and Nuclear waxy Introns. Int. J. Plant Sci. 2001, 162, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Onus, A.N.; Pickersgill, B. Unilateral Incompatibility in Capsicum (Solanaceae): Occurrence and Taxonomic Distribution. Ann. Bot. 2004, 94, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch Fossils and the Domestication and Dispersal of Chili Peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrizo García, C.; Barfuss, M.H.J.; Sehr, E.M.; Barboza, G.E.; Samuel, R.; Moscone, E.A.; Ehrendorfer, F. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann. Bot. 2016, 118, 35–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CarrizoGarcía, C.; Sterpetti, M.; Volpi, P.; Ummarino, M.; Saccardo, F. Wild capsicums: Identification and in situ analysis of Brazilian species. In Breakthroughs in the Genetics and Breeding of Capsicum and Eggplant; Lanteri, S., Rotino, G.L., Eds.; Eucarpia: Torino, Italy, 2013; pp. 205–213. [Google Scholar]
- Djian-Caporalino, C.; Lefebvre, V.; Sage-Daubéze, A.M.; Palloix, A. Capsicum. In Genetic Resources, Chromosome Engineering, and Crop Improvement: Vegetable Crops; Singh, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 3, pp. 185–243. [Google Scholar]
- van Zonneveld, M.; Ramirez, M.; Williams, D.E.; Petz, M.; Meckelmann, S.; Avila, T.; Bejarano, C.; Ríos, L.; Peña, K.; Jäger, M.; et al. Screening Genetic Resources of Capsicum Peppers in Their Primary Center of Diversity in Bolivia and Peru. PLoS ONE 2015, 10, e0134663. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S.; Garcés-Claver, A.; Mallor, C.; Sáenz de Miera, L.E.; Fayos, O.; Pomar, F.; Merino, F.; Silvar, C. New Insights into Capsicum spp. Relatedness and the Diversification Process of Capsicum annuum in Spain. PLoS ONE 2014, 9, e116276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvar, C.; García-González, C.A. Deciphering Genetic Diversity in the Origins of Pepper (Capsicum spp.) and Comparison with Worldwide Variability. Crop Sci. 2016, 56, 3100–3111. [Google Scholar] [CrossRef]
- Pereira-Dias, L.; Vilanova, S.; Fita, A.; Prohens, J.; Rodríguez-Burruezo, A. Genetic diversity, population structure, and relationships in a collection of pepper (Capsicum spp.) landraces from the Spanish centre of diversity revealed by genotyping-by-sequencing (GBS). Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for the Conduct of Tests, for dIstinctness, Uniformity and Stability. Available online: https://www.upov.int/en/publications/tg-rom/tg076/tg_76_8.pdf (accessed on 13 June 2020).
- Figàs, M.R.; Prohens, J.; Casanova, C.; Fernández-de-Córdova, P.; Soler, S. Variation of morphological descriptors for the evaluation of tomato germplasm and their stability across different growing conditions. Sci. Hortic. 2018, 238, 107–115. [Google Scholar] [CrossRef]
- Lobos, G.A.; Camargo, A.V.; del Pozo, A.; Araus, J.L.; Ortiz, R.; Doonan, J.H. Editorial: Plant Phenotyping and Phenomics for Plant Breeding. Front. Plant Sci. 2017, 8, 2181. [Google Scholar] [CrossRef] [Green Version]
- Brewer, M.T.; Lang, L.; Fujimura, K.; Dujmovic, N.; Gray, S.; van der Knaap, E. Development of a Controlled Vocabulary and Software Application to Analyze Fruit Shape Variation in Tomato and Other Plant Species. Plant Physiol. 2006, 141, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, G.R.; Moyseenko, J.B.; Robbins, M.D.; Morejón, N.H.; Francis, D.M.; van der Knaap, E. Tomato Analyzer: A Useful Software Application to Collect Accurate and Detailed Morphological and Colorimetric Data from Two-dimensional Objects. JoVE J. Vis. Exp. 2010, 37, e1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalo, M.J.; Brewer, M.T.; Anderson, C.; Sullivan, D.; Gray, S.; van der Knaap, E. Tomato Fruit Shape Analysis Using Morphometric and Morphology Attributes Implemented in Tomato Analyzer Software Program. J. Am. Soc. Hortic. Sci. 2009, 134, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, G.R.; Muños, S.; Anderson, C.; Sim, S.-C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the Tomato Germplasm and the Relationship to Fruit Shape Diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fernández-de-Córdova, P.; Fita, A.; Soler, S. Characterization of a collection of local varieties of tomato (Solanum lycopersicum L.) using conventional descriptors and the high-throughput phenomics tool Tomato Analyzer. Genet. Resour. Crop Evol. 2015, 62, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, M.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Andújar, I.; Prohens, J. Phenomics of fruit shape in eggplant (Solanum melongena L.) using Tomato Analyzer software. Sci. Hortic. 2013, 164, 625–632. [Google Scholar] [CrossRef]
- Plazas, M.; Andújar, I.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Conventional and phenomics characterization provides insight into the diversity and relationships of hypervariable scarlet (Solanum aethiopicum L.) and gboma (S. macrocarpon L.) eggplant complexes. Front. Plant Sci. 2014, 5, 318. [Google Scholar] [CrossRef] [Green Version]
- Naegele, R.P.; Mitchell, J.; Hausbeck, M.K. Genetic Diversity, Population Structure, and Heritability of Fruit Traits in Capsicum annuum. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Tripodi, P.; Greco, B. Large Scale Phenotyping Provides Insight into the Diversity of Vegetative and Reproductive Organs in a Wide Collection of Wild and Domesticated Peppers (Capsicum spp.). Plants 2018, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Dias, L.; Fita, A.; Vilanova, S.; Sánchez-López, E.; Rodríguez-Burruezo, A. Phenomics of elite heirlooms of peppers (Capsicum annuum L.) from the Spanish centre of diversity: Conventional and high-throughput digital tools towards varietal typification. Sci. Hortic. 2020, 265, 109245. [Google Scholar] [CrossRef]
- Nankar, A.N.; Tringovska, I.; Grozeva, S.; Todorova, V.; Kostova, D. Application of high-throughput phenotyping tool Tomato Analyzer to characterize Balkan Capsicum fruit diversity. Sci. Hortic. 2020, 260, 1–12. [Google Scholar] [CrossRef]
- Nankar, A.N.; Tringovska, I.; Grozeva, S.; Ganeva, D.; Kostova, D. Tomato Phenotypic Diversity Determined by Combined Approaches of Conventional and High-Throughput Tomato Analyzer Phenotyping. Plants 2020, 9, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary Metabolites of Capsicum Species and Their Importance in the Human Diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Mendes, N.; Gonçalves, É.C.B.d.A. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Wabaidur, S.M.; Khan, M.R.; Abdel Ghafar, A.; Habila, M.A.; Ahmed, Y.B.H. Determination of capsaicinoids in Capsicum species using ultra performance liquid chromatography-mass spectrometry. J. Sep. Sci. 2012, 35, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.-Z.; et al. Vitamin Variation in Capsicum Spp. Provides Opportunities to Improve Nutritional Value of Human Diets. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Gnayfeed, M.H.; Daood, H.G.; Biacs, P.A.; Alcaraz, C.F. Content of bioactive compounds in pungent spice red pepper (paprika) as affected by ripening and genotype. J. Sci. Food Agric. 2001, 81, 1580–1585. [Google Scholar] [CrossRef]
- Carvalho, A.V.; Mattietto, R.A.; Rios, A.O.; Maciel, R.A.; Moresco, K.S.; Oliveira, T.C.S. Bioactive compounds and antioxidant activity of pepper (Capsicum spp.) genotypes. J. Food Sci. Technol. 2015, 11, 7457–7464. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Morel, D.W.; Lin, C.Y. Cellular biochemistry of oxysterols derived from the diet or oxidation in vivo. J. Nutr. Biochem. 1996, 7, 495–506. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Z.; Wu, C.-T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant Activities of Different Colored Sweet Bell Peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of Environments on the Accumulation of Capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581. [Google Scholar] [CrossRef]
- Tripodi, P.; Cardi, T.; Bianchi, G.; Migliori, C.A.; Schiavi, M.; Rotino, G.L.; Lo Scalzo, R. Genetic and environmental factors underlying variation in yield performance and bioactive compound content of hot pepper varieties (Capsicum annuum) cultivated in two contrasting Italian locations. Eur. Food Res. Technol. 2018, 244, 1555–1567. [Google Scholar] [CrossRef]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits Consumed in the Western Coastal Region of Ecuador as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Balladares, D.; Castañeda-Terán, M.; Granda-Albuja, M.G.; Tejera, E.; Iturralde, G.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits, Tubers and Legumes Traditionally Consumed in the Andean Regions of Ecuador as a Source of Health-Promoting Compounds. Plant Foods Hum. Nutr. 2019, 74, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Afolayan, A.J. Comparison of nutritional, antioxidant vitamins and capsaicin contents in Capsicum annuum and C. frutescens. Int. J. Veg. Sci. 2020, 26, 190–207. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef]
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.J.; Ríos, L.; Peña, K.; Ugas, R.; Quinonez, L.; Mueller-Seitz, E.; Petz, M. Compositional Characterization of Native Peruvian Chili Peppers (Capsicum spp.). J. Agric. Food Chem. 2013, 61, 2530–2537. [Google Scholar] [CrossRef]
- AOAC Official Method 995.03. Capsaicinoids in Capsicum and their extractives. In AOAC Official Methods of Analysis, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000; pp. 14–16. [Google Scholar]
- Sarpras, M.S.; Gaur, R.; Sharma, V.; Chhapekar, S.S.; Das, J.; Kumar, A.; Yadava, S.K.; Nitin, M.; Brahma, V.; Abraham, S.K.; et al. Comparative Analysis of Fruit Metabolites and Pungency Candidate Genes Expression between Bhut Jolokia and Other Capsicum Species. PLoS ONE 2016, 11, 1–19. [Google Scholar]
- Bogusz, S.; Libardi, S.H.; Dias, F.F.; Coutinho, J.P.; Bochi, V.C.; Rodrigues, D.; Melo, A.M.; Godoy, H.T. Brazilian Capsicum peppers: Capsaicinoid content and antioxidant activity. J. Sci. Food Agric. 2018, 98, 217–224. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.C.d.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative Study of Capsaicinoid Composition in Capsicum Peppers Grown in Brazil. Int. J. Food Prop. 2016, 19, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of Different Capsicum Varieties by Evaluation of Their Capsaicinoids Content by High Performance Liquid Chromatography, Determination of Pungency and Effect of High Temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef] [PubMed]
- Souza-Sora, G.T.; Haminiuk, C.W.I.; Vieira da Silva, M.; Ferreira Zielinski, A.A.; Almeida Gonçalves, G.; Bracht, A.; Peralta, R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT—Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Meckelmann, S.W.; Jansen, C.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Phytochemicals in native Peruvian Capsicum pubescens (Rocoto). Eur. Food Res. Technol. 2015, 241, 817–825. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, H.; González-Hernández, V.A.; Cruz-Pérez, A.B.; Pérez-Grajales, M.; Gutiérrez-Espinosa, M.A.; Gardea-Béjar, A.A.; Gómez-Lim, M.Á. Inheritance of capsaicinoids in manzano hot chili pepper (Capsicum pubescens R. and P.). Agrociencia 2010, 44, 655–665. [Google Scholar]
- Kollmannsberger, H.; Rodríguez-Burruezo, A.; Nitz, S.; Nuez, F. Volatile and capsaicinoid composition of ají (Capsicum baccatum) and rocoto (Capsicum pubescens), two Andean species of chile peppers. J. Sci. Food Agric. 2011, 91, 1598–1611. [Google Scholar] [CrossRef]
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Capsaicinoids, flavonoids, tocopherols, antioxidant capacity and color attributes in 23 native Peruvian chili peppers (Capsicum spp.) grown in three different locations. Eur. Food Res. Technol. 2015, 240, 273–283. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Gurnani, N.; Gupta, M.; Mehta, D.; Mehta, B.K. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). J. Taibah Univ. Sci. 2016, 10, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, M.N.; Wolf, W.; Tevini, D.; Jung, G. Influence of processing parameters on the drying of spice paprika. J. Food Eng. 2001, 49, 63–72. [Google Scholar] [CrossRef]
- da Silveira Agostini-Costa, T.; da Silva Gomes, I.; Palhares de Melo, L.A.M.; Reifschneider, F.J.B.; da Costa Ribeiro, C.S. Carotenoid and total vitamin C content of peppers from selected Brazilian cultivars. J. Food Compos. Anal. 2017, 57, 73–79. [Google Scholar] [CrossRef]
- FDA, Center for Food Safety and Applied Nutrition: Labeling & Nutrition-Guidance for Industry: A Food Labeling Guide (14. Appendix F: Calculate the Percent Daily Value for the Appropriate Nutrients). Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm064928.html (accessed on 14 April 2020).
- Paran, I.; Fallik, E. Breeding for Fruit Quality in Pepper (Capsicum spp.). In Breeding for Fruit Quality; Jenks, M.A., Bebeli, B.J., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 307–322. [Google Scholar]
- Bozokalfa, M.K.; Esiyok, D.; Turhan, K. Patterns of phenotypic variation in a germplasm collection of pepper (Capsicum annuum L.) from Turkey. Span. J. Agric. Res. 2009, 7, 83–95. [Google Scholar] [CrossRef]
- Rivera, A.; Monteagudo, A.B.; Igartua, E.; Taboada, A.; García-Ulloa, A.; Pomar, F.; Riveiro-Leira, M.; Silvar, C. Assessing genetic and phenotypic diversity in pepper (Capsicum annuum L.) landraces from North-West Spain. Sci. Hortic. 2016, 203, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Asnin, L.; Park, S.W. Isolation and Analysis of Bioactive Compounds in Capsicum Peppers. Crit. Rev. Food Sci. Nutr. 2015, 55, 254–289. [Google Scholar] [CrossRef]
- Qin, C.; Changshui, Y.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Z.; Gu, X.; Mao, S.; Li, X.; Chadœuf, J.; Palloix, A.; Wang, L.; Zhang, B. Genetic diversity of pepper (Capsicum spp.) germplasm resources in China reflects selection for cultivar types and spatial distribution. J. Integr. Agric. 2016, 15, 1991–2001. [Google Scholar] [CrossRef]
- Silvar, C.; García-González, C.A. Screening old peppers (Capsicum spp.) for disease resistance and pungency-related traits. Sci. Hortic. 2017, 218, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Xue-Jun, C.; Rong, F.; Kun-Hua, Z.; Nan-Sheng, M.; Chang-Lin, H. Major gene plus polygene inheritance analysis of fruit traits in pepper. Acta Bot. Boreali-Occident. Sin. 2012, 32, 246–251. [Google Scholar]
- Colonna, V.; D’Agostino, N.; Garrison, E.; Albrechtsen, A.; Meisner, J.; Facchiano, A.; Cardi, T.; Tripodi, P. Genomic diversity and novel genome-wide association with fruit morphology in Capsicum, from 746k polymorphic sites. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPGRI; AVRDC; CATIE. Descriptors for Capsicum (Capsicum spp.); International Plant Genetic Resources Institute: Rome, Italy, 1995. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Barbero, G.F.; Palma, M.; Barroso, C.G. Determination of capsaicinoids in peppers by microwave-assisted extraction–high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 2006, 578, 227–233. [Google Scholar] [CrossRef] [PubMed]
- SPSS. SPSS Statistics for Windows, Version 17.0.; SPSS Inc.: Chicago, IL, USA, 2008. [Google Scholar]
- Abdi, H. The Bonferonni and Šidák Corrections for Multiple Comparisons. In Encyclopedia of Measurement and Statistics; Salkind, N.J., Ed.; Sage: Thousand Oaks, CA, USA, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
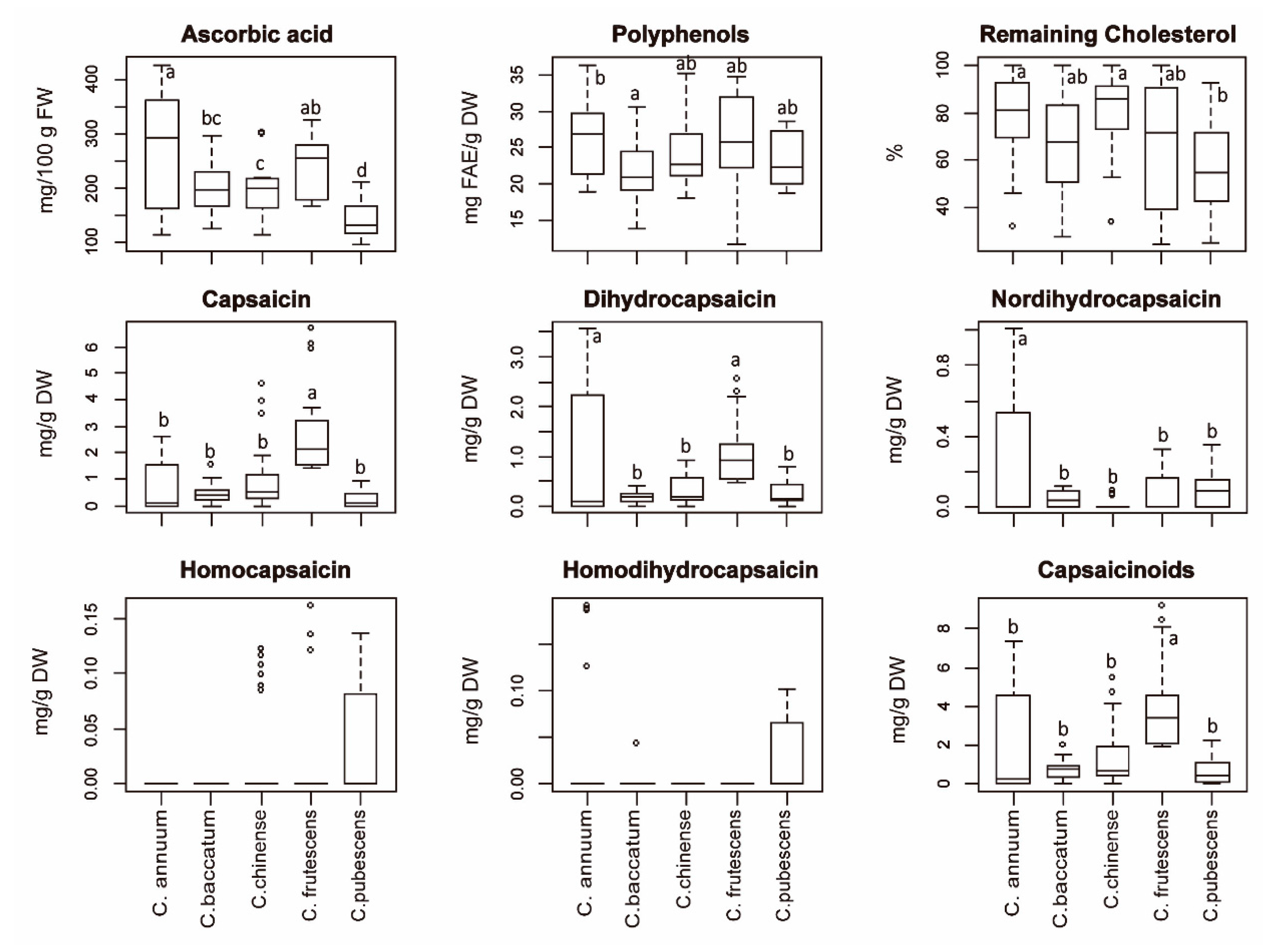


| Ascorbic Acid | Polyphenols | Cholesterol | Cap | DHCap | NDHCap | HCap | HDHCap | Capsaicinoids | |
|---|---|---|---|---|---|---|---|---|---|
| Sum of squares | 236373.1 | 348.2 | 8144.4 | 87.2 | 15.4 | 0.7 | 0.024 | 0.016 | 159.8 |
| Mean squares | 59093.3 | 87.0 | 2036.1 | 21.8 | 3.9 | 0.2 | 0.006 | 0.004 | 39.9 |
| F value † | 16.4 *** | 3.7 ** | 5.0 ** | 22.8 *** | 9.9 *** | 7.1 *** | 4.9 ns | 4.0 ns | 15.2 *** |
| Trait | Sum of Squares | F Value † | C. annuum | C. baccatum | C. chinense | C. frutescens | C. pubescens | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHE | 53049.3 | 44.93 *** | 72.06 | (17.6) | b | 65.71 | (33.0) | b | 67.55 | (25.1) | b | 87.39 | (18.5) | a | 48.69 | (26.7) | c |
| PWI | 28384.1 | 33.85 *** | 67.59 | (7.0) | c | 87.66 | (18.0) | a | 78.33 | (16.9) | b | 65.84 | (17.7) | c | 77.95 | (27.1) | b |
| STE | 27092.0 | 38.48 *** | 17.95 | (49.1) | b | 12.84 | (82.7) | b,c | 12.94 | (96.9) | b,c | 33.11 | (73.5) | a | 7.41 | (41.2) | c |
| SDI | 730.9 | 43.09 *** | 5.58 | (41.0) | b | 7.35 | (29.9) | a | 7.31 | (29.1) | a | 6.29 | (38.2) | b | 3.71 | (17.0) | c |
| MLL | 1563.4 | 62.05 *** | 12.68 | (26.2) | c | 11.24 | (20.7) | d | 14.50 | (11.3) | b | 16.69 | (16.7) | a | 11.92 | (21.2) | c,d |
| MLW | 258.3 | 24.30 *** | 6.224 | (24.8) | b | 6.749 | (31.8) | b | 7.744 | (17.1) | a | 8.401 | (14.3) | a | 6.351 | (21.7) | b |
| CLE | 13.706 | 22.32 *** | 1.744 | (24.7) | a | 1.412 | (28.1) | b,c | 1.519 | (30.1) | b | 1.254 | (12.9) | c | 1.760 | (23.0) | a |
| ALE | 4.571 | 1.45 ns | 3.086 | (27.4) | - | 3.308 | (24.7) | - | 3.167 | (28.5) | - | 3.090 | (30.7) | - | 3.029 | (31.3) | - |
| FILE | 33.5 | 9.78 *** | 4.100 | (20.0) | a | 4.075 | (20.4) | a | 3.811 | (25.8) | a | 3.284 | (33.9) | b | 3.700 | (24.5) | a |
| FLE | 33.5 | 9.78 *** | 8.319 | (36.0) | a | 6.659 | (39.1) | b | 5.162 | (38.7) | c | 2.524 | (49.9) | d | 4.231 | (35.7) | c |
| FWI | 82.7 | 21.33 *** | 2.283 | (48.4) | a | 1.699 | (38.5) | b | 2.431 | (39.2) | a | 1.151 | (61.4) | c | 2.183 | (67.7) | a |
| FWE | 9420.0 | 34.38 *** | 19.05 | (76.6) | a | 10.95 | (53.1) | b | 10.12 | (80.8) | b | 2.83 | (95.2) | c | 8.34 | (56.6) | b |
| FPL | 53.9 | 16.41 *** | 3.693 | (23.3) | a | 3.738 | (26.9) | a | 3.346 | (26.8) | a,b | 2.781 | (39.3) | c | 3.051 | (16.3) | c,b |
| FWT | 185.8 | 39.39 *** | 3.357 | (38.6) | a | 2.825 | (31.0) | b | 3.656 | (36.3) | a | 1.612 | (51.7) | c | 3.286 | (32.0) | a,b |
| NL | 27.8 | 7.96 *** | 2.800 | (33.1) | a | 2.942 | (27.3) | a | 2.811 | (37.3) | a | 2.552 | (41.1) | a,b | 2.200 | (39.9) | b |
| Trait | Sum of Squares | F Value † | C. annuum | C. baccatum | C. chinense | C. frutescens | C. pubescens | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | 4076327.52 | 510.28 *** | 258.41 | (23.6) | a | 163.21 | (32.9) | b | 140.64 | (22.8) | c | 63.62 | (47.7) | e | 111.47 | (29.4) | d |
| A | 395699848.67 | 209.54 *** | 2154.81 | (66.2) | a | 1036.27 | (51.2) | b | 1024.48 | (40.3) | b | 241.20 | (117.4) | d | 609.20 | (50.6) | c |
| WMH | 22387.62 | 88.74 *** | 23.28 | (49.6) | b | 19.44 | (23.9) | c | 25.51 | (38.2) | a | 12.23 | (65.7) | d | 20.01 | (23.6) | c |
| MW | 62417.36 | 297.27 *** | 37.13 | (24.6) | a | 24.23 | (24.4) | c | 28.86 | (28.4) | b | 12.90 | (60.9) | d | 22.93 | (20.4) | c |
| HMW | 407730.65 | 289.78 *** | 80.42 | (28.4) | a | 60.23 | (40.4) | b | 46.68 | (27.8) | c | 21.56 | (51.5) | e | 37.24 | (40.5) | d |
| MH | 556367.80 | 310.73 *** | 89.13 | (29.9) | a | 69.28 | (40.5) | b | 49.93 | (27.0) | c | 22.52 | (50.9) | e | 39.30 | (40.2) | d |
| CH | 631209.90 | 353.83 *** | 97.74 | (25.0) | a | 70.93 | (40.1) | b | 52.95 | (23.6) | c | 24.44 | (46.8) | e | 42.97 | (43.6) | d |
| FSIEI | 394.52 | 109.51 *** | 2.956 | (23.3) | b | 3.203 | (38.6) | a | 1.989 | (43.4) | c | 1.960 | (43.6) | c | 1.957 | (37.0) | c |
| FSIEII | 1433.10 | 164.84 *** | 4.927 | (47.7) | a | 3.715 | (42.0) | b | 2.135 | (49.3) | c | 2.115 | (54.6) | c | 1.943 | (41.8) | c |
| CFSI | 2108.96 | 186.94 *** | 5.977 | (45.6) | a | 4.206 | (42.5) | b | 2.387 | (46.3) | c | 2.502 | (48.7) | c | 2.314 | (45.4) | c |
| PFB | 14.72 | 157.66 *** | 0.841 | (25.3) | a | 0.531 | (21.4) | d | 0.544 | (29.7) | c,d | 0.581 | (20.6) | c | 0.693 | (23.9) | b |
| DFB | 12.80 | 85.90 *** | 1.086 | (14.8) | a | 0.921 | (14.0) | b | 0.842 | (28.3) | c | 0.870 | (33.0) | c | 0.729 | (13.5) | d |
| FST | 37.61 | 43.63 *** | 0.950 | (47.9) | a | 0.582 | (25.2) | b | 0.901 | (83.1) | a | 1.020 | (46.3) | a | 0.995 | (30.0) | a |
| E | 1.07 | 320.09 *** | 0.162 | (32.6) | a | 0.087 | (21.6) | b | 0.090 | (22.9) | b | 0.072 | (24.6) | c | 0.073 | (42.0) | c |
| C | 4.40 | 89.19 *** | 0.344 | (21.0) | a | 0.337 | (41.2) | a | 0.223 | (46.9) | b | 0.227 | (50.9) | b | 0.203 | (40.5) | b |
| R | 1.33 | 80.71 *** | 0.375 | (22.7) | d | 0.448 | (12.2) | b | 0.428 | (14.3) | c | 0.430 | (15.9) | c | 0.491 | (10.9) | a |
| SH | 2.63 | 35.15 *** | 0.498 | (6.7) | a | 0.455 | (19.9) | b | 0.411 | (40.1) | c | 0.347 | (57.9) | d | 0.413 | (34.9) | c |
| PAMI | 646218.10 | 39.37 *** | 162.27 | (57.7) | a | 94.07 | (81.7) | d | 119.68 | (32.1) | c | 117.54 | (46.4) | c | 140.42 | (19.3) | b |
| PAMA | 914210.13 | 54.28 *** | 137.25 | (90.8) | a | 70.31 | (55.0) | c | 105.88 | (41.9) | b | 96.08 | (49.2) | b | 144.54 | (39.6) | a |
| PIA | 1507.27 | 306.59 *** | 3.763 | (67.8) | a | 0.682 | (58.8) | b | 0.692 | (58.7) | b | 0.564 | (120.2) | b | 0.802 | (61.2) | b |
| DAMI | 188471.47 | 20.39 *** | 118.99 | (28.8) | c | 114.58 | (20.9) | c | 147.03 | (38.8) | a | 135.58 | (60.3) | a,b | 131.92 | (17.8) | b |
| DAMA | 60989.22 | 7.09 *** | 121.42 | (65.8) | c | 106.02 | (36.2) | a | 121.67 | (29.4) | c | 112.90 | (39.1) | b,c | 123.47 | (18.9) | c |
| DIA | 2.34 | 46.69 *** | 0.038 | (58.6) | b | 0.021 | (72.0) | b | 0.048 | (119.3) | b | 0.026 | (79.3) | b | 0.153 | (182.1) | a |
| DEP | 10.19 | 34.65 *** | 0.417 | (95.4) | a | 0.245 | (109.7) | b | 0.225 | (89.5) | b | 0.274 | (116.2) | b | 0.088 | (58.9) | c |
| Ob | 5.18 | 81.82 *** | 0.402 | (24.1) | a | 0.356 | (36.7) | b | 0.303 | (50.1) | c | 0.246 | (54.6) | d | 0.204 | (40.8) | e |
| Ov | 4.10 | 47.15 *** | 0.169 | (153.3) | a | 0.000 | (486.2) | d | 0.078 | (185.3) | c | 0.080 | (155.7) | c | 0.126 | (127.9) | b |
| VAs | 31.52 | 340.03 *** | 0.587 | (39.3) | a | 0.263 | (69.9) | b | 0.190 | (45.2) | c | 0.062 | (56.1) | e | 0.144 | (95.4) | d |
| HAob | 128.97 | 144.24 *** | 1.128 | (63.4) | a | 0.746 | (83.7) | b | 0.489 | (50.7) | c | 0.152 | (92.7) | d | 0.217 | (114.0) | d |
| HAov | 29.84 | 61.81 *** | 0.472 | (168.1) | a | 0.000 | (718.5) | d | 0.090 | (165.3) | b,c | 0.045 | (145.6) | c,d | 0.148 | (217.8) | b |
| WWP | 6.55 | 111.26 *** | 0.766 | (13.2) | a | 0.680 | (16.1) | b | 0.605 | (23.9) | c | 0.562 | (22.7) | d | 0.549 | (21.4) | d |
| EC | 0.61 | 71.12 *** | 0.688 | (9.9) | c | 0.743 | (6.5) | b | 0.744 | (5.2) | b | 0.759 | (4.8) | a | 0.752 | (4.4) | a,b |
| PEC | 2.18 | 79.77 *** | 1.018 | (19.0) | a | 0.911 | (3.8) | b | 0.893 | (3.4) | b | 0.905 | (3.5) | b | 0.896 | (3.6) | b |
| DEC | 0.42 | 84.92 *** | 0.929 | (5.6) | a | 0.893 | (2.3) | b | 0.880 | (3.6) | c | 0.868 | (5.0) | d | 0.896 | (3.4) | b |
| FSII | 976.05 | 118.11 *** | 4.159 | (49.0) | a | 3.780 | (41.0) | b | 2.097 | (52.4) | c | 2.205 | (54.6) | c | 2.021 | (53.3) | c |
| ECAI | 6.75 | 156.65 *** | 0.647 | (34.7) | a | 0.467 | (13.8) | b | 0.437 | (11.9) | c,d | 0.418 | (13.4) | d | 0.447 | (11.9) | b,c |
| LD | 101373.30 | 106.07 *** | 35.75 | (38.7) | b | 39.95 | (49.2) | a | 21.36 | (66.3) | c | 21.26 | (62.5) | c | 17.62 | (62.5) | c |
| TPA | 86661426.60 | 204.01 *** | 1024.65 | (69.0) | a | 471.15 | (45.5) | b | 430.75 | (38.9) | b,c | 110.90 | (119.3) | d | 360.73 | (46.6) | c |
| TPAR | 2.51 | 27.54 *** | 0.441 | (1.1) | b | 0.446 | (3.5) | b | 0.556 | (57.4) | a | 0.453 | (1.1) | b | 0.444 | (2.0) | b |
| TPT | 621.97 | 163.19 *** | 3.850 | (43.9) | a | 3.101 | (22.1) | c | 3.523 | (23.9) | b | 1.580 | (52.1) | d | 2.964 | (25.5) | c |
| TPTR | 0.26 | 27.72 *** | 0.209 | (12.0) | b,c | 0.213 | (11.6) | b | 0.241 | (39.1) | a | 0.202 | (12.5) | b,c | 0.200 | (5.0) | c |
| PPB | 2804013.45 | 421.79 *** | 211.31 | (30.9) | a | 130.74 | (35.3) | b | 111.59 | (24.6) | c | 48.97 | (49.5) | e | 91.01 | (25.9) | d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, C.A.; Silvar, C. Phytochemical Assessment of Native Ecuadorian Peppers (Capsicum spp.) and Correlation Analysis to Fruit Phenomics. Plants 2020, 9, 986. https://doi.org/10.3390/plants9080986
García-González CA, Silvar C. Phytochemical Assessment of Native Ecuadorian Peppers (Capsicum spp.) and Correlation Analysis to Fruit Phenomics. Plants. 2020; 9(8):986. https://doi.org/10.3390/plants9080986
Chicago/Turabian StyleGarcía-González, Carlos A., and Cristina Silvar. 2020. "Phytochemical Assessment of Native Ecuadorian Peppers (Capsicum spp.) and Correlation Analysis to Fruit Phenomics" Plants 9, no. 8: 986. https://doi.org/10.3390/plants9080986





