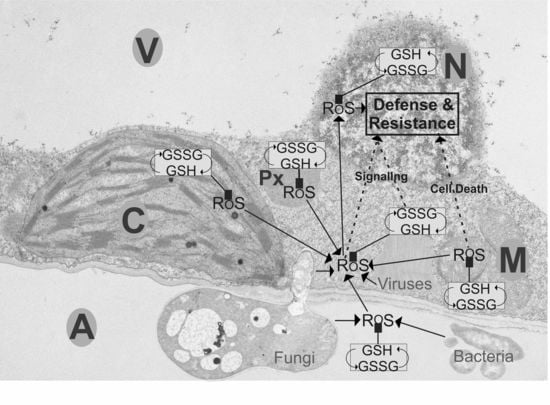
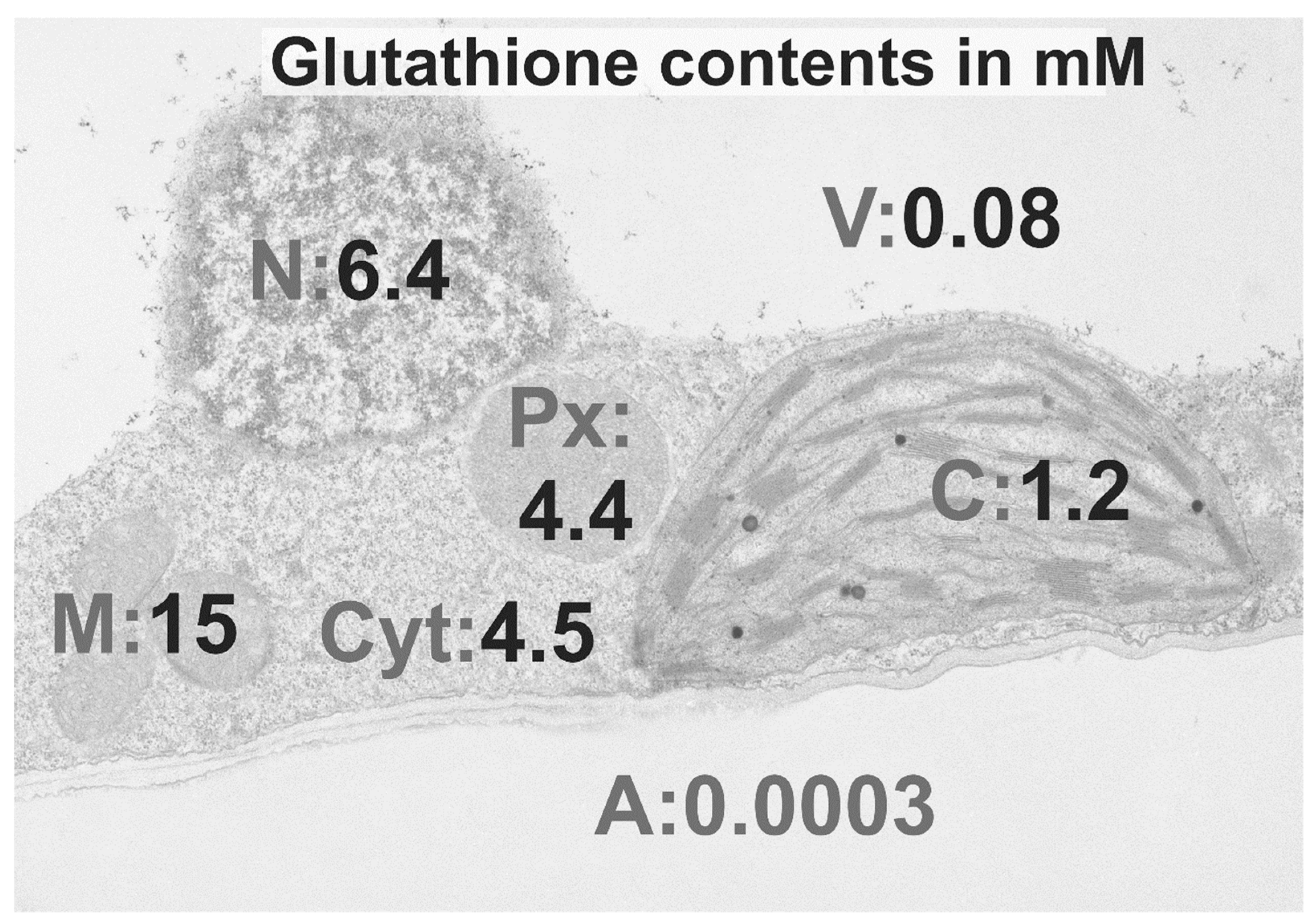
Subcellular Roles of Glutathione in Mediating Plant Defense during Biotic Stress
Abstract
:1. Introduction
2. Fungal Infections
3. Bacterial Infections
4. Viral Infections
5. Mitochondria
6. Chloroplasts and Peroxisomes
7. Apoplast
8. Cytosol
9. Nuclei
10. Concluding Remarks
Funding
Conflicts of Interest
References
- Saijo, Y.; Loo, E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, E.D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Biotic Stress. In Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant Immunity: From Signaling to Epigenetic Control of Defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, A.P.; Ardila, H.D.; Martínez-Peralta, S.T.; Melgarejo-Muñoz, L.M.; Castillejo-Sánchez, M.A.; Jorrín-Novo, J.V. What proteomic analysis of the apoplast tells us about plant-pathogen interactions. Plant Pathol. 2018, 67, 1647–1668. [Google Scholar] [CrossRef]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Hammerbach, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defense against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zogli, P.; Libault, M. Plant response to biotic stress: Is there a common epigenetic response during plant-pathogenic and symbiotic interactions? Plant Sci. 2017, 263, 89–93. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Leary, A.Y.; Sanguankiattichai, N.; Duggan, C.; Tumtas, Y.; Pandey, P.; Segretin, M.E.; Salguero Linares, J.; Savage, Z.D.; Yow, R.J.; Bozkurt, T.O. Modulation of plant autophagy during pathogen attack. J. Exp. Bot. 2018, 69, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Lorang, J.M. Necrotrophic Exploitation and Subversion of Plant Defense: A Lifestyle or Just a Phase, and Implications in Breeding Resistance. Phytopathology 2019, 109, 332–346. [Google Scholar] [CrossRef]
- Mengiste, T. Plant immunity to necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef]
- Collemare, J.; O’Connell, R.J.; Lebrun, M.-H. Nonproteinaceous effectors: The terra incognita of plant–fungal interactions. New Phytol. 2019, 223, 590–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panstruga, R.; Kuhn, H. Mutual interplay between phytopathogenic powdery mildew fungi and other microorganisms. Mol. Plant Pathol. 2019, 20, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Uptake of bacteria into living plant cells, the unifying and distinct feature of the nitrogen-fixing root nodule symbiosis. Curr. Opin. Plant Boil. 2018, 44, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Wipf, D.; Krajinski, F.; Van Tuinen, D.; Recorbet, G.; Courty, P.-E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandaranayaka, E.P.; Frenkel, O.; Elad, Y.; Prusky, D.; Harel, A. Network analysis exposes core functions in major lifestyles of fungal and oomycete plant pathogens. BMC Genom. 2019, 20, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Précigout, P.-A.; Claessen, D.; Makowski, D.; Robert, C. Does the Latent Period of Leaf Fungal Pathogens Reflect Their Trophic Type? A Meta-Analysis of Biotrophs, Hemibiotrophs, and Necrotrophs. Phytopathology 2020, 110, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Bastias, D.A.; Johnson, L.J.; Card, S.D. Symbiotic bacteria of plant-associated fungi: Friends or foes? Curr. Opin. Plant Biol. 2019, 56, 1–8. [Google Scholar] [CrossRef]
- Xin, X.-F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Genet. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Dodueva, I.E.; Lebedeva, M.A.; Kuznetsova, K.A.; Gancheva, M.S.; Paponova, S.S.; Lutova, L.L. Plant tumors: A hundred years of study. Planta 2020, 251, 1–28. [Google Scholar] [CrossRef]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus Latency and the Impact on Plants. Front. Microbiol. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H. Host factors against plant viruses. Mol. Plant Pathol. 2019, 20, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.J.; Hajizadeh, M.; Ohshima, K.; Jones, R.A.C. The Potyviruses: An Evolutionary Synthesis Is Emerging. Viruses 2020, 12, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatineni, S.; Stewart, L.R.; Sanfaçon, H.; Wang, X.; Navas-Castillo, J.; Hajimorad, M.R. Fundamental Aspects of Plant Viruses−An Overview on Focus Issue Articles. Phytopathology 2020, 110, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zellnig, G.; Pöckl, M.H.; Möstl, S.; Zechmann, B. Two and three dimensional characterization of Zucchini Yellow Mosaic Virus induced structural alterations in Cucurbita pepo L. plants. J. Struct. Boil. 2014, 186, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Gullner, G.; Zechmann, B.; Künstler, A.; Király, L. The Signaling Roles of Glutathione in Plant Disease Resistance. In Glutathione in Plant Growth, Development, and Stress Tolerance, 1st ed.; Hossain, M.A., Mostofa, M.G., Diaz-Vivancos, P., Burritt, D.J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 331–357. [Google Scholar] [CrossRef]
- Koch, A.; Kang, H.-G.; Steinbrenner, J.; Dempsey, D.A.; Klessig, D.F.; Kogel, K.-H. MORC Proteins: Novel Players in Plant and Animal Health. Front. Plant Sci. 2017, 8, 1720. [Google Scholar] [CrossRef]
- Van Butselaar, T.; Van den Ackerveken, G. Salicylic Acid Steers the Growth–Immunity Tradeoff. Trends Plant Sci. 2020, 25, 566–576. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Alquéres, S.; Meneses, C.H.S.G.; Rouws, L.F.M.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The Bacterial Superoxide Dismutase and Glutathione Reductase Are Crucial for Endophytic Colonization of Rice Roots byGluconacetobacter diazotrophicusPAL5. Mol. Plant Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Ghanta, S.; Datta, R.; Bhattacharyya, D.; Sinha, R.; Kumar, D.; Hazra, S.; Mazumdar, A.B.; Chattopadhyay, S. Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J. Plant Physiol. 2014, 171, 940–950. [Google Scholar] [CrossRef]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional Analysis of Arabidopsis Mutants Points to Novel Roles for Glutathione in Coupling H2O2 to Activation of Salicylic Acid Accumulation and Signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Künstler, A.; Kátay, G.; Gullner, G.; Király, L. Artificial elevation of glutathione contents in salicylic acid-deficient tobacco (Nicotiana tabacum cv. Xanthi NahG) reduces susceptibility to the powdery mildew pathogen Euoidium longipes. Plant Boil. 2019, 22, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Künstler, A.; Király, L.; Kátay, G.; Enyedi, A.J.; Gullner, G. Glutathione Can Compensate for Salicylic Acid Deficiency in Tobacco to Maintain Resistance to Tobacco Mosaic Virus. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.-C.; Ko, K.; Chang, W.-L.; Kuo, W.-C.; Chen, G.-H.; Lin, T.-P. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Hacham, Y.; Koussevitzky, S.; Kirma, M.; Amir, R. Glutathione application affects the transcript profile of genes in Arabidopsis seedling. J. Plant Physiol. 2014, 171, 1444–1451. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; De Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking cell proliferation to oxidative stress. Free Radic. Boil. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Boil. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Kuźniak, E.; Skłodowska, M. Differential Implication of Glutathione, Glutathione Metabolizing Enzymes and Ascorbate in Tomato Resistance to Pseudomonas syringae. J. Phytopathol. 2004, 152, 529–536. [Google Scholar] [CrossRef]
- Kuźniak, E. Compartment-specific role of the ascorbate-glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. J. Exp. Bot. 2005, 56, 921–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanacker, H.; Carver, T.L.; Foyer, C.H. Pathogen-Induced Changes in the Antioxidant Status of the Apoplast in Barley Leaves. Plant Physiol. 1998, 117, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanacker, H.; Carver, T.L.; Foyer, C.H. Early H2O2 Accumulation in Mesophyll Cells Leads to Induction of Glutathione during the Hyper-Sensitive Response in the Barley-Powdery Mildew Interaction. Plant Physiol. 2000, 123, 1289–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, S.; Rivoal, J. Consequences of Oxidative Stress on Plant Glycolytic and Respiratory Metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; Ende, W.V.D.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef] [Green Version]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [Green Version]
- Künstler, A.; Bacsó, R.; Gullner, G.; Hafez, Y.M.; Király, L. Staying alive—Is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Xing, L.-P.; Wu, G.-J.; Wang, H.-Z.; Wang, X.-E.; Cao, A.-Z.; Chen, P. Plastidial Glutathione Reductase from Haynaldia villosa is an Enhancer of Powdery Mildew Resistance in Wheat (Triticum aestivum). Plant Cell Physiol. 2007, 48, 1702–1712. [Google Scholar] [CrossRef]
- Simon, U.K.; Polanschütz, L.M.; Koffler, B.E.; Zechmann, B. High Resolution Imaging of Temporal and Spatial Changes of Subcellular Ascorbate, Glutathione and H2O2 Distribution during Botrytis cinerea Infection in Arabidopsis. PLoS ONE 2013, 8, e65811. [Google Scholar] [CrossRef] [Green Version]
- Zechmann, B.; Müller, M. Effects of zucchini yellow mosaic virus infection on the subcellular distribution of glutathione and its precursors in a highly tolerant Cucurbita pepo cultivar. Botany 2008, 86, 1092–1100. [Google Scholar] [CrossRef]
- Zechmann, B.; Zellnig, G.; Müller, M. Changes in the Subcellular Distribution of Glutathione during Virus Infection in Cucurbita pepo (L.). Plant Boil. 2005, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B.; Zellnig, G.; Urbanek-Krajnc, A.; Müller, M. Artificial elevation of glutathione affects symptom development in ZYMV-infected Cucurbita pepo L. plants. Arch. Virol. 2006, 152, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Moreno, M.J.; Hernández, J.A.; Diaz-Vivancos, P. Sharka: How do plants respond to Plum pox virus infection? J. Exp. Bot. 2014, 66, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Gullner, C.; Tobias, I.; Fodor, J.; Kömives, U.; Gullner, G.; Komives, T. Elevation of Glutathione Level and Activation of Glutathione-related Enzymes Affect Virus Infection in Tobacco. Free Radic. Res. 1999, 31, 155–161. [Google Scholar] [CrossRef]
- Király, L.; Künstler, A.; Höller, K.; Fattinger, M.; Juhász, C.; Müller, M.; Gullner, G.; Zechmann, B. Sulfate supply influences compartment specific glutathione metabolism and confers enhanced resistance to Tobacco mosaic virus during a hypersensitive response. Plant Physiol. Biochem. 2012, 59, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Höller, K.; Király, L.; Künstler, A.; Müller, M.; Gullner, G.; Fattinger, M.; Zechmann, B. Enhanced Glutathione Metabolism Is Correlated with Sulfur-Induced Resistance in Tobacco mosaic virus–Infected Genetically Susceptible Nicotiana tabacum Plants. Mol. Plant Microbe Interact. 2010, 23, 1448–1459. [Google Scholar] [CrossRef] [Green Version]
- Koffler, B.E.; Bloem, E.; Zellnig, G.; Zechmann, B. High resolution imaging of subcellular glutathione concentrations by quantitative immunoelectron microscopy in different leaf areas of Arabidopsis. Micron 2013, 45, 119–128. [Google Scholar] [CrossRef]
- Wachter, A.; Wolf, S.; Steininger, H.; Bogs, J.; Rausch, T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: Implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 2004, 41, 15–30. [Google Scholar] [CrossRef]
- Ohkama-Ohtsu, N.; Radwan, S.; Peterson, A.; Zhao, P.; Badr, A.; Xiang, C.; Oliver, D.J. Characterization of the extracellular γ-glutamyl transpeptidases, GGT1 and GGT2, in Arabidopsis. Plant J. 2007, 49, 865–877. [Google Scholar] [CrossRef]
- Ohkama-Ohtsu, N.; Zhao, P.; Xiang, C.; Oliver, D.J. Glutathione conjugates in the vacuole are degraded by γ-glutamyl transpeptidase GGT3 in Arabidopsis. Plant J. 2007, 49, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Tolin, S.; Arrigoni, G.; Trentin, A.R.; Veljovic-Jovanovic, S.; Pivato, M.; Zechman, B.; Masi, A. Biochemical and quantitative proteomics investigations in Arabidopsis ggt1 mutant leaves reveal a role for the gamma-glutamyl cycle in plant’s adaptation to environment. Proteomics 2013, 13, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Maughan, S.C.; Pasternak, M.; Cairns, N.; Kiddle, G.; Brach, T.; Jarvis, R.; Haas, F.; Nieuwland, J.; Lim, B.; Müller, C.; et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2331–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechmann, B. Compartment-specific importance of glutathione during abiotic and biotic stress. Front. Plant Sci. 2014, 5, 566. [Google Scholar] [CrossRef] [PubMed]
- Heyneke, E.; Luschin-Ebengreuth, N.; Krajcer, I.; Wolkinger, V.; Müller, M.; Zechmann, B. Dynamic compartment specific changes in glutathione and ascorbate levels in Arabidopsis plants exposed to different light intensities. BMC Plant Boil. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koffler, B.E.; Luschin-Ebengreuth, N.; Stabentheiner, E.; Müller, M.; Zechmann, B. Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Sci. 2014, 227, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Hemetsberger, C.; Herrberger, C.; Zechmann, B.; Hillmer, M.; Döhlemann, G. The Ustilago maydis Effector Pep1 Suppresses Plant Immunity by Inhibition of Host Peroxidase Activity. PLoS Pathog. 2012, 8, e1002684. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S.; Zechmann, B.; Molitor, A.; Trujillo, M.; Petutschnig, E.; Lipka, V.; Kogel, K.-H.; Schäfer, P. Broad-Spectrum Suppression of Innate Immunity Is Required for Colonization of Arabidopsis Roots by the Fungus Piriformospora indica. Plant Physiol. 2011, 156, 726–740. [Google Scholar] [CrossRef] [Green Version]
- Podgórska, A.; Burian, M.; Szal, B. Extra-Cellular but Extra-Ordinarily Important for Cells: Apoplastic Reactive Oxygen Species Metabolism. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Amirsadeghi, S.; Robson, C.A.; Vanlerberghe, G.C. The role of the mitochondrion in plant responses to biotic stress. Physiol. Plant. 2007, 129, 253–266. [Google Scholar] [CrossRef]
- Su, T.; Li, W.; Wang, P.; Ma, C. Dynamics of Peroxisome Homeostasis and Its Role in Stress Response and Signaling in Plants. Front. Plant Sci. 2019, 10, 705. [Google Scholar] [CrossRef] [Green Version]
- Wagner, S.; Van Aken, O.; Elsässer, M.; Schwarzländer, M. Mitochondrial Energy Signaling and Its Role in the Low-Oxygen Stress Response of Plants. Plant Physiol. 2018, 176, 1156–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Großkinsky, D.K.; Koffler, B.E.; Roitsch, T.G.; Maier, R.; Zechmann, B. Compartment-specific antioxidative defense in Arabidopsis against virulent and avirulent Pseudomonas syringae. Phytopathology 2012, 102, 662–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowden, R.G.; Watson, S.J.; Jarvis, P. The role of chloroplasts in plant pathology. Essays Biochem. 2017, 62, 21–39. [Google Scholar] [CrossRef]
- Thapa, S.P.; Davis, E.W.; Lyu, Q.; Weisberg, A.J.; Stevens, D.M.; Clarke, C.R.; Coaker, G.; Chang, J.H. The Evolution, Ecology, and Mechanisms of Infection by Gram-Positive, Plant-Associated Bacteria. Annu. Rev. Phytopathol. 2019, 57, 341–365. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, S.; Noctor, G. Myo-inositol abolishes salicylic acid-dependent cell death and pathogen defence responses triggered by peroxisomal hydrogen peroxide. New Phytol. 2010, 188, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; Van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal Hydrogen Peroxide Is Coupled to Biotic Defense Responses by ISOCHORISMATE SYNTHASE1 in a Daylength-Related Manner. Plant Physiol. 2010, 153, 1692–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, G.; Rodriguez, M.C.; Venturuzzi, A.L.; Asurmendi, S. Modulation of host plant immunity by Tobamovirus proteins. Ann. Bot. 2016, 119, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, K.I.; Eskelin, K.; Lõhmus, A.; Mäkinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [CrossRef] [Green Version]
- Mäkinen, K. Plant susceptibility genes as a source for potyvirus resistance. Ann. Appl. Boil. 2019, 176, 122–129. [Google Scholar] [CrossRef]
- Senda, K.; Ogawa, K. Induction of PR-1 Accumulation Accompanied by Runaway Cell Death in the lsd1 Mutant of Arabidopsis is Dependent on Glutathione Levels but Independent of the Redox State of Glutathione. Plant Cell Physiol. 2004, 45, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; García, B.M.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2011, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vivancos, P.; Clemente-Moreno, M.J.; Rubio, M.; Olmos, E.; García, J.A.; Martínez-Gómez, P.; Hernández, J.A. Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. J. Exp. Bot. 2008, 59, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Rahoutei, J.; García-Luque, I.; Barón, M. Inhibition of photosynthesis by viral infection: Effect on PSII structure and function. Physiol. Plant. 2000, 110, 286–292. [Google Scholar] [CrossRef]
- Zechmann, B.; Zellnig, G.; Müller, M. Cytological modifications in zucchini yellow mosaic virus (ZYMV)-infected Styrian pumpkin plants. Arch. Virol. 2003, 148, 1119–1133. [Google Scholar] [CrossRef]
- Chang, R.; Jang, C.J.H.; Branco-Price, C.; Nghiem, P.; Bailey-Serres, J. Transient MPK6 activation in response to oxygen deprivation and reoxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol. Boil. 2011, 78, 109–122. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Vanlerberghe, G.C. Alternative oxidase impacts the plant response to biotic stress by influencing the mitochondrial generation of reactive oxygen species. Plant Cell Environ. 2012, 36, 721–732. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial Reactive Oxygen Species. Contribution to Oxidative Stress and Interorganellar Signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Greenberg, J.T. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 2005, 18, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuźniak, E. The effect of Botrytis cinerea infection on the antioxidant profile of mitochondria from tomato leaves. J. Exp. Bot. 2004, 55, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, N.; Pike, S.; Pallardy, S.; Gassmann, W.; Zhang, S. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007, 51, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vã¡squez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, M.; Lim, B.; Wirtz, M.; Hell, R.; Cobbett, C.S.; Meyer, A.J. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J. 2007, 53, 999–1012. [Google Scholar] [CrossRef]
- Ghanta, S.; Bhattacharyya, D.; Sinha, R.; Banerjee, A.; Chattopadhyay, S. Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta 2011, 233, 895–910. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, D.; Datta, R.; Hazra, S.; Bhattacharyya, D.; Mazumdar, A.B.; Mukhopadhyay, R.; Sultana, A.; Chattopadhyay, S. Integrated transcriptomic and proteomic analysis of Arabidopsis thaliana exposed to glutathione unravels its role in plant defense. Plant Cell Tissue Organ Cult. 2014, 120, 975–988. [Google Scholar] [CrossRef]
- Ball, L.; Accotto, G.P.; Bechtold, U.; Creissen, G.; Funck, D.; Jiménez, A.; Kular, B.; Leyland, N.; Mejia-Carranza, J.; Reynolds, H.; et al. Evidence for a Direct Link between Glutathione Biosynthesis and Stress Defense Gene Expression in Arabidopsis. Plant Cell 2004, 16, 2448–2462. [Google Scholar] [CrossRef] [Green Version]
- Dubreuil-Maurizi, C.; Vitecek, J.; Marty, L.; Branciard, L.; Frettinger, P.; Wendehenne, D.; Meyer, A.J.; Mauch, F.; Poinssot, B. Glutathione Deficiency of the Arabidopsis Mutant pad2-1 Affects Oxidative Stress-Related Events, Defense Gene Expression, and the Hypersensitive Response. Plant Physiol. 2011, 157, 2000–2012. [Google Scholar] [CrossRef] [Green Version]
- Parisy, V.; Poinssot, B.; Owsianowski, L.; Buchala, A.; Glazebrook, J.; Mauch, F. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2006, 49, 159–172. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.; Dong, X. Inducers of Plant Systemic Acquired Resistance Regulate NPR1 Function through Redox Changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.; Trujillo-Hernandez, J.A.; Reichheld, J.-P. Thiol Based Redox Signaling in Plant Nucleus. Front. Plant Sci. 2018, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B.; Zellnig, G.; Müller, M. Virus-Induced Changes in the Subcellular Distribution of Glutathione Precursors in Cucurbita pepo (L.). Plant Boil. 2007, 9, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Shekariesfahlan, A.; Ageeva, A.; Mengel, A.; Von Toerne, C.; Durner, J.; Lindermayr, C. Identification of nuclear target proteins for S-nitrosylation in pathogen-treated Arabidopsis thaliana cell cultures. Plant Sci. 2015, 238, 115–126. [Google Scholar] [CrossRef]
- Morisse, S.; Zaffagnini, M.; Gao, X.-H.; Lemaire, S.; Marchand, C.H. Insight into protein S-nitrosylation in Chlamydomonas reinhardtii. Antioxid. Redox Signal. 2014, 21, 1271–1284. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, M.E.; Mauriès, A.; Maes, A.; Tourasse, N.J.; Hamon, M.; Lemaire, S.; Marchand, C.H. The Deep Thioredoxome in Chlamydomonas reinhardtii: New Insights into Redox Regulation. Mol. Plant 2017, 10, 1107–1125. [Google Scholar] [CrossRef]
- Waszczak, C.; Akter, S.; Eeckhout, M.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K.; et al. Sulfenome mining in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 11545–11550. [Google Scholar] [CrossRef] [Green Version]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Morisse, S.; Trost, P.; Lemaire, S. Redox Regulation in Photosynthetic Organisms: Focus on Glutathionylation. Antioxid. Redox Signal. 2012, 16, 567–586. [Google Scholar] [CrossRef] [Green Version]
- Zaffagnini, M.; De Mia, M.; Morisse, S.; Di Giacinto, N.; Marchand, C.H.; Maes, A.; Lemaire, S.; Trost, P. Protein S-nitrosylation in photosynthetic organisms: A comprehensive overview with future perspectives. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 952–966. [Google Scholar] [CrossRef]
- De Simone, A.; Hubbard, R.; De La Torre, N.V.; Velappan, Y.; Wilson, M.; Considine, M.J.; Soppe, W.J.; Foyer, C.H. Redox Changes During the Cell Cycle in the Embryonic Root Meristem of Arabidopsis thaliana. Antioxid. Redox Signal. 2017, 27, 1505–1519. [Google Scholar] [CrossRef] [Green Version]
- Vivancos, P.D.; Wolff, T.; Markovic, J.; Pallardó, F.V.; Foyer, C.H. A nuclear glutathione cycle within the cell cycle. Biochem. J. 2010, 431, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Kocsy, G.; Tari, I.; Vanková, R.; Zechmann, B.; Gulyas, Z.; Poór, P.; Galiba, G. Redox control of plant growth and development. Plant Sci. 2013, 211, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, P.D.; Dong, Y.; Ziegler, K.; Markovic, J.; Pallardó, F.V.; Pellny, T.K.; Verrier, P.J.; Foyer, C.H. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010, 64, 825–838. [Google Scholar] [CrossRef] [PubMed]


| Botrytis cinerea | ||||
|---|---|---|---|---|
| Infection site | Adjacent to Infection Site | |||
| 12 hpi | 48 hpi | 12 hpi | 48 hpi | |
| Mitochondria | ns | −39 *** | ns | ns |
| Chloroplasts | 141 *** | 92 *** | ns | 358 *** |
| Nuclei | 79 *** | ns | ns | 132 *** |
| Peroxisomes | 122 ** | ns | 93 *** | 227 *** |
| Cytosol | 132 ** | ns | ns | 95 *** |
| Pseudomonas syringae | ||||||
|---|---|---|---|---|---|---|
| Virulent | Avirulent | |||||
| 12 hpi | 24 hpi | 48 hpi | 12 hpi | 24 hpi | 48 hpi | |
| Mitochondria | 44 * | ns | −29 * | 29 * | ns | ns |
| Chloroplasts | 73 * | 133 * | −48 * | 25 * | ns | −40 * |
| Nuclei | ns | 158 * | 70 * | 53 * | 70 * | 38 * |
| Peroxisomes | ns | 452 * | −48 * | 139 * | 258 * | −39 * |
| Cytosol | ns | 307 * | ns | 143 * | 160 * | −41 * |
| ZYMV | TMV | ||||||
|---|---|---|---|---|---|---|---|
| Susceptible (14 dpi) | Tolerant (14 dpi) | Susceptible | Resistant | ||||
| OTC | 7 dpi | 14 dpi | 1 dpi | 4 dpi | |||
| Mitochondria | ns | ns | 57 ** | 125 *** | 35.8 ** | ns | −17 * |
| Chloroplasts | 50 * | 39 * | 163 *** | 246 *** | −23.4 * | ns | 39 * |
| Nuclei | 115 * | ns | 202 *** | 203 *** | ns | 26 * | 28 * |
| Peroxisomes | 81 * | ns | 247 *** | 265 *** | 88 *** | ns | ns |
| Cytosol | 195 * | ns | 163 *** | 195 *** | ns | ns | ns |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechmann, B. Subcellular Roles of Glutathione in Mediating Plant Defense during Biotic Stress. Plants 2020, 9, 1067. https://doi.org/10.3390/plants9091067
Zechmann B. Subcellular Roles of Glutathione in Mediating Plant Defense during Biotic Stress. Plants. 2020; 9(9):1067. https://doi.org/10.3390/plants9091067
Chicago/Turabian StyleZechmann, Bernd. 2020. "Subcellular Roles of Glutathione in Mediating Plant Defense during Biotic Stress" Plants 9, no. 9: 1067. https://doi.org/10.3390/plants9091067