Expression Fluctuations of Genes Involved in Carbohydrate Metabolism Affected by Alterations of Ethylene Biosynthesis Associated with Ripening in Banana Fruit
Abstract
1. Introduction
2. Results
2.1. Transcriptomic Analysis of As1, As2, and WT in Targeted Silencing of Two Paralogous ACC Oxidase Genes in Banana
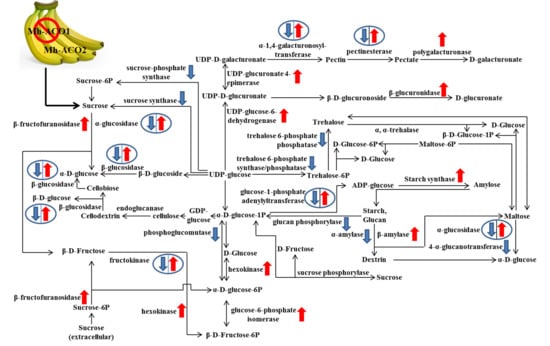
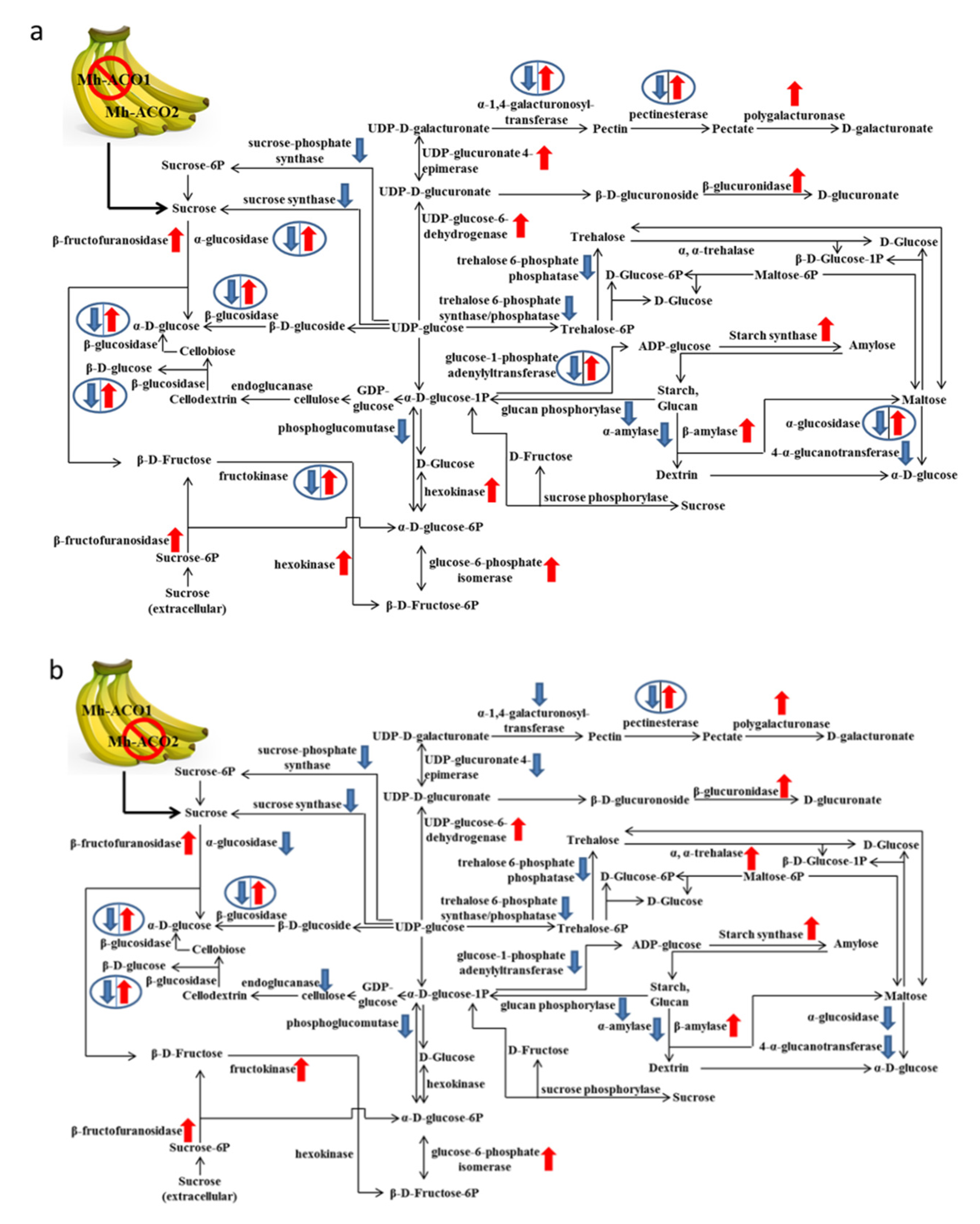
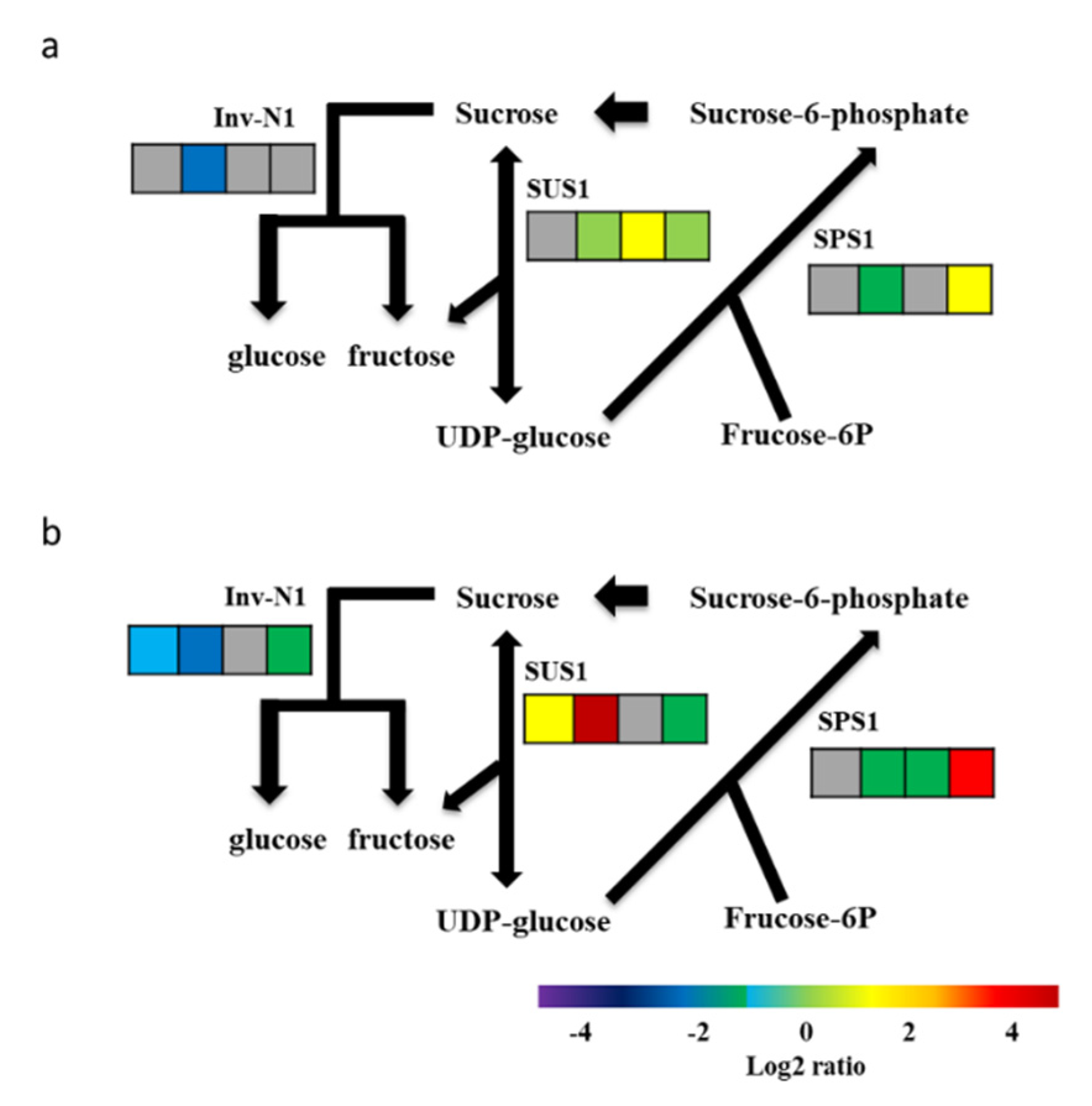
2.2. Expression Profiles of Selected Relevant Genes in Starch and Sucrose Metabolism
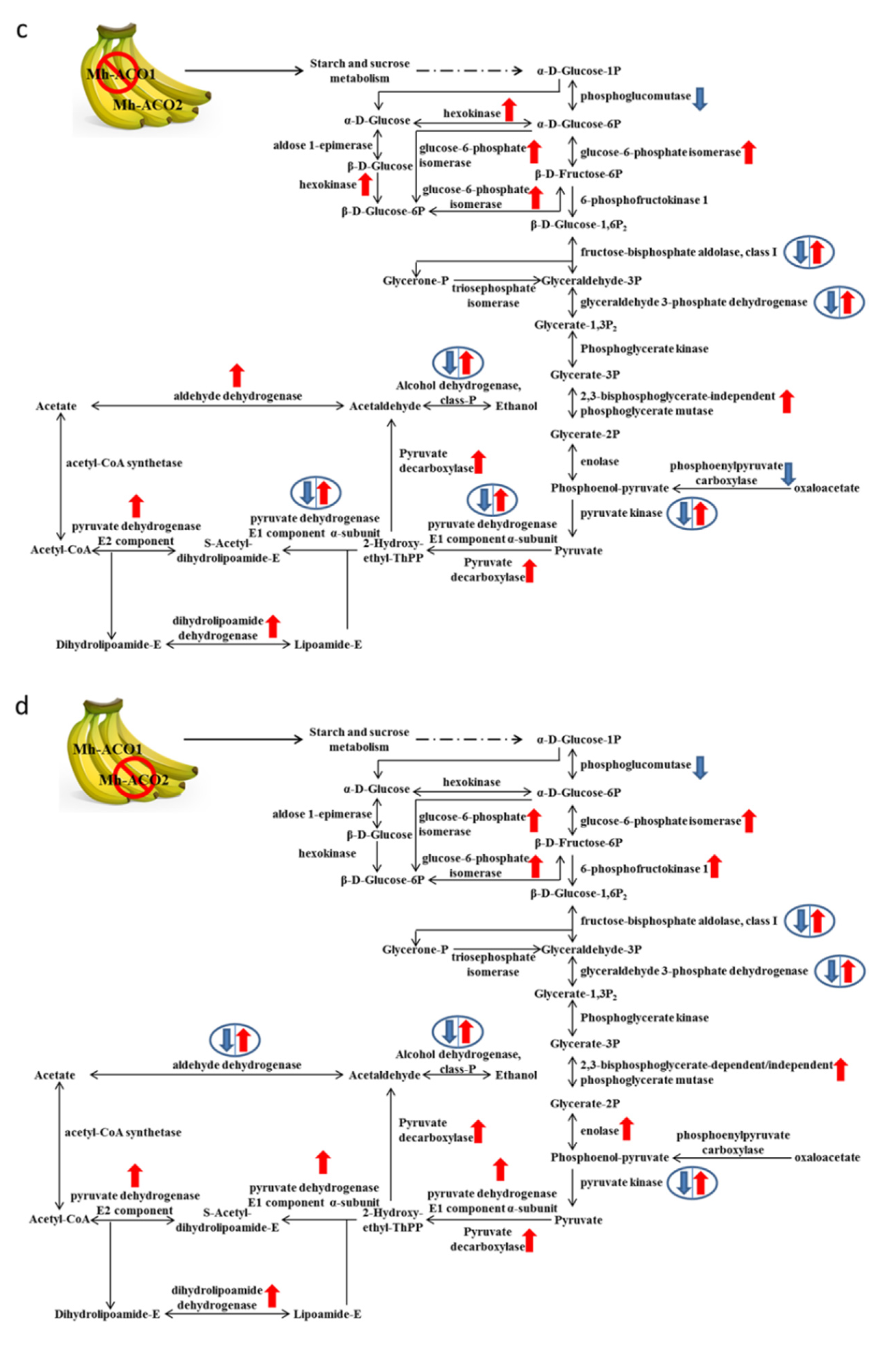
2.3. Expression Profiles of Selected Relevant Genes in Glycolytic Metabolism
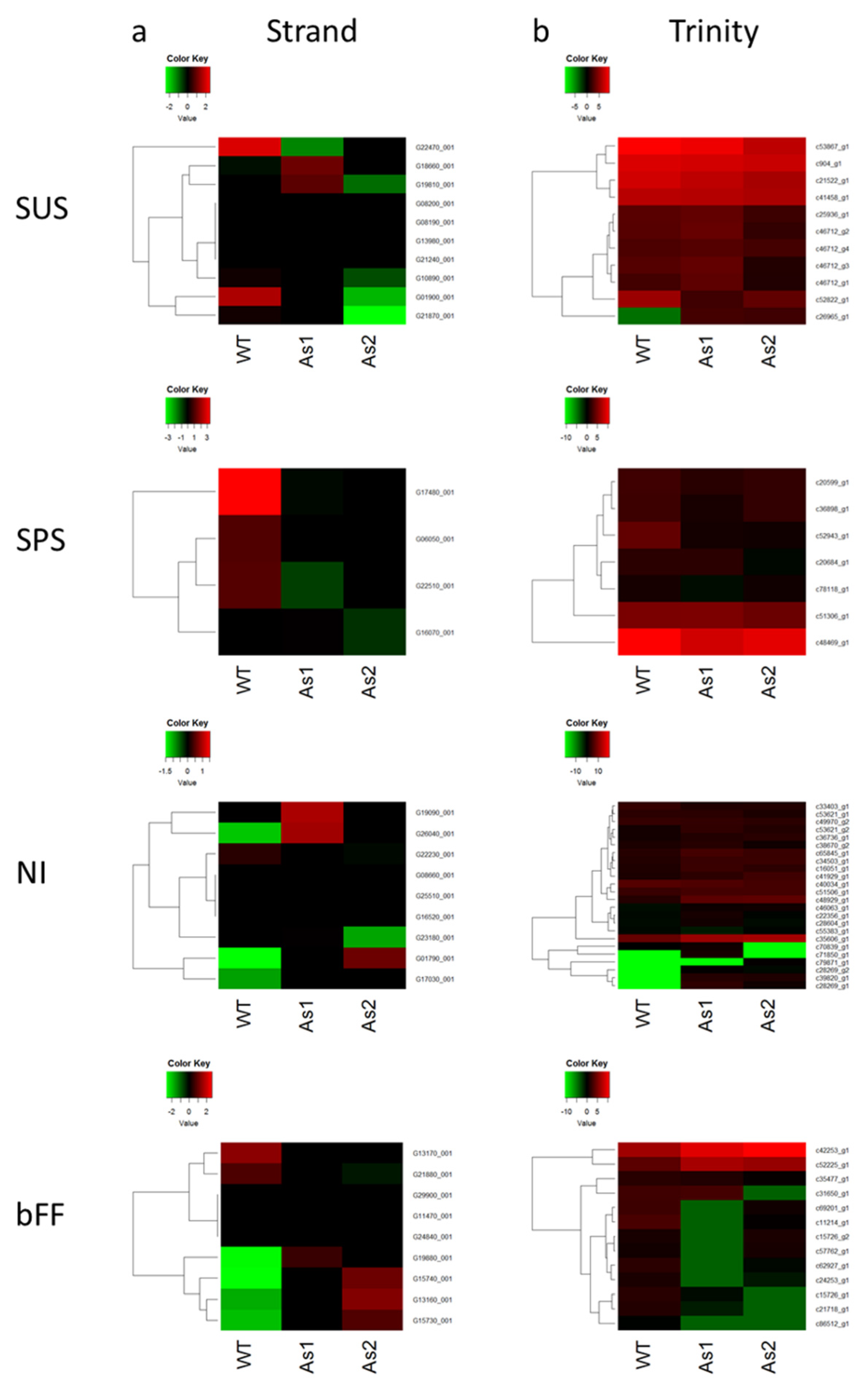
2.4. Gene Expressions of Sucrose Synthase, Sucrose Phosphate Synthase, and Neutral Invertase as Analyzed by Avadis and Trinity
2.5. Expression Profiles of the Sucrose Biosynthesis Pathway Genes Based on Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Data
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Total RNA Extraction and Whole-Transcriptome Deep Sequencing
4.3. Real-Time Quantitative RT-PCR
4.4. R Programming for Heat Map Illustration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, K.M.; Carvalho, V.D.D.; Cal-vidal, J. Physical changes during ripening of silver bananas. J. Food Sci. 1979, 44, 1254–1255. [Google Scholar] [CrossRef]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochemical properties, and digestibility—A review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Gibert, O.; Dufour, D.; Giraldo, A.; Sanchez, T.; Reynes, M.; Pain, J.P.; Gonzalez, A.; Fernandez, A.; Diaz, A. Differentiation between cooking bananas and dessert bananas. 1. Morphological and compositional characterization of cultivated Colombian Musaceae (Musa sp.) in relation to consumer preferences. J. Agric. Food Chem. 2009, 57, 7857–7869. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Rodrigues, S. Ultrasound as pre-treatment for drying of fruits: Dehydration of banana. J. Food Eng. 2007, 82, 261–267. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Lajolo, F.M. Starch breakdown during banana ripening: Sucrose synthase and sucrose phosphate synthase. J. Agric. Food Chem. 1995, 43, 347–351. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, Q.; Jia, C.; Liu, J.; Hu, W.; Jin, Z.; Xu, B. Soluble starch synthase III-1 in amylopectin metabolism of banana fruit: Characterization, expression, enzyme activity, and functional analyses. Front. Plant Sci. 2017, 8, 454. [Google Scholar] [CrossRef]
- Ball, K.L.; Green, J.H.; Rees, T.A. Glycolysis at the climacteric of bananas. Eur. J. Biochem. 1991, 197, 265–269. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, Q.; Liu, J.; Xu, B.; Jin, Z. The AGPase family proteins in banana: Genome-wide identification, phylogeny, and expression analyses reveal their involvement in the development, ripening, and abiotic/biotic stress responses. Int. J. Mol. Sci. 2017, 18, 1581. [Google Scholar] [CrossRef]
- Cheng, J.; Khan, M.A.; Qiu, W.-M.; Li, J.; Zhou, H.; Zhang, Q.; Guo, W.; Zhu, T.; Peng, J.; Sun, F. Diversification of genes encoding granule-bound starch synthase in monocots and dicots is marked by multiple genome-wide duplication events. PLoS ONE 2012, 7, e30088. [Google Scholar] [CrossRef]
- Batra, R.; Saripalli, G.; Mohan, A.; Gupta, S.; Gill, K.S.; Varadwaj, P.K.; Balyan, H.S.; Gupta, P.K. Comparative analysis of AGPase genes and encoded proteins in eight monocots and three dicots with emphasis on wheat. Front. Plant Sci. 2017, 8, 19. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Roy, S.; Das, R.; Sengupta, D.N. Differential transcriptional regulation of banana sucrose phosphate synthase gene in response to ethylene, auxin, wounding, low temperature and different photoperiods during fruit ripening and functional analysis of banana SPS gene promoter. Planta 2008, 229, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Roy, S.; Sengupta, D.N. A comparative study of cultivar differences in sucrose phosphate synthase gene expression and sucrose formation during banana fruit ripening. Postharvest Biol. Technol. 2009, 54, 15–24. [Google Scholar] [CrossRef]
- Pua, E.; Lim, S.; Liu, P.; Liu, J. Expression of a UDP glucose pyrophosphorylase cDNA during fruit ripening of banana (Musa acuminata). Funct. Plant Biol. 2000, 27, 1151–1159. [Google Scholar] [CrossRef]
- Xia, Y.; Kuan, C.; Chiu, C.H.; Chen, X.J.; Do, Y.Y.; Huang, P.L. Global transcriptomic analysis of targeted silencing of two paralogous ACC oxidase genes in banana. Int. J. Mol. Sci. 2016, 17, 1632. [Google Scholar] [CrossRef]
- Garcia, E.; Lajolo, F.M. Starch transformation during banana ripening: The amylase and glucosidase behavior. J. Food Sci. 2010, 53, 1181–1186. [Google Scholar] [CrossRef]
- Yang, S.F.; Ho, H.K. Biochemical studies on post-ripening of banana. J. Chin. Chem. Soc. 2013, 5, 71–85. [Google Scholar] [CrossRef]
- Gao, H.; Huang, S.; Dong, T.; Yang, Q.; Yi, G. Analysis of resistant starch degradation in postharvest ripening of two banana cultivars: Focus on starch structure and amylases. Postharvest Biol. Technol. 2016, 119, 1–8. [Google Scholar] [CrossRef]
- Nascimento, J.R.O.D.; Júnior, A.V.; Bassinello, P.Z.; Cordenunsi, B.R.; Mainardi, J.A.; Purgatto, E.; Lajolo, F.M. Beta-amylase expression and starch degradation during banana ripening. Postharvest Biol. Technol. 2006, 40, 41–47. [Google Scholar] [CrossRef]
- Mcglasson, W.B.; Vendrell, J.P.M.; Brady, C.J. Metabolic studies with banana fruit slices I. Changes in the incorporation of 14c labelled compounds in response to cutting. Aust. J. Biol. Sci. 1971, 24, 7–14. [Google Scholar] [CrossRef]
- Nakamura, Y. Biosynthesis of reserve starch. In Starch: Metabolism and Structure; Springer: Tokyo, Japan, 2015; pp. 161–209. [Google Scholar]
- Mohnen, D. Biosynthesis of pectins and galactomannans. In Comprehensive Natural Products Chemistry; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1999; Volume 3, pp. 497–527. [Google Scholar]
- Fromm, H.J.; Hargrove, M. Essentials of Biochemistry; Springer: Berlin/Heidelberg, Germany, 2012; pp. 257–277. [Google Scholar]
- Surendranathan, K.K.; Iyer, M.G.; Nair, P.M. Mechanism of action of a dimeric phosphofructokinase from banana: Role of magnesium on its kinetics and regulation. Plant Sci. 1992, 81, 29–36. [Google Scholar] [CrossRef]
- Turner, W.L.; Plaxton, W.C. Purification and characterization of pyrophosphate- and ATP-dependent phosphofructokinases from banana fruit. Planta 2003, 217, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Commonwealth Scientific and Industrial Research Organization (CSIRO) Division of Food Research. Banana Ripening Guide/Division of Food Research; Commonwealth Scientific and Industrial Research Organization: Sydney, Australia, 1972; Volume 8, pp. 1–12.
- Hu, H.-L.; Do, Y.-Y.; Huang, P.-L. Expression profiles of a MhCTR1 gene in relation to banana fruit ripening. Plant Physiol. Biochem. 2012, 56, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Xian, A.; Fan, L.; Raychowdhury, R.; Zeng, Q. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. Degseq: An R package for identifying differentially expressed genes from RNA-Seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Australia, 2015; Available online: https://www.r-project.org/ (accessed on 22 August 2017).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Chiu, C.-H.; Do, Y.-Y.; Huang, P.-L. Expression Fluctuations of Genes Involved in Carbohydrate Metabolism Affected by Alterations of Ethylene Biosynthesis Associated with Ripening in Banana Fruit. Plants 2020, 9, 1120. https://doi.org/10.3390/plants9091120
Xia Y, Chiu C-H, Do Y-Y, Huang P-L. Expression Fluctuations of Genes Involved in Carbohydrate Metabolism Affected by Alterations of Ethylene Biosynthesis Associated with Ripening in Banana Fruit. Plants. 2020; 9(9):1120. https://doi.org/10.3390/plants9091120
Chicago/Turabian StyleXia, Yan, Chien-Hsiang Chiu, Yi-Yin Do, and Pung-Ling Huang. 2020. "Expression Fluctuations of Genes Involved in Carbohydrate Metabolism Affected by Alterations of Ethylene Biosynthesis Associated with Ripening in Banana Fruit" Plants 9, no. 9: 1120. https://doi.org/10.3390/plants9091120
APA StyleXia, Y., Chiu, C.-H., Do, Y.-Y., & Huang, P.-L. (2020). Expression Fluctuations of Genes Involved in Carbohydrate Metabolism Affected by Alterations of Ethylene Biosynthesis Associated with Ripening in Banana Fruit. Plants, 9(9), 1120. https://doi.org/10.3390/plants9091120