Simulated Photovoltaic Solar Panels Alter the Seed Bank Survival of Two Desert Annual Plant Species
Abstract
:1. Introduction
2. Results
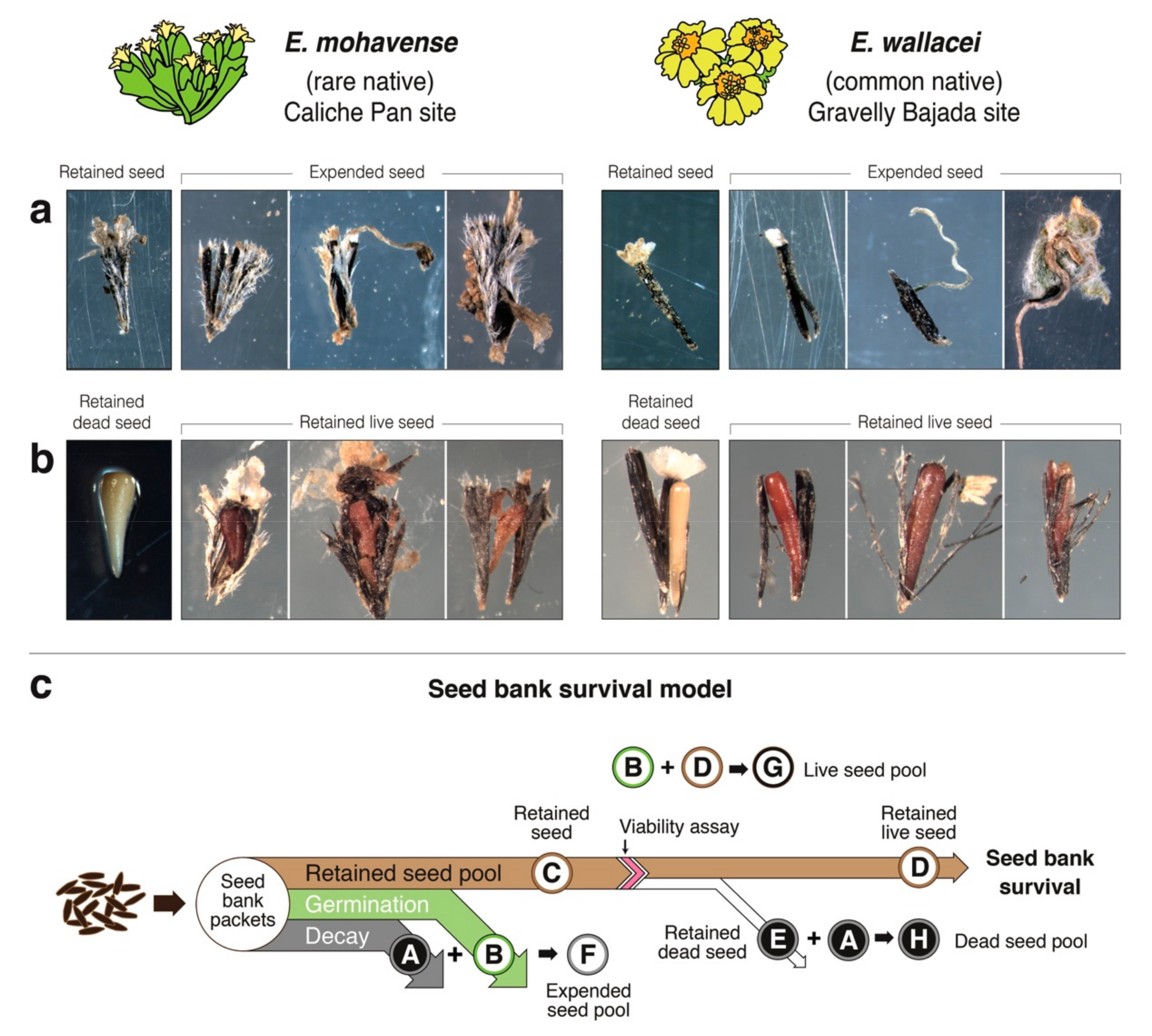
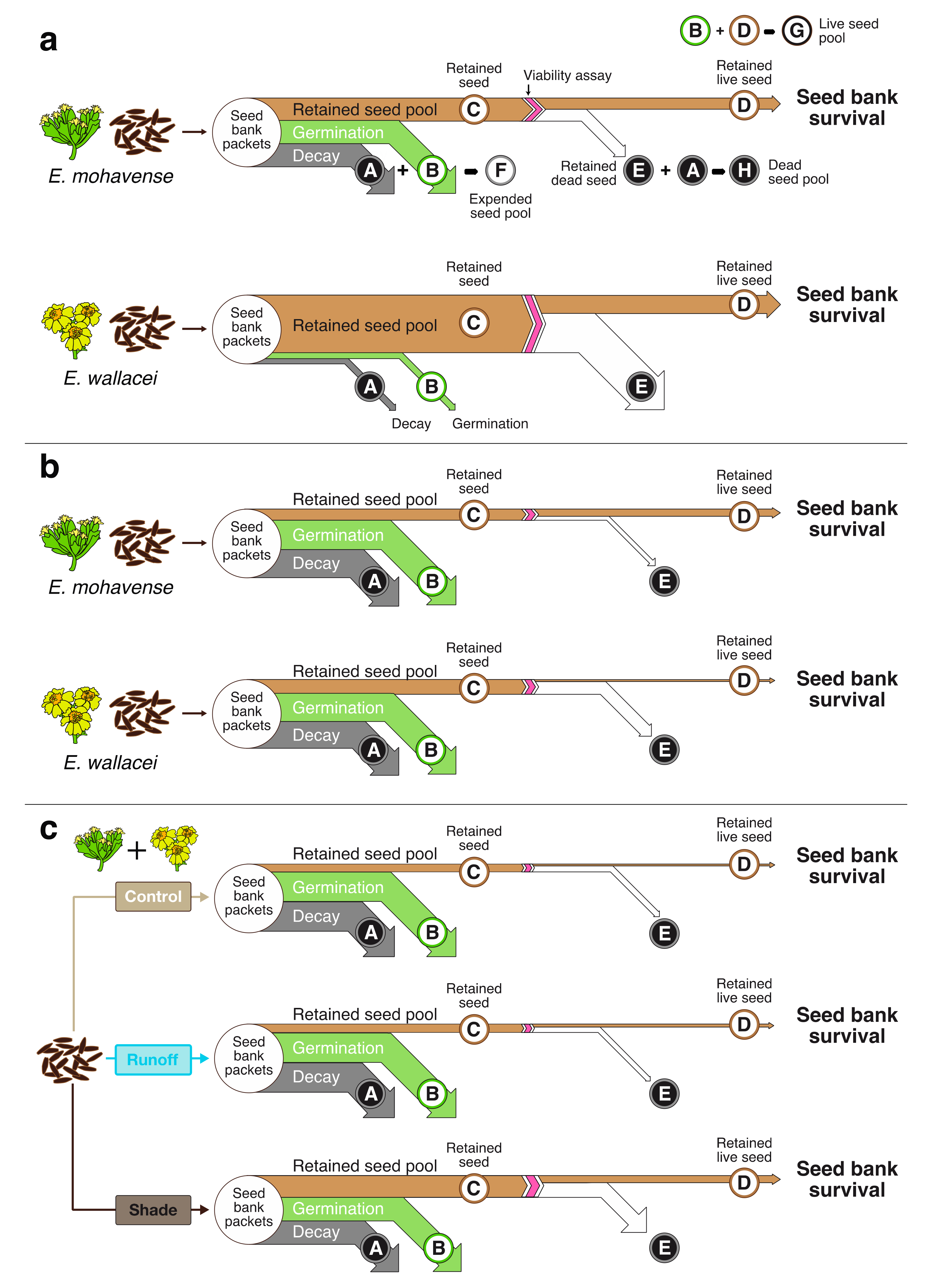
2.1. Seed Bank Survival Conceptual Model
Seed Bank Survival
2.2. Seed Bank of a Rare and Common Annual Plant Congener Pair
2.3. Seed Bank-Environment Interactions
3. Discussion
3.1. Simulated Photovoltaic Solar Panels and Seed Bank Survival
3.2. Seed Bank Survival of a Rare and Common Annual Plant Congener
4. Materials and Methods
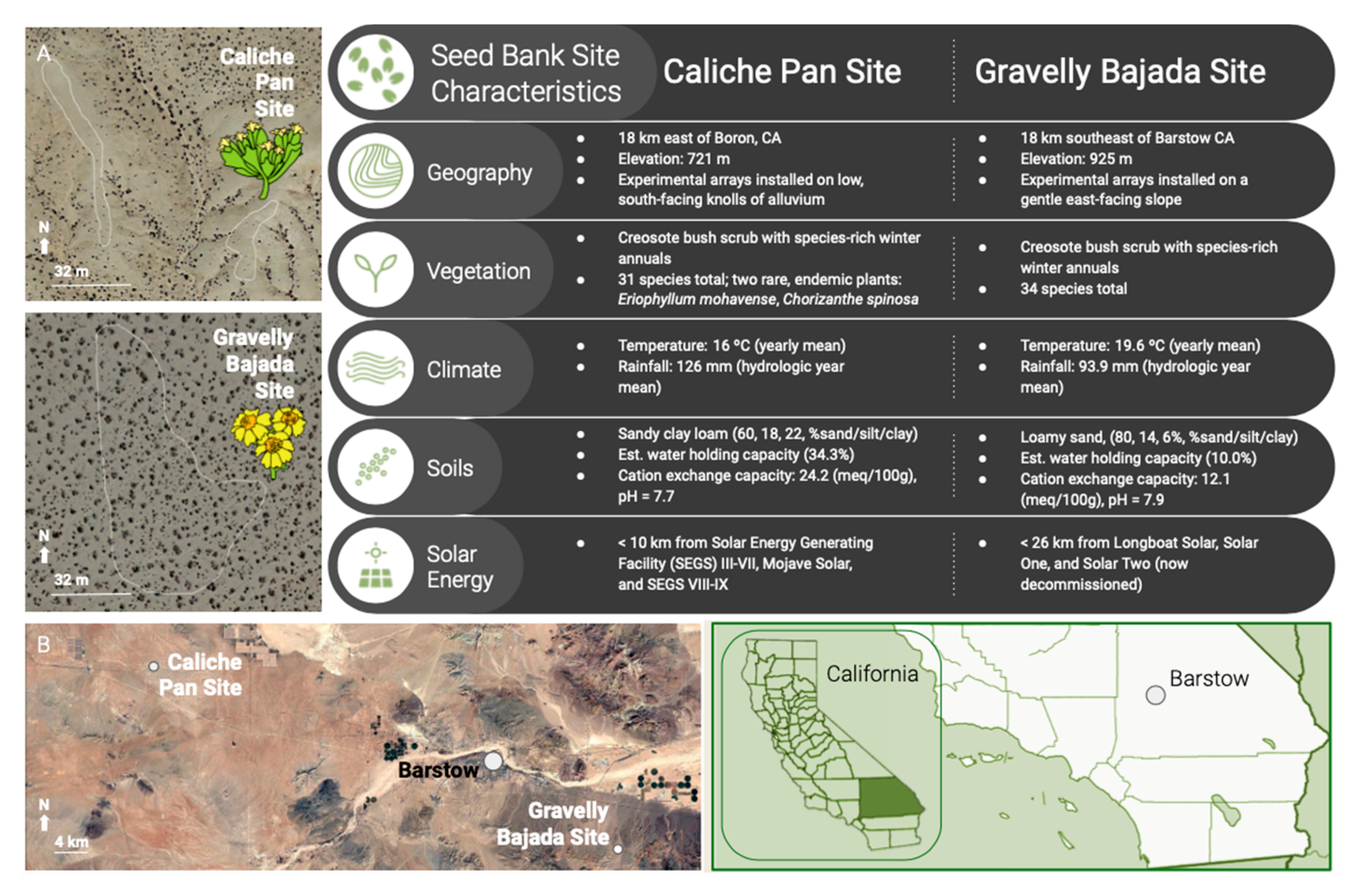
4.1. Study Sites
4.2. Artificial Photovoltaic Installation and Experimental Design
4.3. Study Organisms
4.4. Seed Acquisition for Seed Bank Packets
4.5. Seed Bank Packet Construction
4.6. Staining Assays
4.7. Seed Bank Survival Conceptual Model and Methods
4.8. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandel, B.; Gutiérrez, A.G.; Reich, P.B.; Schrodt, F.; Dickie, J.; Kattge, J. Estimating the missing species bias in plant trait measurements. J. Veg. Sci. 2015, 26, 828–838. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.O.; Cross, A.T.; et al. A research agenda for seed-trait functional ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef] [Green Version]
- Wisheu, I.C.; Keddy, P.A. Seed banks of a rare wetland plant community: Distribution patterns and effects of human-induced disturbance. J. Veg. Sci. 1991, 2, 181–188. [Google Scholar] [CrossRef]
- Moriuchi, K.S.; Venable, D.L.; Pake, C.E.; Lange, T. Direct measurement of the seed bank age structure of a Sonoran Desert annual plant. Ecology 2000, 81, 1133–1138. [Google Scholar] [CrossRef]
- Saatkamp, A.; Poschlod, P.; Venable, D.L. The functional role of soil seed banks in natural communities. In Seeds: The Ecology of Regeneration in Plant Communities; Oxford University Press: Oxford, UK, 2014; pp. 263–295. [Google Scholar]
- LaForgia, M.L.; Spasojevic, M.J.; Case, E.J.; Latimer, A.M.; Harrison, S.P. Seed banks of native forbs, but not exotic grasses, increase during extreme drought. Ecology 2018, 99, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, J.A. Thermal requirements underpinning germination allude to risk of species decline from climate warming. Plants 2020, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.M.; Scholl, J.P.; Basinger, U.; Huxman, T.E.; Venable, D.L. Functional trait trade-off and species abundance: Insights from a multi-decadal study. Ecol. Lett. 2019, 22, 583–592. [Google Scholar] [CrossRef]
- Tielbörger, K.; Prasse, R. Do seeds sense each other? Testing for density-dependent germination in desert perennial plants. Oikos 2009, 118, 792–800. [Google Scholar] [CrossRef]
- Sartor, C.E.; Marone, L. A plurality of causal mechanisms explains the persistence or transience of soil seed banks. J. Arid Environ. 2010, 74, 303–306. [Google Scholar] [CrossRef]
- Rehbein, J.A.; Watson, J.E.M.; Lane, J.L.; Sonter, L.J.; Venter, O.; Atkinson, S.C.; Allan, J.R. Renewable energy development threatens many globally important biodiversity areas. Glob. Chang. Biol. 2020, 26, 3040–3051. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Hoffacker, M.K.; Murphy-Mariscal, M.L.; Wu, G.; Allen, M.F. Solar energy development impacts on land cover change and protected areas. Proc. Natl. Acad. Sci. USA 2015, 112, 13579–13584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore-O’Leary, K.A.; Hernandez, R.R.; Johnston, D.S.; Abella, S.R.; Tanner, K.E.; Swanson, A.C.; Kreitler, J.; Lovich, J.E. Sustainability of utility-scale solar energy—Critical ecological concepts. Front. Ecol. Environ. 2017, 15, 385–394. [Google Scholar] [CrossRef]
- Holmes, P.M.; Newton, R.J. Patterns of seed persistence in South African fynbos. Plant Ecol. 2004, 172, 143–158. [Google Scholar] [CrossRef]
- Ooi, M.K.J.; Auld, T.D.; Whelan, R.J. Distinguishing between persistence and dormancy in soil seed banks of three shrub species from fire-prone southeastern Australia. J. Veg. Sci. 2007, 18, 405. [Google Scholar] [CrossRef]
- Auld, T.D.; Keith, D.A.; Bradstock, R.A. Patterns in longevity of soil seedbanks in fire-prone communities of south-eastern Australia. Aust. J. Bot. 2000, 48, 539–548. [Google Scholar] [CrossRef]
- Sotomayor, D.A.; Gutiérrez, J.R. Seed bank of desert annual plants along an aridity gradient in the southern Atacama coastal desert. J. Veg. Sci. 2015, 26, 1148–1158. [Google Scholar] [CrossRef]
- Caballero, I.; Olano, J.M.; Loidi, J.; Escudero, A. Seed bank structure along a semi-arid gypsum gradient in Central Spain. J. Arid Environ. 2003, 55, 287–299. [Google Scholar] [CrossRef]
- Van Mourik, T.A.; Stomph, T.J.; Murdoch, A.J. Why high seed densities within buried mesh bags may overestimate depletion rates of soil seed banks. J. Appl. Ecol. 2005, 42, 299–305. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Small, J.L.; Torvell, L. Edaphic factors influence the longevity of seeds in the soil. Plant Ecol. 2012, 213, 57–65. [Google Scholar] [CrossRef]
- Rivera, D.; Jáuregui, B.M.; Peco, B. The fate of herbaceous seeds during topsoil stockpiling: Restoration potential of seed banks. Ecol. Eng. 2012, 44, 94–101. [Google Scholar] [CrossRef]
- Reichman, O.J. Spatial and Temporal Variation of Seed Distributions in Sonoran Desert Soils. J. Biogeogr. 1984, 11, 1–11. [Google Scholar] [CrossRef]
- Kemp, P.R. Seed Banks and Vegetation Processes in Deserts. In Ecology of Soil Seed Banks; Academic Press: Cambridge, MA, USA, 1989; pp. 257–281. [Google Scholar]
- Cabin, R.J.; Marshall, D.L.; Mitchell, R.J. The demographic role of soil seed banks. II. Investigations of the fate of experimental seeds of the desert mustard Lesquerella fendleri. J. Ecol. 2000, 88, 293–302. [Google Scholar] [CrossRef]
- Angert, A.L.; Huxman, T.E.; Barron-Gafford, G.A.; Gerst, K.L.; Venable, D.L. Linking growth strategies to long-term population dynamics in a guild of desert annuals. J. Ecol. 2007, 95, 321–331. [Google Scholar] [CrossRef]
- Huxman, T.E.; Kimball, S.; Angert, A.L.; Gremer, J.R.; Barron-Gafford, G.A.; Lawrence Venable, D. Understanding past, contemporary, and future dynamics of plants, populations, and communities using Sonoran desert winter annuals. Am. J. Bot. 2013, 100, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Borgy, B.; Choler, P. Trait databases: Misuses and precautions. J. Veg. Sci. 2015, 26, 826–827. [Google Scholar] [CrossRef]
- Saatkamp, A.; Affre, L.; Baumberger, T.; Dumas, P.J.; Gasmi, A.; Gachet, S.; Arène, F. Soil depth detection by seeds and diurnally fluctuating temperatures: Different dynamics in 10 annual plants. Plant Soil 2011, 349, 331–340. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. High temperature requirements for afterripening in seeds of winter annuals. New Phytol. 1976, 77, 619–624. [Google Scholar] [CrossRef]
- Li, Y.M.; Shaffer, J.P.; Hall, B.; Ko, H. Soil-borne fungi influence seed germination and mortality, with implications for coexistence of desert winter annual plants. PLoS ONE 2019, 14, e0224417. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.H.; Reichman, O.J.; Davidson, D.W. Granivory in desert ecosystems. Annu. Rev. Ecol. Syst. 1979, 10, 201–227. [Google Scholar] [CrossRef]
- Saatkamp, A.; Affre, L.; Dutoit, T.; Poschlod, P. The seed bank longevity index revisited: Limited reliability evident from a burial experiment and database analyses. Ann. Bot. 2009, 104, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K. The functional ecology of soil seed banks. In Seeds: The Ecology of Regeneration in Plant Communities; Oxford University Press: Oxford, UK, 2009; pp. 215–235. [Google Scholar]
- Adondakis, S.; Venable, D.L. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology 2004, 85, 2582–2590. [Google Scholar] [CrossRef] [Green Version]
- Parker, V.T.; Simpson, R.L.; Leck, M.A. Pattern and Process in the Dynamics of Seed Banks. In Ecology of Soil Seed Banks; Academic Press: Cambridge, MA, USA, 1989; pp. 367–384. [Google Scholar]
- Lyons, K.G.; Brigham, C.A.; Traut, B.H.; Schwartz, M.W. Rare species and ecosystem functioning. Conserv. Biol. 2005, 19, 1019–1024. [Google Scholar] [CrossRef]
- Lavergne, S.; Garnier, E.; Debussche, M. Do rock endemic and widespread plant species differ under the Leaf-Height-Seed plant ecology strategy scheme? Ecol. Lett. 2003, 6, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The ecophysiology of seed persistence: A mechanistic view of the journey to germination or demise. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef]
- Tanner, K.E.; Moore-O’Leary, K.; Parker, I.M.; Pavlik, B.M.; Hernandez, R.R. Microhabitats associated with solar energy development alter demography of two desert annuals. under review.
- Hernandez, R.R.; Hoffacker, M.K.; Field, C.B. Efficient use of land to meet sustainable energy needs. Nat. Clim. Chang. 2015, 5, 353–358. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Hoffacker, M.K.; Murphy-Mariscal, M.L.; Wu, G.C.; Allen, M.F. Land-sparing opportunities for solar energy development in agricultural landscapes: A case study of the Central Valley. Environ. Sci. Technol. 2017, 51, 14472–14482. [Google Scholar]
- Hernandez, R.R.; Armstrong, A.; Burney, J.; Ryan, G.; Moore-O’Leary, K.; Diédhiou, I.; Grodsky, S.M.; Saul-Gershenz, L.; Davis, R.; Macknick, J.; et al. Techno–ecological synergies of solar energy for global sustainability. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Parker, S.S.; Cohen, B.S.; Moore, J. Impact of solar and wind development on conservation values in the Mojave Desert. PLoS ONE 2018, 13, e0207678. [Google Scholar] [CrossRef] [Green Version]
- Grodsky, S.M.; Leary, K.A.M.; Hernandez, R.R. From butterflies to bighorns: Multi-dimensional species-species and species-process interactions may inform sustainable solar energy development in desert ecosystems. In Proceedings of the 31st Annual Desert Symposium, California State University Desert Studies Center, Zzyzx, CA, USA, 14–15 April 2017; pp. 322–327. [Google Scholar]
- Grodsky, S.M.; Hernandez, R.R. Reduced ecosystem services of desert plants from ground-mounted solar energy. Nat. Sustain. 2020, 1–8. [Google Scholar] [CrossRef]
- Haegel, N.M.; Atwater, H.; Barnes, T.; Breyer, C.; Burrell, A.; Chiang, Y.-M.; De Wolf, S.; Dimmler, B.; Feldman, D.; Glunz, S.; et al. Terawatt-scale photovoltaics: Transform global energy. Science. 2019, 364, 836–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, K.E.; Moore-O’Leary, K.A.; Parker, I.M.; Pavlik, B.M.; Hernandez, R.R. Simulated solar panels create altered microhabitats in desert landforms. Ecosphere 2020, 11, e03089. [Google Scholar] [CrossRef]
- Cohen, D. Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 1966, 12, 119–129. [Google Scholar] [CrossRef]
- Salguero-Gómez, R.; Siewert, W.; Casper, B.B.; Tielbörger, K. A demographic approach to study effects of climate change in desert plants. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3100–3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Chiquoine, L.P.; Abella, S.R. Soil seed bank assay methods influence interpretation of non-native plant management. Appl. Veg. Sci. 2018, 21, 626–635. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, J.M.; Baskin, C.C.; Hidayati, S.N. Defining transient and persistent seed banks in species with pronounced seasonal dormancy and germination patterns. Seed Sci. Res. 2005, 15, 189–196. [Google Scholar] [CrossRef]
- Fox, J.; Weisbery, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Carruthers, E.; Lewis, K.; Mccue, T.; Westley, P. Generalized Linear Models: Model Selection, Diagnostics, and Overdispersion; Memorial University of Newfoundland: St. John’s, NL, Canada, 2008; pp. 1–21. [Google Scholar]
- Kalisz, S.; McPeek, M.A. Demography of an age-structured annual: Resampled projection matrices, elasticity analyses, and seed bank effects. Ecology 1992, 73, 1082–1093. [Google Scholar] [CrossRef]
- Pluntz, M.; Le Coz, S.; Peyrard, N.; Pradel, R.; Choquet, R.; Cheptou, P.O. A general method for estimating seed dormancy and colonisation in annual plants from the observation of existing flora. Ecol. Lett. 2018, 21, 1311–1318. [Google Scholar] [CrossRef]
- Tevis, L. A population of desert Ephemerals germinated by less than one inch of rain. Ecology 1958, 39, 688–695. [Google Scholar] [CrossRef]
- Beatley, J.C. Phenological events and their environmental triggers in Mojave Desert ecosystems. Ecology 1974, 55, 856–863. [Google Scholar] [CrossRef]
- Filazzola, A.; Lortie, C.J. A systematic review and conceptual framework for the mechanistic pathways of nurse plants. Glob. Ecol. Biogeogr. 2014, 23, 1335–1345. [Google Scholar] [CrossRef]
- Kos, M.; Poschlod, P. Seeds use temperature cues to ensure germination under nurse-plant shade in Xeric Kalahari Savannah. Ann. Bot. 2007, 99, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macknick, J.; Beatty, B.; Hill, G. Overview of Opportunities for Co-Location of Solar Energy Technologies and Vegetation; NREL/TP-6A20-60240; National Renewable Energy Laboratory: Golden, CO, USA, 2013.
- Yang, Y.; Hobbie, S.E.; Hernandez, R.R.; Fargione, J.; Grodsky, S.M.; Tilman, D.; Zhu, Y.-G.; Luo, Y.; Smith, T.M.; Jungers, J.M.; et al. Restoring Abandoned Farmland to Mitigate Climate Change on A Full Earth. One Earth 2020, in press. [Google Scholar] [CrossRef]
- Schafer, M.; Kotanen, P.M. The influence of soil moisture on losses of buried seeds to fungi. Acta Oecologica 2003, 24, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Mordecai, E.A. Soil moisture and fungi affect seed survival in California Grassland annual plants. PLoS ONE 2012, 7, e39083. [Google Scholar] [CrossRef] [Green Version]
- Tanner, K.E. Plant Response to Land Use Change in Two Iconically Stressful Habitats: California’s Desert Solar Fields and Restored Coastal Salt Marshes; University of California: Santa Cruz, CA, USA, 2020. [Google Scholar]
- Cohen, D. Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J. Theor. Biol. 1967, 16, 1–14. [Google Scholar] [CrossRef]
- Grime, J.P.; Mason, G.; Curtis, A.V.; Rodman, J.; Band, S.R. A Comparative Study of Germination Characteristics in a Local Flora. J. Ecol. 1981, 69, 1017. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Leck, M.A. Ecology of Soil Seed Banks; Academic Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Del Castillo, R.F. Factors influencing the genetic structure of Phacelia dubia, a species with a seed bank and large fluctuations in population size. Heredity 1994, 72, 446–458. [Google Scholar] [CrossRef] [Green Version]
- Venable, D.L.; Kimball, S. Population and community dynamics in variable environments: The desert annual system. In Temporal Dynamics and Ecological Process; Cambridge University Press: Cambridge, UK, 2012; pp. 140–164. [Google Scholar]
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Philippi, T. Bet-hedging germination of desert annuals: Variation among populations and maternal effects in Lepidium lasiocarpum. Am. Nat. 1993, 142, 488–507. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Jones, C.S. The comparative importance of overland runoff and mean annual rainfall to shrub communities of the Mojave Desert. Bot. Gaz. 1984, 145, 116–124. [Google Scholar] [CrossRef]
- Hamerlynck, E.; Huxman, T.E.; McAuliffe, J.R.; Smith, S.D. Carbon isotope discrimination and foliar nutrient status of Larrea tridentata (creosote bush) in contrasting Mojave Desert soils. Oecologia 2004, 138, 210–215. [Google Scholar] [CrossRef]
- Vandergast, A.G.; Inman, R.D.; Barr, K.R.; Nussear, K.E.; Esque, T.C.; Hathaway, S.A.; Wood, D.A.; Medica, P.A.; Breinholt, J.W.; Stephen, C.L.; et al. Evolutionary Hotspots in the Mojave Desert. Diversity 2013, 5, 293–319. [Google Scholar] [CrossRef] [Green Version]
- Thorne, J.H.; Viers, J.H.; Price, J.; Stoms, D.M. Spatial Patterns of Endemic Plants in California. Nat. Areas J. 2009, 29, 344–366. [Google Scholar] [CrossRef]
- Baldwin, B.; Goldman, D.H.; Keil, D.J.; Patterson, R.; Rosatti, T.J. The Jepson Manual: Vascular Plants of California; University of California Press: Berkeley, CA, USA, 2012. [Google Scholar]
- California Native Plant Society: Calscape-Barstow Woolly Sunflower. Available online: https://calscape.org/Eriophyllum-mohavense-() (accessed on 1 May 2020).
- Mooring, J.S. Experimental hybridizations of Eriophyllum annuals (Asteraceae, Helenieae). Am. J. Bot. 2002, 89, 1973–1983. [Google Scholar] [CrossRef]
- Consortium of California Herbaria The Calflora Database: Eriophyllum mohavense. Available online: https://www.calflora.org/cgi-bin/species_query.cgi?where-calrecnum=3439 (accessed on 1 May 2020).
- State of California Desert Renewable Energy Conservation Plan. Available online: https://www.energy.ca.gov/programs-and-topics/programs/desert-renewable-energy-conservation-plan (accessed on 1 May 2020).
- Colville, L.; Pritchard, H.W. Seed life span and food security. New Phytol. 2019, 224, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Freas, K.E.; Kemp, P.R. Some relationships between environmental reliability and seed dormancy in desert annual plants. J. Ecol. 1983, 71, 211. [Google Scholar] [CrossRef]
- Porter, R.H.; Durrell, M.; Romm, H.J. The use of 2,3,5-Triphenyl-tetrazoliumchloride as a measure of seed germinability. Plant Physiol. 1947, 22, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Pake, C.E.; Venable, D.L. Seed banks in desert annuals: Implications for persistence and coexistence in variable environments. Ecology 1996, 77, 1427–1435. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means, Version 1.3.4; R Package. 2019. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 May 2020).
- Shoemaker, W.R.; Lennon, J.T. Evolution with a seed bank: The population genetic consequences of microbial dormancy. Evol. Appl. 2018, 11, 60–75. [Google Scholar] [CrossRef] [PubMed]


| Species | Seed Cohort | Number of Seed Bank Packets | Number of Seeds Per Packet | Total Seeds | |
|---|---|---|---|---|---|
| (a) | E. mohavense | 2015 | 90 | 18 | 1620 |
| 2016 | 180 | 9 | 1620 | ||
| (b) | E. wallacei | 2015 | 90 | 14 | 1260 |
| 2016 | 180 | 2 | 360 |
| Predictor | Sum Sq. | Df | F-Value | p-Value |
|---|---|---|---|---|
| year | 190.94 | 1 | 108.77 | <0.001 |
| species | 52.38 | 1 | 29.84 | <0.001 |
| microhabitat | 17.94 | 2 | 5.11 | 0.007 |
| seed cohort | 3.88 | 1 | 2.21 | 0.139 |
| year × species | 63.07 | 1 | 35.92 | <0.001 |
| year × microhabitat | 20.17 | 2 | 5.74 | 0.004 |
| species × microhabitat | 1.86 | 2 | 0.53 | 0.589 |
| year × seed cohort | 0.30 | 1 | 0.17 | 0.681 |
| species × seed cohort | 29.83 | 1 | 16.99 | <0.001 |
| microhabitat × seed cohort | 0.55 | 2 | 0.16 | 0.855 |
| year × species × microhabitat | 2.76 | 2 | 0.79 | 0.457 |
| year × species × seed cohort | 0.05 | 1 | 0.03 | 0.869 |
| year × microhabitat × seed cohort | 0.70 | 2 | 0.20 | 0.819 |
| species × microhabitat × seed cohort | 0.02 | 2 | 0.01 | 0.994 |
| year × species × microhabitat × seed cohort | 0.38 | 2 | 0.11 | 0.897 |
| residuals | 330.04 | 188 | NA | NA |
| Predictor | Sum Sq. | Df | F-Value | p-Value |
|---|---|---|---|---|
| Year | 2.97 | 1 | 2.90 | 0.089 |
| species | 41.99 | 1 | 41.00 | <0.001 |
| microhabitat | 0.00 | 2 | 0.00 | 1.000 |
| seed cohort | 2.02 | 1 | 1.97 | 0.161 |
| year × species | 11.48 | 1 | 11.21 | 0.001 |
| year × microhabitat | 0.00 | 2 | 0.00 | 1.000 |
| species × microhabitat | 0.00 | 2 | 0.00 | 1.000 |
| year × seed cohort | 4.55 | 1 | 4.45 | 0.035 |
| species × seed cohort | 0.87 | 1 | 0.85 | 0.357 |
| microhabitat × seed cohort | 0.00 | 2 | 0.00 | 1.000 |
| year × species × microhabitat | 0.00 | 2 | 0.00 | 1.000 |
| year × species × seed cohort | 7.95 | 1 | 7.76 | 0.006 |
| year × microhabitat × seed cohort | 0.00 | 2 | 0.00 | 1.000 |
| species × microhabitat × seed cohort | 0.00 | 2 | 0.00 | 1.000 |
| year × species × microhabitat × seed cohort | 0.00 | 2 | 0.00 | 1.000 |
| residuals | 595.00 | 581 | NA | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, R.R.; Tanner, K.E.; Haji, S.; Parker, I.M.; Pavlik, B.M.; Moore-O’Leary, K.A. Simulated Photovoltaic Solar Panels Alter the Seed Bank Survival of Two Desert Annual Plant Species. Plants 2020, 9, 1125. https://doi.org/10.3390/plants9091125
Hernandez RR, Tanner KE, Haji S, Parker IM, Pavlik BM, Moore-O’Leary KA. Simulated Photovoltaic Solar Panels Alter the Seed Bank Survival of Two Desert Annual Plant Species. Plants. 2020; 9(9):1125. https://doi.org/10.3390/plants9091125
Chicago/Turabian StyleHernandez, Rebecca R., Karen E. Tanner, Sophia Haji, Ingrid M. Parker, Bruce M. Pavlik, and Kara A. Moore-O’Leary. 2020. "Simulated Photovoltaic Solar Panels Alter the Seed Bank Survival of Two Desert Annual Plant Species" Plants 9, no. 9: 1125. https://doi.org/10.3390/plants9091125







