Chemical Compounds, Pharmacological and Toxicological Activity of Brugmansia suaveolens: A Review
Abstract
:1. Introduction
2. Botany
2.1. Taxonomical Classification
2.2. Distribution
2.3. Ethnobotany
3. Phytochemistry
3.1. Alkaloids
3.2. Volatile Compounds
3.3. Phenolic Compounds, Coumarin, and Flavonoids
3.4. Steroids
3.5. Hydrocarbons
4. Pharmacological Activity
4.1. Antinociceptive
4.2. Antimicrobial
4.3. Nematicide
4.4. Cytotoxicity
4.5. Muscle Relaxer
5. Toxicity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Plants List. “Brugmansia suaveolens”. 2020. Available online: http://www.theplantlist.org./tpl1.1/record/kew-26840192020 (accessed on 18 August 2020).
- Chellemi, D.O.; Webster, C.G.; Baker, C.A.; Annamalai, M.; Achor, D.; Adkins, S. Widespread occurrence and low genetic diversity of Colombian datura virus in Brugmansia suggest an anthropogenic role in virus selection and spread. Plant Dis. 2011, 95, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Monroy-Ortiz, C.; Monroy, R. Las Plantas, Compañeras de Siempre: La Experiencia en Morelos, 1st ed.; UAEM, Centro de Investigaciones Biológicas de la Conabio Conanp: Cuernavaca, Morelos, Mexico, 2006. [Google Scholar]
- Encarnación-Dimayuga, R.; Altamirano, L.; Maki, K.A. Screening of medicinal plants from Baja California Sur (Mexico) by their effects on smooth muscle contractility. Pharm. Biol. 1998, 36, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Furlan, V.; Kujawska, M.; Hilgert, N.I.; Pochettino, M.L. To what extent are medicinal plants shared between country home gardens and urban ones? A case study from Misiones, Argentina. Pharm. Biol. 2016, 54, 1628–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Feo, V. The ritual use of Brugmansia species in traditional Andean medicine in northern Peru. Econ. Bot. 2004, 58, S221–S229. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. A townesmith toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Biset, J.; Cañigueral, S. Plants as medicinal stressors, the case of depurative practices in Chazuta valley (Peruvian Amazonia). J. Ethnopharmacol. 2013, 145, 67–76. [Google Scholar] [CrossRef]
- Parker, A.; Goulart Peraza, G.; Sena, J.; Sinnott Silva, E.; Flores Soares, M.C.; Cezar Vaz, M.R.; Badiale Furlong, L.; Muccillo-Baisch, A.L. Antinociceptive effects of the aqueous extract of Brugmansia suaveolens flowers in mice. Biol. Res. Nurs. 2007, 8, 234–239. [Google Scholar] [CrossRef]
- Lucinda, N.; Inoue-Nagata, A.K.; Kitajima, E.W.; Nagata, T. Complete genome sequence of Brugmansia suaveolens mottle virus, a potyvirus from an ornamental shrub. Arch. Virol. 2010, 155, 1729–1732. [Google Scholar] [CrossRef]
- Hudák, J.; Walles, B.; Vennigerholz, F. The transmitting tissue in Brugmansia suaveolens L.: Ultrastructure of the stylar transmitting tissue. Ann. Bot. 1993, 71, 177–186. [Google Scholar] [CrossRef]
- Jayawickreme, K.P.; Janaka, K.V.C.; Subasinghe, S. Unknowing ingestion of Brugmansia suaveolens leaves presenting with signs of anticholinergic toxicity: A case report. J. Med. Case Rep. 2019, 13, 1–4. [Google Scholar] [CrossRef]
- Oktavia, A.I.; Indriani, S.; Jati, B. Ethnobotanical study of toxic plants in Ngadiwono Village, Tosari District, Pasuruan Regency, East Java. Indones. J. Environ. Sustain. Dev. 2017, 8, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Batoro, J.; Siswanto, D. Ethnomedicinal survey of plants used by local society in Poncokusumo district, Malang, East Java Province, Indonesia. Asian J. Med. Biol. Res. 2017, 3, 158–167. [Google Scholar] [CrossRef]
- IjazaI, F.; IqbalbI, Z.; Ur RahmancI, I.; AlamdI, J.; Mulk KhaneI, S.; Mujtaba ShahfI, G.; KhangI, K.; AfzalhI, A. Investigation of traditional medicinal floral knowledge of Sarban Hills, Abbottabad, KP, Pakistan. J. Ethnopharmacol. 2016, 179, 208–233. [Google Scholar] [CrossRef] [PubMed]
- Chetri, B.K.; Wangdi, P.; Penjor, T. Ethnomedicinal practices in Kilikhar, Mongar. Asian Plant Res. J. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Bye, R.; Sosa, V. Molecular phylogeny of the jimsonweed genus Datura (Solanaceae). Syst. Bot. 2013, 38, 818–829. [Google Scholar] [CrossRef]
- Safford, W.E. Synopsis of the genus Datura. J. Washingt. Acad. Sci. 1921, 11, 173–189. Available online: http://www.jstor.org/stable/24532461 (accessed on 25 March 2020).
- Kujawska, M. Forms of medical pluralism among the Polish Community in Misiones, Argentina. Anthropol. Med. 2016, 23, 205–219. [Google Scholar] [CrossRef]
- Hosking, J.R.; Conn, B.J.; Lepschi, B.J.; Barker, C.H. Plant species first recognised as naturalised or naturalising for New South Wales in 2004 and 2005. Cunninghamia 2011, 12, 85–114. [Google Scholar]
- Anthony, S.J.; Zuchowski, W.; Setzer, W.N. Composition of the floral essential oil of Brugmansia suaveolens. Rec. Nat. Prod. 2009, 3, 76. [Google Scholar]
- Stashenko, E.E.; Martínez, J.R. Sampling flower scent for chromatographic analysis. J. Sep. Sci. 2008, 31, 2022–2031. [Google Scholar] [CrossRef]
- Mosquera-Yuqui, F.; Garrido, P.; Flores, F.J. Molecular characterization and complete genome of alstroemeria mosaic virus (AlMV). Virus Genes 2020, 56, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, C.E.; Barbosa, E.P.; Freitas, A.V.L. Immature stages of Pagyris cymothoe cymothoe (Hewitson, 1855) (Lepidoptera, Danainae, Ithomiini). Trop. Zool. 2013, 26, 145–153. [Google Scholar] [CrossRef]
- Danton, P.; Perrier, C.; de Reyes, G.M. Nouveau catalogue de la flore vasculaire de l’archipel Juan Fernández (Chili) Nuevo catálogo de la flora vascular del Archipiélago Juan Fernández (Chile). Acta Bot. Gall. 2006, 153, 399–587. [Google Scholar] [CrossRef]
- Zhao, F.; Lim, S.; Yoo, R.H.; Lim, H.S.; Kwon, S.Y.; Lee, S.H.; Moon, J.S. Complete genome sequence of a South Korean isolate of Brugmansia mosaic virus. Arch. Virol. 2013, 58, 2019–2022. [Google Scholar] [CrossRef]
- Mai, N.T. Quantitative analysis of scopolamine in Brugmansia suaveolens by HPLC-MS method. J. Multidiscip. Eng. Sci. Technol. 2017, 4, 8176–8179. [Google Scholar]
- Lin, T.J.; Nelson, L.S.; Tsai, J.L.; Hung, D.Z.; Hu, S.C.; Chan, H.M.; Deng, J.F. Common toxidromes of plant poisonings in Taiwan. Clin. Toxicol. 2009, 47, 161–168. [Google Scholar] [CrossRef]
- Sajeli Begum, A.; Sahai, M.; Fujimoto, Y.; Asai, K.; Schneider, K.; Nicholson, G.; Suessmuth, R. A new kaempferol diglycoside from Datura suaveolens Humb. & Bonpl. ex. Willd. Nat. Prod. Res. 2006, 20, 1231–1236. [Google Scholar]
- Suganda, A.G.; Nishiyama, Y.; Yamakawa, T.; Sugiyama, N. Random amplified polymorphic DNA analysis to distinguish Brugmansia suaveolens, B. candida and B. versicolor. Plant Biotechnol. 2006, 23, 519–520. [Google Scholar] [CrossRef]
- Sevketoglu, E.; Tatlı, B.; Tuğcu, B.; Demirelli, Y.; Hatipoglu, S. An unusual cause of fulminant Guillain-Barré syndrome: Angel’s trumpet. Pediatr. Neurol. 2010, 43, 368–370. [Google Scholar] [CrossRef]
- Haegi, L. Taxonomic account of Datura L.(Solanaceae) in Australia with a note on Brugmansia Pers. Aust. J. Bot. 1976, 24, 415–435. [Google Scholar] [CrossRef]
- Sykes, W.R. Checklist of dicotyledons naturalised in New Zealand 10. Polemoniales and Boraginaceae. N. Z. J. Bot. 1981, 19, 311–317. [Google Scholar] [CrossRef]
- Otim, A.S.; Kajobe, R.; Abila, P.P.; Kasangaki, P.; Echodu, R. Important plants for honey production in four agro ecological zones of Uganda. Bee World 2019, 96, 81–86. [Google Scholar] [CrossRef]
- Doncheva, T.; Berkov, S.; Philipov, S. Comparative study of the alkaloids in tribe Datureae and their chemosystematic significance. Biochem. Syst. Ecol. 2006, 34, 478–488. [Google Scholar] [CrossRef]
- Zayed, R.; and Wink, M. Induction of tropane alkaloid formation in transformed root cultures of Brugmansia suaveolens (Solanaceae). Z. Nat. C 2004, 59, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, A.; Darók, J.; Bacskay, I.; Felinger, A.; Jakab, G. Protein and alkaloid patterns of the floral nectar in some solanaceous species. Acta Biol. Hung. 2015, 66, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Malandraki, I.; Papachristopoulou, M.; Vassilakos, N. First report of potato spindle tuber viroid (PSTVd) in ornamental plants in Greece. New Dis. Rep. 2010, 21, 9. [Google Scholar] [CrossRef] [Green Version]
- Vennigerholz, F. The transmitting tissue in Brugmansia suaveolens: Immunocytochemical localization of pectin in the style. Protoplasma 1992, 171, 117–122. [Google Scholar] [CrossRef]
- Bennett, B.C. Hallucinogenic plants of the Shuar and related indigenous groups in Amazonian Ecuador and Peru. Brittonia 1992, 44, 483–493. [Google Scholar] [CrossRef]
- Laferriere, J.E. Medicinal plants of the Lowland Inga people of Colombia. Int. J. Pharmacogn. 1994, 32, 90–94. [Google Scholar] [CrossRef]
- Schultes, E.R.; Plowman, T. The ethnobotany of Brugmansia. J. Ethnopharmacol. 1979, 1, 147–164. [Google Scholar] [CrossRef]
- Lalzarzovi, S.T.; Lalramnghinglova, H. Traditional use of medicinal plants found within Aizawl city in Mizoram, India. Pleione 2016, 10, 269–277. [Google Scholar]
- Reis, R.B.; Bragagnolo, F.S.; Gianeti, T.M.R.; Rodrigues, S.A.; Funari, C.S.; Gonçalves, G.G.; Ming, L.C. Brugmansia suaveolens leaf productivity and alkaloid contents under different doses of organic fertilizer. J. Agric. Sci. 2019, 11, 341–349. [Google Scholar] [CrossRef]
- Carlini, E.A.; Maia, L.O. Plant and fungal hallucinogens as toxic and therapeutic agents. Toxinology 2015, 1, 1–44. [Google Scholar]
- Rohman, F.; Juma, D.H.; Utomo, S.R.; Arifah, S.N.; Putra, W.E. Plants diversity as a medicinal plants by the Tengger Tribe, Bromo Tengger Semeru National Park, East Java, Indonesia. EurAsian J. Biosci. 2019, 13, 2293–2298. [Google Scholar]
- Freitas, A.V.L.; Trigo, J.R.; Brown, K.S.; Witte, L.; Hartmann, T.; Barata, L.E.S. Tropane and pyrrolizidine alkaloids in the ithomiines Placidula euryanassa and Miraleria cymothoe (Lepidoptera: Nymphalidae). Chemoecology 1996, 7, 61–67. [Google Scholar] [CrossRef]
- Sakunthala, P.; Charles, A.; Kesavan, D.; Ramani, V.A. Phytochemical screening and adsorption studies of Brugmansia suaveolens. Chem. Sci. Rev. Lett. 2013, 29, 319–322. [Google Scholar]
- Nandakumar, A.; Vaganan, M.M.; Sundararaju, P.; Udayakumar, R. Phytochemical analysis and nematicidal activity of ethanolic leaf extracts of Datura metel, Datura innoxia and Brugmansia suaveolens against Meloidogyne incognita. Asian J. Biol. 2017, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Marvin, “MarvinSketch 18.04”. 2018. Available online: http://www.chemaxon.com (accessed on 25 March 2020).
- Alam, E.A. In vitro cultures for the production of some anticancer agents. Life Sci. J. 2013, 10, 297–310. [Google Scholar]
- Pinto, C.F.; Salinas, S.; Flores-Prado, L.; Echeverría, J.; Niemeyer, H.M. Sequestration of tropane alkaloids from Brugmansia suaveolens (Solanaceae) by the treehopper Alchisme grossa (Hemiptera: Membracidae). Biochem. Syst. Ecol. 2016, 66, 161–165. [Google Scholar] [CrossRef]
- Andreola, B.; Piovan, A.; Da Dalt, L.; Filippini, R.; Cappelletti, E. Unilateral mydriasis due to Angel’s Trumpet. Clin. Toxicol. 2008, 46, 329–331. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.N.; Sartoratto, A.; Trigo, J.R. Scopolamine in Brugmansia suaveolens (Solanaceae): Defense, allocation, costs, and induced response. J. Chem. Ecol. 2007, 33, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.T. Investigation on chemical constituents of the Brugmansia suaveolens flowers. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 10021–10024. [Google Scholar]
- Geller, F.; Murillo, R.; Steinhauser, L.; Heinzmann, B.; Albert, K.; Merfort, I.; Laufer, S. Four new flavonol glycosides from the leaves of Brugmansia suaveolens. Molecules 2014, 19, 6727–6736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muccillo-Baisch, A.L.; Parker, A.G.; Cardoso, G.P.; Cezar-Vaz, M.R.; Flores Soares, M.C. Evaluation of the analgesic effect of aqueous extract of Brugmansia suaveolens flower in mice: Possible mechanism involved. Biol. Res. Nurs. 2010, 11, 345–350. [Google Scholar] [CrossRef]
- Vilani, A.; Lozano, E.R.; Potrich, M.; de Gouvea, A.; Martins Costa Maia, F.; Angeli Alves, L.F.; Dall Agnol de Lima, J. Activity of plant aqueous extracts on Bacillus thuringiensis and their interactions on Anticarsia gemmatalis (Lepidoptera: Erebinae). Semin. Ciências Agrárias 2017, 38, 1051–1057. [Google Scholar] [CrossRef]
- Santos, I.D.; Souza, F.; Akisue, G.; da Coelho, S.F.A.; Coelho, M.D.G. Evaluation of ovicidal and larvicidal activity of ten plant extracts against Ancylostoma spp. Rev. Patol. Trop. 2013, 42, 209–216. [Google Scholar]
- Doan, U.V.M.; Wu, L.; Phua, D.H.; Mendez Rojas, B.; Yang, C.C. Datura and Brugmansia plants related antimuscarinic toxicity: An analysis of poisoning cases reported to the Taiwan poison control center. Clin. Toxicol. 2019, 57, 246–253. [Google Scholar] [CrossRef]
- Isbister, G.K.; Oakley, P.A.; Dawson, H.; Whyte, I.M. Presumed Angel’s trumpet (Brugmansia) poisoning: Clinical effects and epidemiology. Emerg. Med. 2003, 15, 376–382. [Google Scholar] [CrossRef]
- Fuchs, J.; Rauber-Lüthy, C.; Kupferschmidt, H.; Kupper, J.; Kullak-Ublick, G.A.; Ceschi, A. Acute plant poisoning: Analysis of clinical features and circumstances of exposure. Clin. Toxicol. 2011, 49, 671–680. [Google Scholar] [CrossRef] [Green Version]


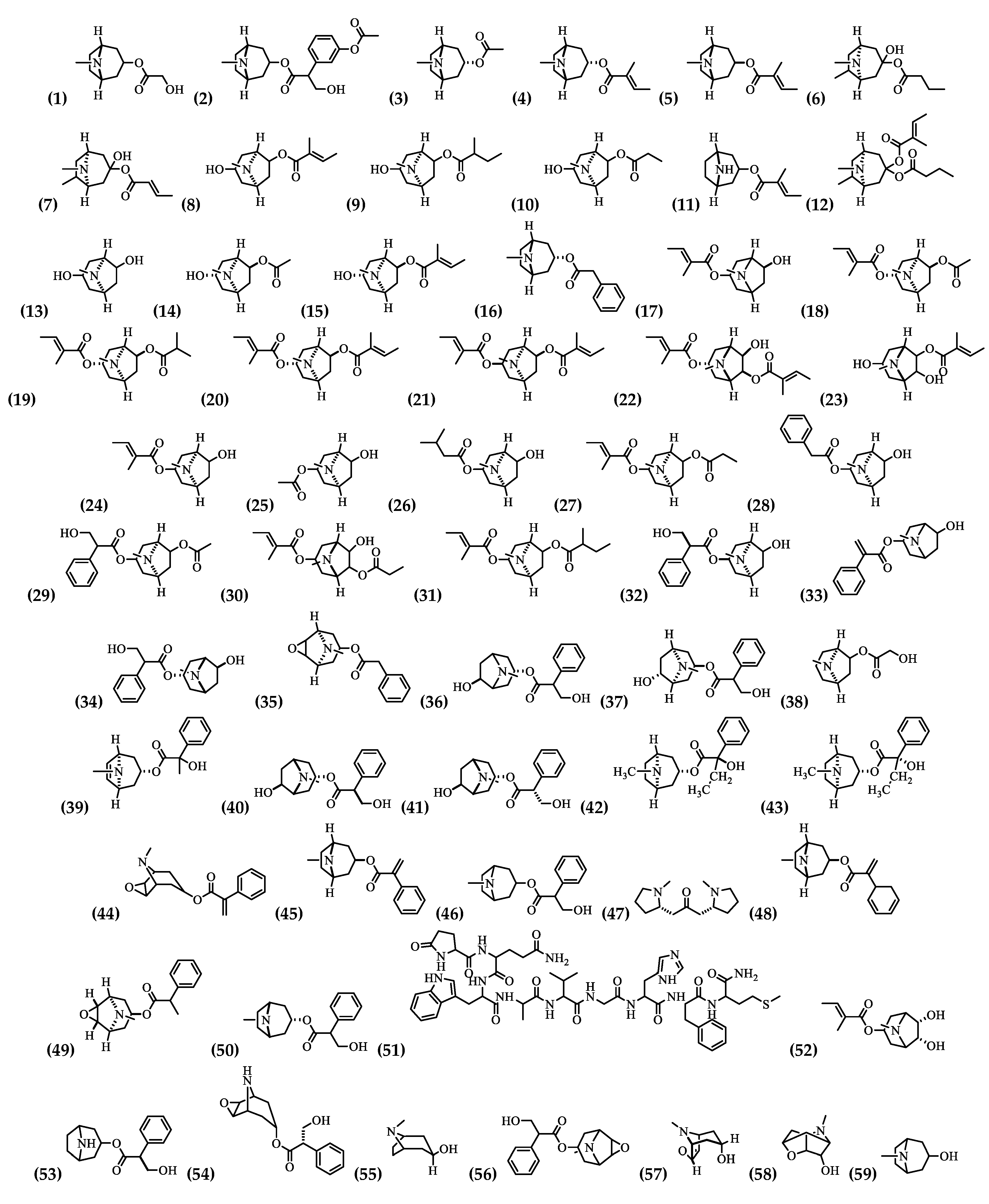




| Kingdom | Plantae |
|---|---|
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Order | Solanales |
| Family | Solanaceae |
| Subfamilia | Solanoideae |
| Tribe | Datureae |
| Genus | Brugmansia |
| Species | B. suaveolens [1] |
| No. | Compound Name | Parts Used |
|---|---|---|
| (1) | 3-(Hydroxyacetoxy)-tropane | Roots [35] |
| (2) | 3-(3′Acetoxytropoyloxy)-tropane | Flowers, Roots [35] |
| (3) | 3α-Acetoxytropane | Root cultivation [36] |
| (4) | 3α-Tigloyloxytropane | Flowers and Roots [35] |
| (5) | 3β-Tigloyloxytropane | Flowers [35] |
| (6) | 3-Hydroxy-6-methylbutyryloxy-tropane | Roots [35] |
| (7) | 3-Hydroxy-6-methyl-butenoyl-oxytropane | Flowers [47] |
| (8) | 3-Hydroxy-6-tigloyloxytropane | Root cultivation [36] |
| (9) | 3-Hydroxy-6-(2-methyl butyryloxy)-tropane | Root cultivation [36], Flowers, Roots [35] |
| (10) | 3-Hydroxy-6-propionyl-oxytropane | Flowers [47] |
| (11) | 3-Tigloyloxynortropane | Flowers [35] |
| (12) | 3-Tigloyloxy-6-methylbutyryloxytropane | Flowers, Roots [35] |
| (13) | 3,6-Dihydroxytropane | Flowers [47] |
| (14) | 3α-Hydroxy-6β-acetoxytropane | Flowers, Roots [35] |
| (15) | 3α-Hydroxy-6β-tigloyloxytropane | Flowers, Roots [35] |
| (16) | 3α-Phenylacetoxytropane | Roots [35] |
| (17) | 3α-Tigloyloxy-6β-hydroxytropane | Flowers, Roots [35] |
| (18) | 3α-Tigloyloxy-6β-acetoxytropane | Flowers [35] |
| (19) | 3α-Tigloyloxy-6β-isobutyryloxytropane | Flowers, Roots [35] |
| (20) | 3α,6β-Ditigloyloxytropane | Flowers, Roots [35] and Root cultivation [36] |
| (21) | 3β,6β-Ditigloyloxytropane | Roots [35] |
| (22) | 3α,6β-Ditigloyloxy-7β-hydroxytropane | Flowers [35] |
| (23) | 3,6-dihydroxy-7-tigloyloxytropane | Flowers [35] |
| (24) | 3-Tigloyloxy-6-hydroxytropane | Root cultivation [36] |
| (25) | 3-Acetoxy-6-hydroxytropane | Root cultivation [36] |
| (26) | 3-Isovaleryloxy-6-hydroxytropane | Roots [35] |
| (27) | 3-Tigloyloxy-6-propionyloxytropane | Flowers, and Roots [35] |
| (28) | 3-Phenylacetoxy-6-hydroxytropane | Flowers [35] |
| (29) | 3-Tropoyloxy-6-acetoxytropane | Flowers [35] |
| (30) | 3-Tigloyloxy-6-propionyloxy-7-hydroxytropane | Flowers, Roots [35] |
| (31) | 3-Tigloyloxy-6-(2-methylbutyryloxy)-tropane | Root cultivation [36] and Roots [35] |
| (32) | 7-Hydroxyhyoscyamine | Flowers [35] |
| (33) | 6-Hydroxyapoatropine | Flowers [47], Root cultivation [36], Roots [35] |
| (34) | 6-Hydroxyhyoscyamine | Flowers, Roots [35] |
| (35) | 3-Phenylacetoxy-6,7-epoxytropane | Flowers [52] |
| (36) | 6β-Hydroxyhyoscyamine | Root cultivation [36] |
| (37) | 7β-Hydroxyhyoscyamine | Root cultivation [36] |
| (38) | 6-Hydroxyacetoxytropane | Flowers, Roots [35] |
| (39) | 6,7-Dehydronoratopine | Flowers [47] |
| (40) | 6R-Hydroxyhyoscyamine | Flowers [47] |
| (41) | 6S-Hydroxyhyoscyamine | Flowers [47] |
| (42) | 6R-Hydroxynorhyoscyamine | Flowers [47] |
| (43) | 6S-Hydroxynorhyoscyamine | Flowers [47] |
| (44) | Aposcopolamine | Flowers [47], Root cultivation [36], and Roots [35] |
| (45) | Apoatropine | Flowers [47], Root cultivation [36], and Roots [35] |
| (46) | Atropine | Root cultivation [36], and Corolla [53] |
| (47) | Cuscohygrine | Root cultivation [36] |
| (48) | Dihydroapoatropine | Flowers [47] |
| (49) | Dihydroaposcopolamine | Flowers [47] |
| (50) | Hyoscyamine | Root cultivation [36], Corolla [53], Roots [35], and Flowers [47] |
| (51) | Litorine | Flowers, and Roots [35] |
| (52) | Meteloidine | Flowers [35] |
| (53) | Norhyoscyamine | Flowers [47] |
| (54) | Norscopolamine | Flowers [47] |
| (55) | Pseudotropine | Root cultivation [36], Flowers, Roots [35] |
| (56) | Scopolamine | Ripe flowers, and immature flowers and fruits [54], Corolla [53], Flowers [47], Roots [35], and Flowers nectar [37] |
| (57) | Scopine | Root cultivation [36], Flowers [47] |
| (58) | Scopoline | Root cultivation [36] |
| (59) | Tropine | Flowers, and Roots [35] |
| No. | Compound Name | Parts Used | No. | Compound Name | Parts Used |
|---|---|---|---|---|---|
| (60) | Hexanol | Flowers [22] | (85) | α-Tujene | Flowers [22] |
| (61) | (Z)-3-Hexen-1-ol | Flowers [22] | (86) | Sabinene | Flowers [22] |
| (62) | Citronellol | Flowers [22] | (87) | trans-Sabinene hydrate | Flowers [22] |
| (63) | Geraniol | Flowers [22] | (88) | 1,8-cineol | Flowers [22] |
| (64) | (trans, trans)-Farnesol | Flowers [22] | (89) | α-Pinene | Flowers [22] |
| (65) | Linalool | Flowers [21] | (90) | β-Pinene | Flowers [22] |
| (66) | (E)-Nerolidol | Flowers [21] | (91) | Theaspirane A | Flowers [21] |
| (67) | Hexanal | Flowers [22] | (92) | 2-Isobutyl-3-methoxypyrazine | Flowers [21] |
| (68) | Heptanal | Flowers [21] | (93) | Indole | Flowers [22] |
| (69) | Octanal | Flowers [21] | (94) | Benzyl alcohol | Flowers [22] |
| (70) | Nonanal | Flowers [22] | (95) | Phenethyl alcohol | Flowers [22] |
| (71) | Decanal | Flowers [22] | (96) | Benzaldehyde | Flowers [22] |
| (72) | Neral | Flowers [22] | (97) | Phenylacetaldehyde | Flowers [21] |
| (73) | Geranial | Flowers [22] | (98) | 4-Methoxy benzaldehyde | Flowers [22] |
| (74) | Citronellal | Flowers [22] | (99) | Methyl benzoate | Flowers [22] |
| (75) | Farnesal | Flowers [22] | (100) | Methyl salicylate | Flowers [22] |
| (76) | 6-Methyl hept-5-en-2-one | Flowers [22] | (101) | 3-phenyl lactic acid | Leaves [29] |
| (77) | cis-β-Ocimene | Flowers [22] | (102) | Benzyl benzoate | Flowers [22] |
| (78) | β-Myrcene | Flowers [22] | (103) | Benzyl salicylate | Flowers [22] |
| (79) | Allo-ocimene | Flowers [22] | (104) | 3-(3-indolyl) lactic acid | Leaves [29] |
| (80) | trans-β-Ocimene | Flowers [22] | (105) | Indole-3-lactic acid methyl ester | Leaves [29] |
| (81) | Terpinolene | Flowers [22] | (106) | Megastigmatrienone I | Flowers [21] |
| (82) | γ-Terpinene | Flowers [21] | (107) | Megastigmatrienone II | Flowers [21] |
| (83) | Terpinen-4-ol | Flowers [22] | (108) | Megastigmatrienone III | Flowers [21] |
| (84) | α-Terpineol | Flowers [22] | (109) | Megastigmatrienone IV | Flowers [21] |
| No. | Compound Name | Parts Used |
|---|---|---|
| (110) | Acanthoside B | Flowers [55] |
| (111) | Scopoletin 7-O-β-d-galactopyranoside | Flowers [55] |
| (112) | Kaempferol | Flowers [55] |
| (113) | Kaempferol 3-O-α-l-arabinopyranoside | Leaves [29] |
| (114) | kaempferol 3-O-α-l-arabinopyranosyl-7-O-β-d-glucopyranoside | Leaves [29] |
| (115) | Kaempferol 3-O-β-d-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside | Leaves [56] |
| (116) | Kaempferol 3-O-β-d-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside-7-O-β-d-glucopyranoside | Leaves [56] |
| (117) | Kaempferol 3-O-β-d-[6′′′-O-(E-caffeoyl)]-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside-7-O-β-d-glucopyranoside | Leaves [56] |
| (118) | Kaempferol 3-O-β-d-[2′′′-O-(E-caffeoyl)]-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside-7-O-β-d-glucopyranoside | Leaves [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petricevich, V.L.; Salinas-Sánchez, D.O.; Avilés-Montes, D.; Sotelo-Leyva, C.; Abarca-Vargas, R. Chemical Compounds, Pharmacological and Toxicological Activity of Brugmansia suaveolens: A Review. Plants 2020, 9, 1161. https://doi.org/10.3390/plants9091161
Petricevich VL, Salinas-Sánchez DO, Avilés-Montes D, Sotelo-Leyva C, Abarca-Vargas R. Chemical Compounds, Pharmacological and Toxicological Activity of Brugmansia suaveolens: A Review. Plants. 2020; 9(9):1161. https://doi.org/10.3390/plants9091161
Chicago/Turabian StylePetricevich, Vera L., David Osvaldo Salinas-Sánchez, Dante Avilés-Montes, Cesar Sotelo-Leyva, and Rodolfo Abarca-Vargas. 2020. "Chemical Compounds, Pharmacological and Toxicological Activity of Brugmansia suaveolens: A Review" Plants 9, no. 9: 1161. https://doi.org/10.3390/plants9091161
APA StylePetricevich, V. L., Salinas-Sánchez, D. O., Avilés-Montes, D., Sotelo-Leyva, C., & Abarca-Vargas, R. (2020). Chemical Compounds, Pharmacological and Toxicological Activity of Brugmansia suaveolens: A Review. Plants, 9(9), 1161. https://doi.org/10.3390/plants9091161










