Determination of the Ecotoxicity of Herbicides Roundup® Classic Pro and Garlon New in Aquatic and Terrestrial Environments
Abstract
1. Introduction
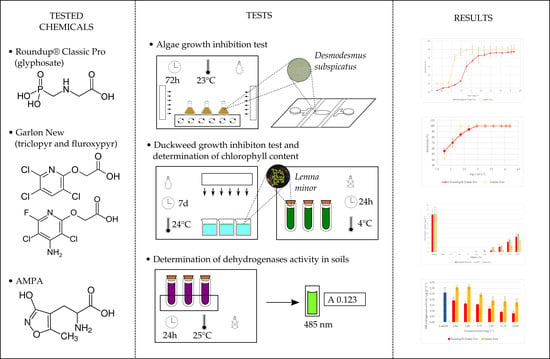
2. Materials and Methods
2.1. Chemicals
2.2. Organisms
2.3. Experimental Design
2.3.1. Algal Growth Inhibition Test
2.3.2. Duckweed Growth Inhibition Test
2.3.3. The Determination of Dehydrogenase Activity in Soil
2.4. Statistical Analysis
3. Results and Discussion
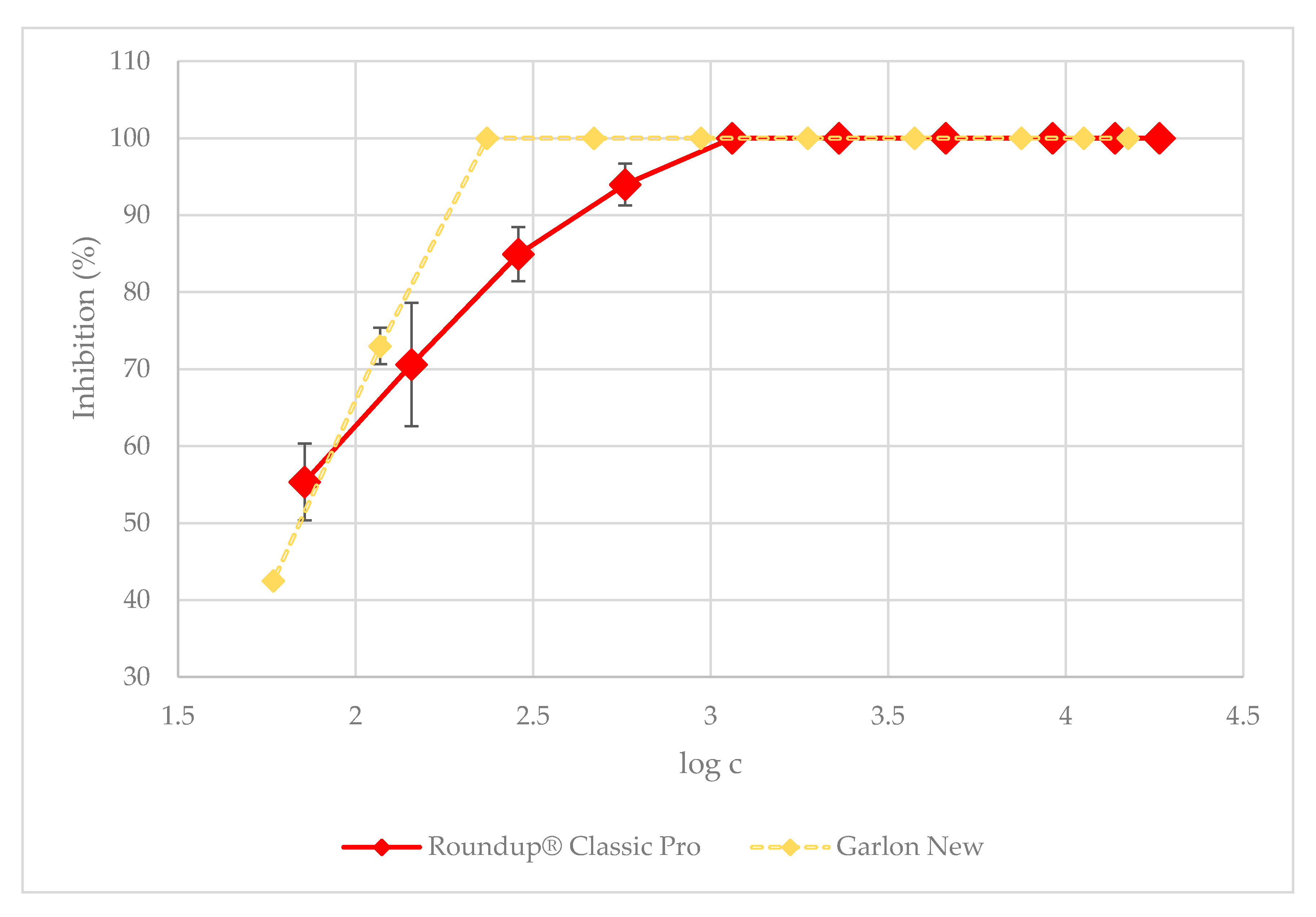
3.1. Green Algae Acute Toxicity Test
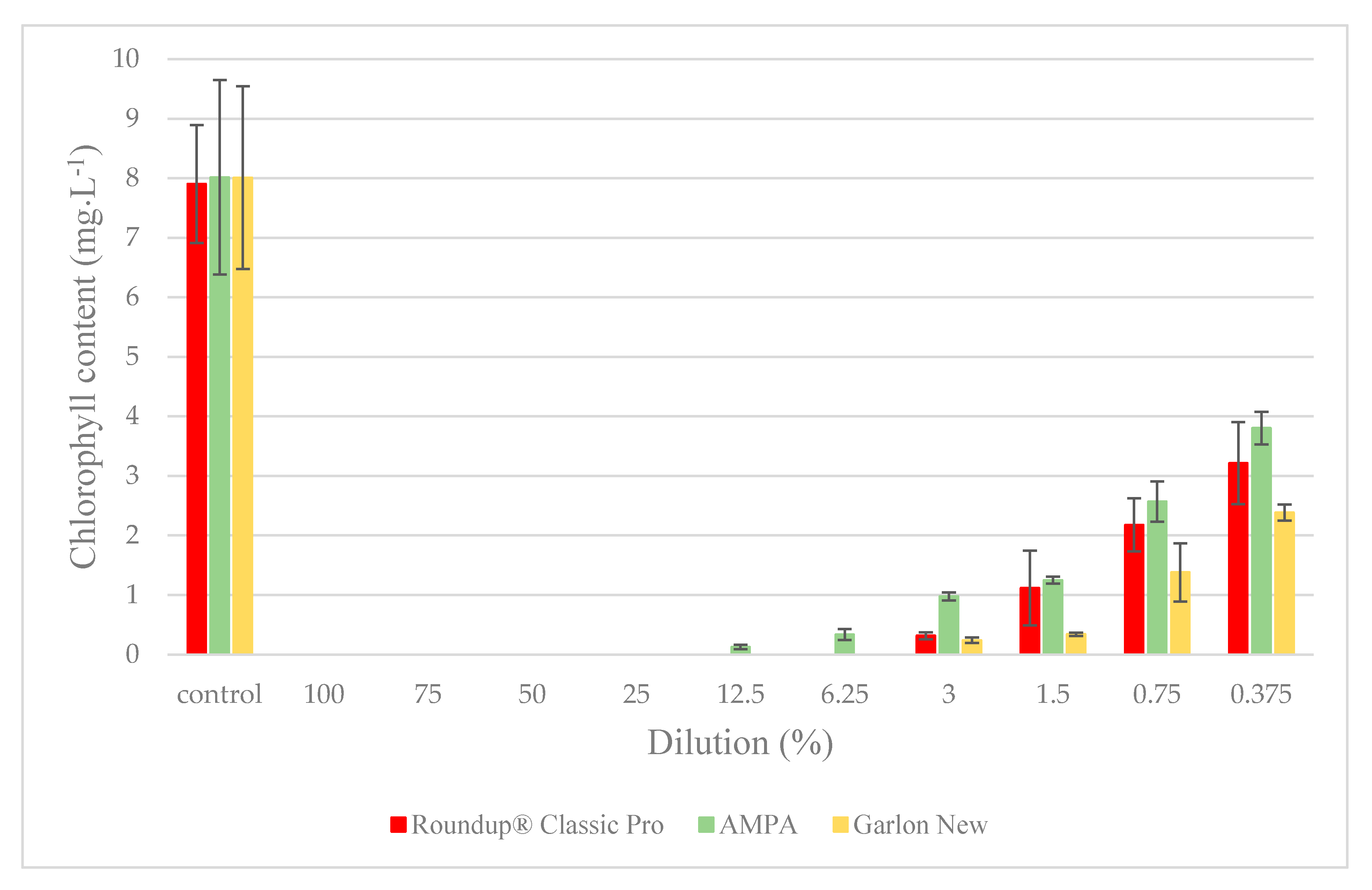
3.2. Duckweed Acute Toxicity Test
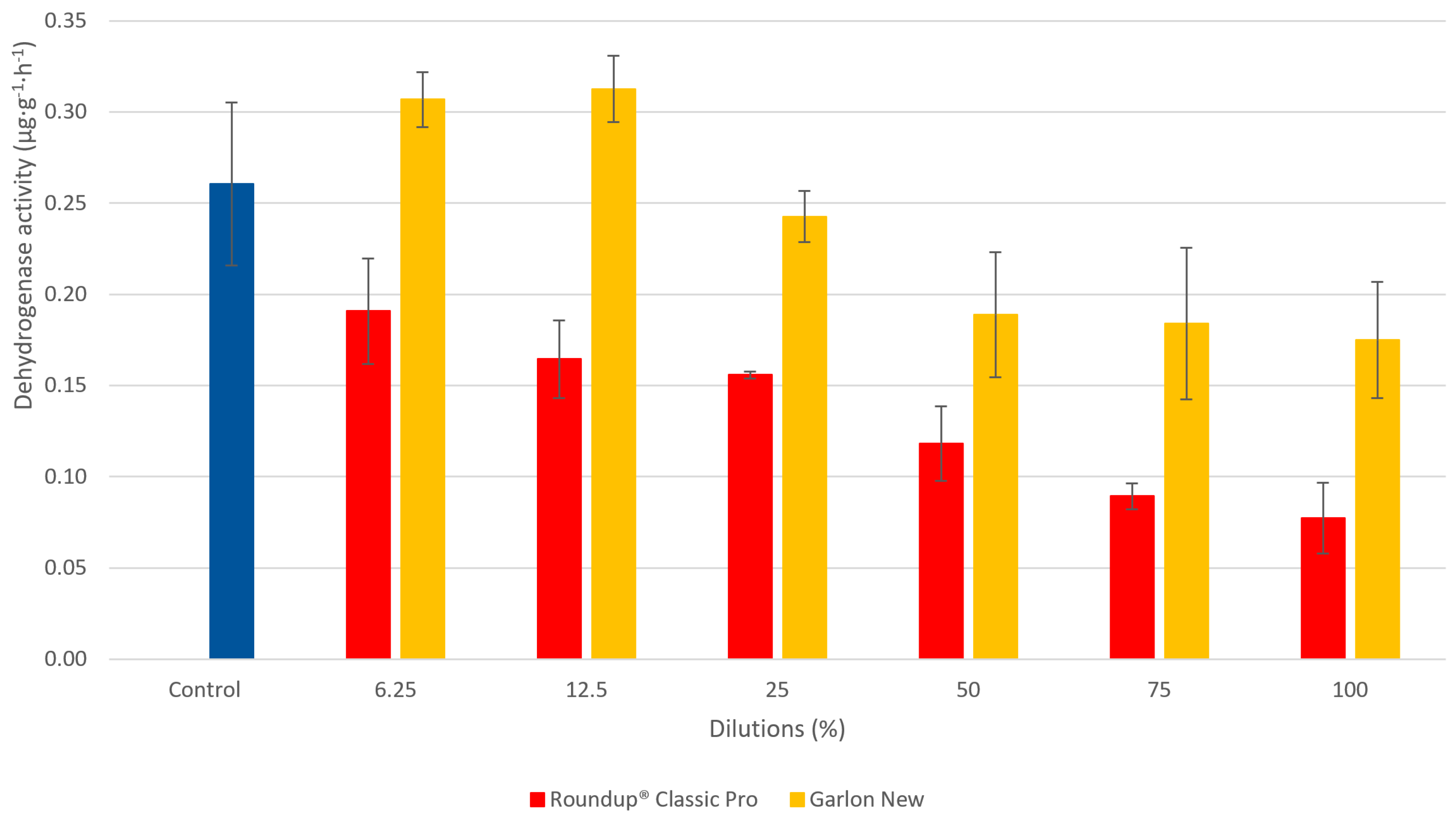
3.3. The Determination of Dehydrogenase Activity in Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, S.; Georgieva, E.; Velcheva, I.; Iliev, I.; Vasileva, T.; Bivolarski, V.; Tomov, S.; Nyeste, K.; Antal, L.; Yancheva, V. Multi-biomarker assessment in common carp (Cyprinus carpio, Linnaeus 1758) liver after acute chlorpyrifos exposure. Water 2020, 12, 1837. [Google Scholar] [CrossRef]
- Cavalcante, D.; Martinez, C.B.R.; Sofia, S.H. Genotoxic effects of Roundup (R) on the fish Prochilodus lineatus. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 655, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Cavas, T.; Konen, S. Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 2007, 22, 263–268. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Thompson, D.G.; Capell, S.S.; Thomas, D.R.; Staznik, B. Field-evaluation of triclopyr ester toxicity to fish. Arch. Environ. Contam. Toxicol. 1995, 28, 18–26. [Google Scholar] [CrossRef]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef]
- Woodburn, A.T. Glyphosate: Production, pricing and use worldwide. Pest Manag. Sci. 2000, 56, 309–312. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Ware, G.W. The Pesticide Book; Meister Media Worldwide: Willoughby, OH, USA, 2004; Volume 6. [Google Scholar]
- Schonbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyvuvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376–1380. [Google Scholar] [CrossRef]
- Mottier, A.; Kientz-Bouchart, V.; Serpentini, A.; Lebel, J.M.; Jha, A.N.; Costil, K. Effects of glyphosate-based herbicides on embryo-larval development and metamorphosis in the Pacific oyster, Crassostrea gigas. Aquat. Toxicol. 2013, 128, 67–78. [Google Scholar] [CrossRef]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Seralini, G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Freitas-Silva, L.D.; Araujo, T.O.D.; Nunes-Nesi, A.; Ribeiro, C.; Costa, A.C.; da Silva, L.C. Evaluation of morphological and metabolic responses to glyphosate exposure in two neotropical plant species. Ecol. Indic. 2020, 113, 11. [Google Scholar] [CrossRef]
- Tahir, H.M.; Basheer, T.; Ali, S.; Yaqoob, R.; Naseem, S.; Khan, S.Y. Effect of pesticides on biological control potential of neoscona theisi (Araneae: Araneidae). J. Insect Sci. 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Korenko, S.; Niedobova, J.; Kolarova, M.; Hamouzova, K.; Kysilkova, K.; Michalko, R. The effect of eight common herbicides on the predatory activity of the agrobiont spider Pardosa agrestis. Biocontrol 2016, 61, 507–517. [Google Scholar] [CrossRef]
- Verderame, M.; Scudiero, R. How glyphosate impairs liver condition in the field lizard podarcis siculus (Rafinesque-Schmaltz, 1810): Histological and molecular evidence. BioMed Res. Int. 2019, 2019, 4746283. [Google Scholar] [CrossRef] [PubMed]
- Santadino, M.; Coviella, C.; Momo, F. Glyphosate sublethal effects on the population dynamics of the earthworm eisenia fetida (Savigny, 1826). Water Air Soil Pollut. 2014, 225, 8. [Google Scholar] [CrossRef]
- Samal, S.; Mishra, C.S.K.; Sahoo, S. Dermal, histological anomalies with variations in enzyme activities of the earthworms Lampito mauritii and Drawida willsi after short term exposure to organophosphate pesticides. ISJ Invertebr. Surviv. J. 2020, 17, 117–128. [Google Scholar]
- Antoniolli, Z.I.; Redin, M.; Souza, E.L.D.; Pocojeski, E. Heavy metal, pesticides and fuels: Effect in the population of collembola in the soil. Cienc. Rural 2013, 43, 992–998. [Google Scholar] [CrossRef]
- Grunewald, K.; Schmidt, W.; Unger, C.; Hanschmann, G. Behavior of glyphosate and aminomethylphosphonic acid (AMPA) in soils and water of reservoir Radeburg II catchment (Saxony/Germany). J. Plant Nutr. Soil Sci. 2001, 164, 65–70. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate degradation in glyphosate-resistant and -susceptible crops and weeds. J. Agric. Food Chem. 2011, 59, 5835–5841. [Google Scholar] [CrossRef]
- Barrett, K.A.; McBride, M.B. Oxidative degradation of glyphosate and aminomethylphosphonate by manganese oxide. Environ. Sci. Technol. 2005, 39, 9223–9228. [Google Scholar] [CrossRef] [PubMed]
- Pizzul, L.; Castillo, M.D.; Stenstrom, J. Degradation of glyphosate and other pesticides by ligninolytic enzymes. Biodegradation 2009, 20, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Vreeken, R.J.; Speksnijder, P.; Bobeldijk-Pastorova, I.; Noij, T.H.M. Selective analysis of the herbicides glyphosate and aminomethylphosphonic acid in water by on-line solid-phase extraction high-performance liquid chromatography electrospray ionization mass spectrometry. J. Chromatogr. A 1998, 794, 187–199. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation(1). J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Grandcoin, A.; Piel, S.; Baures, E. AminoMethylPhosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef]
- Rodrigues, L.D.; Costa, G.G.; Tha, E.L.; Silva, L.R.d.; Oliveira, R.d.; Leme, D.M.; Cestari, M.M.; Grisolia, C.K.; Valadares, M.C.; Oliveira, G.A.R.d. Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 94–101. [Google Scholar] [CrossRef]
- Merey, G.V.; Manson, P.S.; Mehrsheikh, A.; Sutton, P.; Levine, S.L. Glyphosate and aminomethylphosphonic acid chronic risk assessment for soil biota. Environ. Toxicol. Chem. 2016, 35, 2742–2752. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Howe, C.M.; Berrill, M.; Pauli, B.D.; Helbing, C.C.; Werry, K.; Veldhoen, N. Toxicity of glyphosate-based pesticides to four North American frog species. Environ. Toxicol. Chem. 2004, 23, 1928–1938. [Google Scholar] [CrossRef]
- Bonansea, R.I.; Filippi, I.; Wunderlin, D.A.; Marino, D.J.G.; Ame, M.V. The fate of glyphosate and AMPA in a freshwater endorheic basin: An ecotoxicological risk assessment. Toxics 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Getsinger, K.D.; Petty, D.G.; Madsen, J.D.; Skogerboe, J.G.; Houtman, B.A.; Haller, W.T.; Fox, A.M. Aquatic dissipation of the herbicide triclopyr in Lake Minnetonka, Minnesota. Pest Manag. Sci. 2000, 56, 388–400. [Google Scholar] [CrossRef]
- Petty, D.G.; Getsinger, K.D.; Woodburn, K.B. A review of the aquatic environmental fate of triclopyr and its major metabolites. J. Aquat. Plant Manag. 2003, 41, 69–75. [Google Scholar]
- Isbister, K.M.; Lamb, E.G.; Stewart, K.J. Herbicide toxicity testing with non-target boreal plants: The sensitivity of achillea millefolium L. and chamerion angustifolium L. to triclopyr and imazapyr. Environ. Manag. 2017, 60, 136–156. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Holmes, S.B.; Behmer, D.J. Effects of the Herbicides hexazinone and triclopyr ester on aquatic insects. Ecotox. Environ. Safe 1992, 23, 364–374. [Google Scholar] [CrossRef]
- Guilherme, S.; Santos, M.A.; Gaivao, I.; Pacheco, M. Genotoxicity evaluation of the herbicide Garlon((R)) and its active ingredient (triclopyr) in fish (Anguilla anguilla L.) using the comet assay. Environ. Toxicol. 2015, 30, 1073–1081. [Google Scholar] [CrossRef]
- Yahnke, A.E.; Grue, C.E.; Hayes, M.P.; Pearman-Gillman, S. Effects of the herbicide triclopyr on metamorphic northern red-legged frogs. Environ. Toxicol. Chem. 2017, 36, 2316–2326. [Google Scholar] [CrossRef]
- Curtis, A.N.; Bidart, M.G. Effects of chemical management for invasive plants on the performance of Lithobates pipiens tadpoles. Environ. Toxicol. Chem. 2017, 36, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Berrill, M.; Bertram, S.; McGillivray, L.; Kolohon, M.; Pauli, B. Effects of low concentrations of forest-use pesticides on frog embryous and tadpoles. Environ. Toxicol. Chem. 1994, 13, 657–664. [Google Scholar] [CrossRef]
- Perez-Lucas, G.; Vela, N.; Abellan, M.; Fenoll, J.; Navarro, S. Use of index-based screening models to evaluate the leaching of triclopyr and fluroxypyr through a loam soil amended with vermicompost. Bull. Environ. Contam. Toxicol. 2020, 104, 497–502. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lucas, G.; Aliste, M.; Vela, N.; Garrido, I.; Fenoll, J.; Navarro, S. Decline of fluroxypyr and triclopyr residues from pure, drinking and leaching water by photo-assisted peroxonation. Process Saf. Environ. Protect. 2020, 137, 358–365. [Google Scholar] [CrossRef]
- Morais, E.C.; Lattuada, R.M.; Correa, G.G.; Brambilla, R.; Santos, J.H.Z.D. Evaluating the effect of pharmaceuticals encapsulated in silica by the sol-gel method on algal growth inhibition. J. Sol-Gel Sci. Technol. 2020, 94, 628–636. [Google Scholar] [CrossRef]
- Pokora, W.; Bascik-Remisiewicz, A.; Tukaj, S.; Kalinowska, R.; Pawlik-Skowronska, B.; Dziadziuszko, M.; Tukaj, Z. Adaptation strategies of two closely related Desmodesmus armatus (green alga) strains contained different amounts of cadmium: A study with light-induced synchronized cultures of algae. J. Plant Physiol. 2014, 171, 69–77. [Google Scholar] [CrossRef]
- Guidony, N.S.; Lopes, F.M.; Guimaraes, P.S.; Escarrone, A.L.V.; Souza, M.M. Can short-term exposure to copper and atrazine be cytotoxic to microalgae? Environ. Sci. Pollut. Res. 2020, 27, 27961–27970. [Google Scholar] [CrossRef]
- Boutin, C.; Freemark, K.E.; Keddy, C.J. Overview and rationale for developing regulatory guidelines for nontarget plant-testing with chemical pesticides. Environ. Toxicol. Chem. 1995, 14, 1465–1475. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, Z.; Feng, G.; Zhao, Y. Analysis on enrichment of aquatic plants response to different heavy metal ions in polluted water taking duckweed as an example. Appl. Ecol. Environ. Res. 2019, 17, 3469–3482. [Google Scholar] [CrossRef]
- Shirinpur-Valadi, A.; Hatamzadeh, A.; Sedaghathoor, S. Study of the accumulation of contaminants by Cyperus alternifolius, Lemna minor, Eichhornia crassipes, and Canna x generalis in some contaminated aquatic environments. Environ. Sci. Pollut. Res. 2019, 26, 21340–21350. [Google Scholar] [CrossRef]
- Kostopoulou, S.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 12. [Google Scholar] [CrossRef]
- Ubuza, L.J.A.; Padero, P.C.S.; Nacalaban, C.M.N.; Tolentino, J.T.; Alcoran, D.C.; Tolentino, J.C.; Ido, A.L.; Mabayo, V.I.F.; Arazo, R.O. Assessment of the potential of duckweed (Lemna minor L.) in treating lead-contaminated water through phytoremediation in stationary and recirculated set-ups. Environ. Eng. Res. 2020, 25, 977–982. [Google Scholar] [CrossRef]
- Chen, D.Q.; Zhang, H.; Wang, Q.L.; Shao, M.; Li, X.Y.; Chen, D.M.; Zeng, R.S.; Song, Y.Y. Intraspecific variations in cadmium tolerance and phytoaccumulation in giant duckweed (Spirodela polyrhiza). J. Hazard. Mater. 2020, 395, 10. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, E.; Sharma, P. Chromium and cadmium removal from wastewater using duckweed-Lemna gibba L. and ultrastructural deformation due to metal toxicity. Int. J. Phytoremediat. 2019, 21, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Osama, R.; Awad, H.M.; Ibrahim, M.G.; Tawfik, A. Mechanistic and economic assessment of polyester wastewater treatment via baffled duckweed pond. J. Water Process. Eng. 2020, 35, 10. [Google Scholar] [CrossRef]
- Pertile, M.; Antunes, J.E.L.; Araujo, F.F.; Mendes, L.W.; Van den Brink, P.J.; Araujo, A.S.F. Responses of soil microbial biomass and enzyme activity to herbicides imazethapyr and flumioxazin. Sci. Rep. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Bennicelli, R.P.; Szafranek-Nakonieczna, A.; Wolinska, A.; Stepniewska, Z.; Bogudzinska, M. Influence of pesticide (glyphosate) on dehydrogenase activity, pH, Eh and gases production in soil (laboratory conditions). Int. Agrophys. 2009, 23, 117–122. [Google Scholar]
- Sebiomo, A.; Ogundero, V.W.; Bankole, S.A. Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr. J. Biotechnol. 2011, 10, 770–778. [Google Scholar]
- Bold, H.C.; Bischoff, H.W. Phycological Studies IV. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas Publication: Austin, TX, USA, 1963; p. 95, No. 6318. [Google Scholar]
- ISO. Water Quality-Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae (ISO 8692:2012); Office for Standards, Metrology and Testing: Prague, Czechia, 2012; p. 24. [Google Scholar]
- ISO. Water Quality-Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna minor)-Duckweed Growth Inhibition Test (ISO 20079:2005); Czech Standards Institute: Prague, Czechia, 2007; p. 28. [Google Scholar]
- Technical Committee ISO/TC 190, S.q., Subcommittee SC4. Soil Quality-Determination of Dehydrogenases Activity in Solis-Part 1: Method Using Triphenyltetrazolium Chloride (TTC) (ISO 23753-1:2019); European Comittee for Standardization: Brussels, Belgium, 2019; p. 9. [Google Scholar]
- Wellburn, A.R. The spectral determination of Chlorophyll-a and Chlorophyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- ISO. Soil QUALITY-SAMPLING-Part 6: Guidance on the Collection, Handling and Storage of Soil under Aerobic Conditions for the Assessment of Microbiological Processes, Biomass and Diversity in the Laboratory (ISO 10381-6:2009); Office for Standards, Metrology and Testing: Prague, Czechia, 2011; p. 12. [Google Scholar]
- ISO. Soil Quality-Determination of pH (ISO 10390:2005); Office for Standards, Metrology and Testing: Prague, Czechia, 2011; p. 12. [Google Scholar]
- ISO. Soil Quality-Determination of Dry Matter and Water Content on a Mass Basis-Gravimetric Method (ISO 11465:1993); Czech Standards Institute: Prague, Czechia, 1998; p. 7. [Google Scholar]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 107. [Google Scholar] [CrossRef]
- Nagai, T. Sensitivity differences among seven algal species to 12 herbicides with various modes of action. J. Pestic. Sci. 2019, 44, 225–232. [Google Scholar] [CrossRef]
- Saenz, M.E.; Marzio, W.D.D. Ecotoxicity of herbicide Glyphosate to four chlorophyceaen freshwater algae. Limnetica 2009, 28, 149–158. [Google Scholar]
- Ma, J. Differential sensitivity to 30 herbicides among populations of two green algae Scenedesmus obliquus and Chlorella pyrenoidosa. Bull. Environ. Contam. Toxicol. 2002, 68, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, E.; Ferraz, D.; Sabater, C.; Carrasco, J.M. Effect of glyphosate on growth of four freshwater species of phytoplankton: A microplate bioassay. Bull. Environ. Contam. Toxicol. 2009, 82, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Daouk, S.; Copin, P.J.; Rossi, L.; Chevre, N.; Pfeifer, H.R. Dynamics and environmental risk assessment of the herbicide glyphosate and its metabolite AMPA in a small vineyard river of the Lake Geneva catchment. Environ. Toxicol. Chem. 2013, 32, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Peterson, H.G.; Boutin, C.; Martin, P.A.; Freemark, K.E.; Ruecker, N.J.; Moody, M.J. Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquat. Toxicol. 1994, 28, 275–292. [Google Scholar] [CrossRef]
- Zhang, S.A.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef]
- Sikorski, L.; Baciak, M.; Bes, A.; Adomas, B. The effects of glyphosate-based herbicide formulations on Lemna minor, a non-target species. Aquat. Toxicol. 2019, 209, 70–80. [Google Scholar] [CrossRef]
- Kielak, E.; Sempruch, C.; Mioduszewska, H.; Klocek, J.; Leszczynski, B. Phytotoxicity of roundup ultra 360 SL in aquatic ecosystems: Biochemical evaluation with duckweed (Lemna minor L.) as a model plant. Pest. Biochem. Physiol. 2011, 99, 237–243. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: Is the mitochondrial electron transport chain a target of this herbicide? Environ. Pollut. 2016, 218, 402–409. [Google Scholar] [CrossRef]
- Zhou, J.A.; Wu, Z.H.; Yu, D.; Yang, L. Toxicity of the herbicide flurochloridone to the aquatic plants Ceratophyllum demersum and Lemna minor. Environ. Sci. Pollut. Res. 2020, 27, 3923–3932. [Google Scholar] [CrossRef]
- Sobrero, M.C.; Rimoldi, F.; Ronco, A.E. Effects of the glyphosate active ingredient and a formulation on Lemna gibba L. at different exposure levels and assessment end-points. Bull. Environ. Contam. Toxicol. 2007, 79, 537–543. [Google Scholar] [CrossRef]
- Cowgill, U.M.; Milazzo, D.P.; Landenberger, B.D. A Comparison of the effect of triclopyr triethylamine salt on 2 species of duckweed (Lemna) examined for a 7-Day and 14-Day test period. Water Res. 1989, 23, 617–623. [Google Scholar] [CrossRef]
- Authority, E.F.S. Conclusion on the peer review of the pesticide risk assessment of the active substance fluroxypyr (evaluated variant fluroxypyr-meptyl). EFSA J. 2011, 9, 91. [Google Scholar] [CrossRef]
- Campos, J.A.; Peco, J.D.; Garcia-Noguero, E. Antigerminative comparison between naturally occurring naphthoquinones and commercial pesticides. Soil dehydrogenase activity used as bioindicator to test soil toxicity. Sci. Total Environ. 2019, 694, 7. [Google Scholar] [CrossRef]
- Zabaloy, M.C.; Garland, J.L.; Gomez, M.A. An integrated approach, to evaluate the impacts of the herbicides glyphosate, 2,4-D and metsulfuron-methyl on soil microbial communities in the Pampas region, Argentina. Appl. Soil Ecol. 2008, 40, 1–12. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Borggaard, O.K.; Bang, M. Influence of soil composition on adsorption of glyphosate and phosphate by contrasting Danish surface soils. Eur. J. Soil Sci. 2004, 55, 183–191. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Hanson, K.G.; Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’Donovan, J.T.; Johnson, E.N.; Gan, Y.; Irvine, R.B.; Monreal, M.A. Soil microbial biomass, functional diversity and enzyme activity in glyphosate-resistant wheat-canola rotations under low-disturbance direct seeding and conventional tillage. Soil Biol. Biochem. 2007, 39, 1418–1427. [Google Scholar] [CrossRef]
- Zofia, W.A.S. Dehydrogenase activity in the soil environment. Dehydrogenases 2012. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Monteiro, R.T.R.; Abarkeli, R.B. Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 2003, 52, 799–804. [Google Scholar] [CrossRef]
- Garcia-Perez, J.A.; Alarcon-Gutierrez, E.; Diaz-Fleischer, F. Interactive effect of glyphosate-based herbicides and organic soil layer thickness on growth and reproduction of the tropical earthworm Pontoscolex corethrurus (Muller, 1857). Appl. Soil Ecol. 2020, 155, 10. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajnaiová, L.; Vurm, R.; Kholomyeva, M.; Kobera, M.; Kočí, V. Determination of the Ecotoxicity of Herbicides Roundup® Classic Pro and Garlon New in Aquatic and Terrestrial Environments. Plants 2020, 9, 1203. https://doi.org/10.3390/plants9091203
Tajnaiová L, Vurm R, Kholomyeva M, Kobera M, Kočí V. Determination of the Ecotoxicity of Herbicides Roundup® Classic Pro and Garlon New in Aquatic and Terrestrial Environments. Plants. 2020; 9(9):1203. https://doi.org/10.3390/plants9091203
Chicago/Turabian StyleTajnaiová, Lucia, Radek Vurm, Marina Kholomyeva, Miroslav Kobera, and Vladimír Kočí. 2020. "Determination of the Ecotoxicity of Herbicides Roundup® Classic Pro and Garlon New in Aquatic and Terrestrial Environments" Plants 9, no. 9: 1203. https://doi.org/10.3390/plants9091203
APA StyleTajnaiová, L., Vurm, R., Kholomyeva, M., Kobera, M., & Kočí, V. (2020). Determination of the Ecotoxicity of Herbicides Roundup® Classic Pro and Garlon New in Aquatic and Terrestrial Environments. Plants, 9(9), 1203. https://doi.org/10.3390/plants9091203