Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.)
Abstract
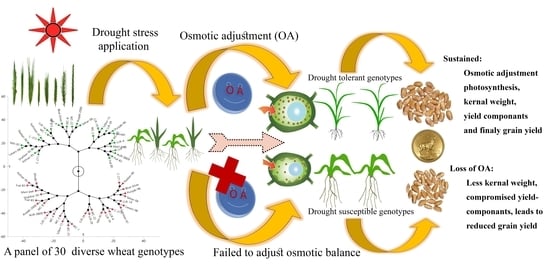
:1. Introduction
2. Results
2.1. Analysis of Variance and Mean Performance
2.2. Principal Component and Biplot Analyses
2.3. Association among OA, DSITKW, Yield and Yield Components
2.4. Variability and Diversity Estimation Analysis.
3. Discussion
3.1. Variability and Effects of Drought Stress on Wheat Genotypes
3.2. Association of Yield with Osmotic Adjustment and Other Yield Components
4. Material and Methods
4.1. Plant Material and Experimental Conditions
4.2. Evaluation and Data Recording
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braun, H.-J.; Atlin, G.; Payne, T. Multi-Location Testing as a Tool to Identify Plant Response to Global Climate Change; Blackwell Publishing: London, UK, 2010. [Google Scholar]
- Pradhan, G.P.; Prasad, P.V.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. High Temperature Tolerance in Aegilops Species and Its Potential Transfer to Wheat; FAO: Rome, Italy, 2012. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Kausar, M.; Shah, N.; Ghafoor, A.; Du, X.; Shah, M.K.N.; Ghafoor, A.; et al. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2020, 9, 105–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmar, S.; Saeed, S.; Khan, M.H.U.; Ullah Khan, S.; Mora-Poblete, F.; Kamran, M.; Faheem, A.; Maqsood, A.; Rauf, M.; Saleem, S.; et al. A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement. Int. J. Mol. Sci. 2020, 21, 5665. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.; Gaulin, E.; Havaux, M.; Acevedo, E.; Monneveux, P. Drought and Heat Responses in the Wild Wheat Relative Aegilops geniculata Roth: Potential Interest for Wheat Improvement. Aegilops Geniculata 2001, 41, 1515–1522. [Google Scholar] [CrossRef]
- Din, A.; Ahmad, M.; Watto, F.M.; Ahmed, S.; Ali, I.; Shah, M.K.N. Drought tolerance screening in thirty common wheat (Triticum aestivum L.) genotypes. Sarhad J. Agric. 2020, 36, 168–177. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [Green Version]
- Ayalew, H.; Ma, X.; Yan, G. Screening Wheat (Triticum spp ) Genotypes for Root Length under Contrasting Water Regimes: Potential Sources of Variability for Drought Resistance Breeding. Agron. Crop Sci. 2015, 201, 189–194. [Google Scholar] [CrossRef]
- Cossani, C.M.; Reynolds, M.P. Physiological traits for improving heat tolerance in wheat. Plant Physiol. 2012, 160, 1710–1718. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.; Copaja, S.V.; Bravo, H.R.; Argandoña, V.H. Relationship between grain yield, osmotic adjustment and benzoxazinone content in Triticum aestivum L. cultivars. Z. fur Naturforsch. Sect. C J. Biosci. 2006, 61, 704–708. [Google Scholar] [CrossRef]
- Bum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of Mannitol-Accumulating Transgenic Wheat to Water Stress and Salinity. Plant Physiol. 2003, 131, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nio, S.A.; Mantilen Ludong, D.P.; Wade, L.J. Comparison of leaf osmotic adjustment expression in wheat (Triticum aestivum L.) under water deficit between the whole plant and tissue levels. Agric. Nat. Resour. 2018, 52, 33–38. [Google Scholar] [CrossRef]
- Naeem, M.K.; Ahmad, M.; Shah, M.K.N.; Kamran, M.; Iqbal, M.S. Character association and path analysis of osmotic adjustment, growth and physiological traits in wheat. J. Anim. Plant Sci. 2016, 26, 680–685. [Google Scholar]
- Morgan, J.M. Increases in grain yield of wheat by breeding for an osmoregulation gene: Relationship to water supply and evaporative demand. Aust. J. Agric. Res. 2000, 51, 971–978. [Google Scholar] [CrossRef]
- Ali, M.; Jensen, C.; Mogensen, V.O.; Andersen, M.; Henson, I.E. Root signalling and osmotic adjustment during soil drying sustains grain yield of field grown wheat. Field Crop. Res. 1999, 62, 35–52. [Google Scholar] [CrossRef]
- Blum, A.; Zhang, J.; Nguyen, H.T. Consistent differences among wheat cultivars in osmotic adjustment and their relationship to plant production. Field Crops Res. 1999, 64, 287–291. [Google Scholar] [CrossRef]
- Teulat, B.; Rekika, D.; Nachit, M.; Monneveux, P. Comparative Osmotic Adjustments in Barley and Tetraploid Wheats; Lavoisier Publishing: Paris, France, 2006; Volume 116. [Google Scholar]
- Blum, A.; Pnuel, Y. Physiological attributes associated with drought resistance of wheat cultivars in a Mediterranean environment. Aust. J. Agric. Res. 1990, 41, 799–810. [Google Scholar] [CrossRef]
- Morgan, J.; Condon, A. Water Use, Grain Yield, and Osmoregulation in Wheat. Aust. J. Plant Physiol. 1986, 13, 523–532. [Google Scholar] [CrossRef]
- Johnson, C.R.T.; Nguyen, H.I.; Croy, L. Osmotic Adjustment and Solute Accumulation in Two Wheat Genotypes Differing in Drought Resistance1; RC Johnson: WSU Pullman, WA, USA, 1984; Volume 24. [Google Scholar]
- Fischer, R.A.; Wood, J.T. Drought Resistance in Spring Wheat Cultivars. III. Yield Associations with Morpho-Physiological Traits; Wiley Interscience: New York, NY, USA, 1979; Volume 30. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.B.; Gurumurthi, K.K.; Panigrahi, A.; Shaw, B. Salinity Induced Changes in Proline Betaine Contents and Synthesis in Two Aquatic Macrophytes Differing in Salt Tolerance. Biol. Plant. 2007, 51, 110–115. [Google Scholar] [CrossRef]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; Hassan, W.; Ahmad, Z.; Qi, C.; Lu, Y.; et al. Response and Tolerance Mechanism of Cotton Gossypium hirsutum L. to Elevated Temperature Stress: A Review. Front. Plant Sci. 2016, 7, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farouk, S. Osmotic adjustment in wheat flag leaf in relation to flag leaf area and grain yield per plant Osmotic adjustment in wheat flag leaf in relation to flag leaf area and grain yield per plant. J. Stress Physiol. Biochem. 2011, 7, 117–138. [Google Scholar]
- Bandehagh, A.; Toorchi, M.; Mohammadi, S.; Chaparzadeh, N.; Salekdeh, G.H.; Kazemnia, H. Growth and Osmotic Adjustment of Canola Genotypes in Response to Salinity; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; Volume 6. [Google Scholar]
- Nguyen, H.; Ranganathan, C.; Blum, A. Breeding for Drought Resistance in Rice: Physiology and Molecular Genetics Considerations. Crop Sci. 1997, 37, 1426–1434. [Google Scholar] [CrossRef]
- Babu, R.C.; Zhang, J.; Blum, A.; Ho, T.H.D.; Wu, R.; Nguyen, H.T. HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Plant Sci. 2004, 166, 855–862. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Prasanna, B.M. REVIEW & INTERPRETATION Analysis of Genetic Diversity in Crop Plants. Salient Stat. Tools 1990, 43, 1235–1248. [Google Scholar]
- Wang, Q.; Zhang, T.; Cui, J.; Wang, X.; Zhou, H. Path and Ridge Regression Analysis of Seed Yield and Seed Yield Components of Russian Wildrye (Psathyrostachys juncea Nevski) under Field Conditions. PLoS ONE 2011, 6, 18245. [Google Scholar] [CrossRef]
- Hoerl, E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I—Selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Javed, I.; Awan, S.I.; Ahmad, H.M.; Rao, A. Assesment of Genetic Diversity in Wheat Synthetic Double Haploids for Yield and Drought Related Traits Through Factor and Cluster Analyses. Plant Gene Trait 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Aziz, A.; Mahmood, T.; Mahmood, Z.; Shazadi, K.; Mujeeb-Kazi, A.; Rasheed, A. Genotypic variation and genotype × environment interaction for yield-related traits in synthetic hexaploid wheats under a range of optimal and heat-stressed environments. Crop Sci. 2018, 58, 295–303. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhang, Q.; Sun, P.; Song, C. Impact of Droughts on Winter Wheat Yield in Different Growth Stages during 2001–2016 in Eastern China. Int. J. Disaster Risk Sci. 2018, 9, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Serraj, R.; Sinclair, T.R. Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araus, J.L.; Serret, M.D.; Edmeades, G.O. Phenotyping maize for adaptation to drought. Front. Physiol. 2012, 3, 305. [Google Scholar] [CrossRef] [Green Version]
- Mariano Cossani, C.; Reynolds, M. Heat Stress Adaptation in Elite Lines Derived from Synthetic Hexaploid Wheat. Crop Sci. 2015, 55, 2719–2735. [Google Scholar] [CrossRef]
- Hays, D.; Do Hwa, J.; Mason, R.; Morgan, G.A.; Finlayson, S. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci. 2007, 172, 1113–1123. [Google Scholar] [CrossRef]
- Leport, L.; Turner, N.C.; French, R.J.; Barr, M.D.; Duda, R.; Davies, S.L.; Tennant, D.; Siddique, K.H.M. Physiological responses of chickpea genotypes to terminal drought in a Mediterranean-type environment. Eur. J. Agron. 1999, 11, 279–291. [Google Scholar] [CrossRef]
- Khlebova, L.P.; Bychkova, O.V.; Titova, A.M.; Rozova, M.A.; Ziborov, A.I. Osmotic adjustment in spring durum wheat pollen grains under induced drought stress. Ukr. J. Ecol. 2018, 8, 213–221. [Google Scholar]
- Zareian, A.; Tabatabaei, S.A. Field Performance of Three Wheat Cultivars under Drought Stress and Potassium Foliar Application Treatments. Electron. J. Biol. 2014, 10, 52–58. [Google Scholar]
- Abid, M.; Ali, S.; Kang Qi, L.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) OPEN. Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; OsBlum, A. Osmotic Adjustment and Growth of Barley Genotypes under Drought Stress. Crop Sci. 1989, 29, 230. [Google Scholar] [CrossRef]
- Reynolds, M. Physiological Breeding II: A field Guide to Wheat Phenotyping; CIMMYT: Mexico, MX, USA, 2015; ISBN 9789706481825. [Google Scholar]
- Martınez-Ballesta, M.C.; Martınez, V.; Carvajal, M. Osmotic Adjustment, Water Relations and Gas Exchange in Pepper Plants Grown under NaCl or KCl. Environ. Exp. Bot. 2004, 52, 161–174. [Google Scholar] [CrossRef]




| Crop | Effects of OA on Drought Tolerance and Yield | Reference |
|---|---|---|
| Wheat | Potassium, glycine betaine and proline contributors to the leaf OA under DS condition and cumulatively assist to the grain yield | [14] |
| Wheat | Osmotic adjustment showed positive direct effects on shoot length, root length, fresh root weight, sugar and glycine betaine under drought stress | [15] |
| Wheat | Plants with better OA capacity and high benzoxazinone content have better field yields | [11] |
| Wheat | Overall, high osmoregulation increases grain yields in response to osmotic stress | [16] |
| Wheat | Osmotic adjustment sustained turgor maintenance and hence the yield-forming processes during moderate and severe water stress | [17] |
| Wheat | Indications of OA exist among wheat cultivars and associated with plant production under drought stress | [18] |
| Wheat and barley | Higher OA was found in genotypes exhibiting high yield stability across contrasting environments. Additionally, relative water content, leaf osmotic potential, and accumulation of soluble sugars were found to be highly related to osmotic adjustment. | [19] |
| Wheat | OA associated with the yield stability during the grain filling and ear growth under the DS condition | [20] |
| Wheat | Plants with higher osmoregulation extract more water from the soil and produce more dry matter and grain yield | [21] |
| Wheat | The yield of genotypes was 17% higher in bread wheat and 7% in durum wheat having higher OA | [21] |
| Wheat | Osmotic adjustment, water use efficiency WUE, and tissue elasticity are selection tools for the improvement of wheat drought tolerance | [22] |
| Wheat | The relationships suggested that direct selection for OA it may increase or decrease yield under drought but it depends on stress intensity | [23] |
| SOV | DF | OA | PH | SL | NTL | NSLS | NGS | TKW | YP |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | 2 | 0.43 ** | 1130.02 ** | 169.80 ** | 84.54 ** | 146.95 ** | 529.78 ** | 917.28 ** | 449.28 ** |
| Genotype | 29 | 0.04 ** | 1433.06 ** | 7.51 ** | 6.18 ** | 10.95 ** | 327.63 ** | 73.64 ** | 8.93 ** |
| G x T | 58 | 0.03 ** | 97.65 NS | 2.53 ** | 0.88 ** | 4.14 | 80.75 * | 48.80 ** | 3.92 ** |
| Error | 178 | 0.002 | 76.93 | 0.79 | 0.55 | 2.77 | 15.68 | 9.61 | 2.25 |
| Variable | Minimum | Maximum | Mean | Std. Deviation | F1 | F2 | F3 |
|---|---|---|---|---|---|---|---|
| OA | 0.4057 | 0.9190 | 0.6841 | 0.1258 | 0.1299 | 0.3386 | 0.4093 |
| NTL | 2.8222 | 9.5167 | 5.1142 | 1.3146 | 0.5478 | 0.1639 | 0.0078 |
| PH | 83.5444 | 140.3778 | 107.7956 | 13.7760 | 0.1402 | 0.1125 | 0.6336 |
| SL | 4.9083 | 13.5083 | 10.7098 | 1.7890 | 0.7571 | 0.0379 | 0.0274 |
| NSLS | 14.7667 | 22.1667 | 18.2166 | 1.9255 | 0.5950 | 0.0192 | 0.0197 |
| NGS | 22.4889 | 54.4889 | 40.3834 | 7.2232 | 0.2302 | 0.5697 | 0.0026 |
| TKW | 20.6670 | 47.6967 | 33.8196 | 5.3032 | 0.4800 | 0.0270 | 0.0244 |
| YP | 3.6333 | 12.2356 | 8.0751 | 2.5313 | 0.8717 | 0.0157 | 0.0014 |
| Environment | R.P Value | NGS | NTL | PH | SL | NSLS | YP | OA | R2 |
|---|---|---|---|---|---|---|---|---|---|
| WW | 0.3 | 0.065651 | −0.91773 | 0.075970 | 0.391445 | −1.10072 | 0.91381 | 10.4632 | 37.69% |
| DS | 0.4 | 0.054466 | −0.81762 | 0.070063 | 0.356197 | −0.979303 | 0.878935 | 10.1628 | 54.54% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, T.; Abdullah, M.; Ahmar, S.; Yasir, M.; Iqbal, M.S.; Yasir, M.; Ur Rehman, S.; Ahmed, S.; Rana, R.M.; Ghafoor, A.; et al. Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.). Plants 2020, 9, 1208. https://doi.org/10.3390/plants9091208
Mahmood T, Abdullah M, Ahmar S, Yasir M, Iqbal MS, Yasir M, Ur Rehman S, Ahmed S, Rana RM, Ghafoor A, et al. Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.). Plants. 2020; 9(9):1208. https://doi.org/10.3390/plants9091208
Chicago/Turabian StyleMahmood, Tahir, Muhammad Abdullah, Sunny Ahmar, Muhammad Yasir, Muhammad Shahid Iqbal, Muhmmad Yasir, Shoaib Ur Rehman, Sulaiman Ahmed, Rashid Mehmood Rana, Abdul Ghafoor, and et al. 2020. "Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.)" Plants 9, no. 9: 1208. https://doi.org/10.3390/plants9091208
APA StyleMahmood, T., Abdullah, M., Ahmar, S., Yasir, M., Iqbal, M. S., Yasir, M., Ur Rehman, S., Ahmed, S., Rana, R. M., Ghafoor, A., Nawaz Shah, M. K., Du, X., & Mora-Poblete, F. (2020). Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.). Plants, 9(9), 1208. https://doi.org/10.3390/plants9091208