Impact of Climate Change on Biodiversity and Implications for Nature-Based Solutions
Abstract
:1. Introduction
2. Methodology
2.1. Building the Datasets
2.2. Climate Impact Model
2.2.1. Upper Thermal-Tolerance Limit and Species Sensitivity–Response Relationship
2.2.2. Correlating IPCC Projections with IUCN Data
2.3. Probabilistic Climate Impact on Biodiversity
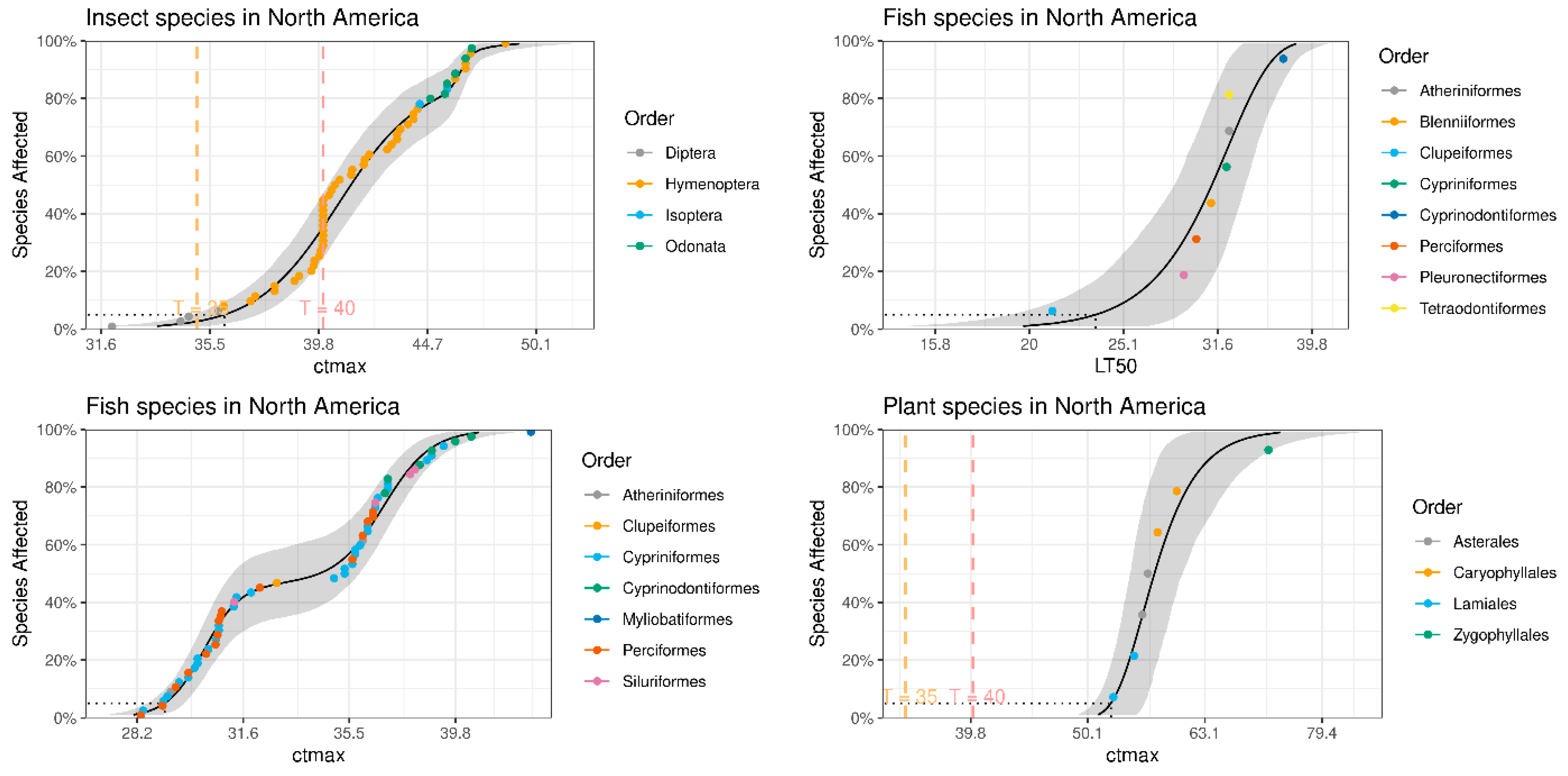
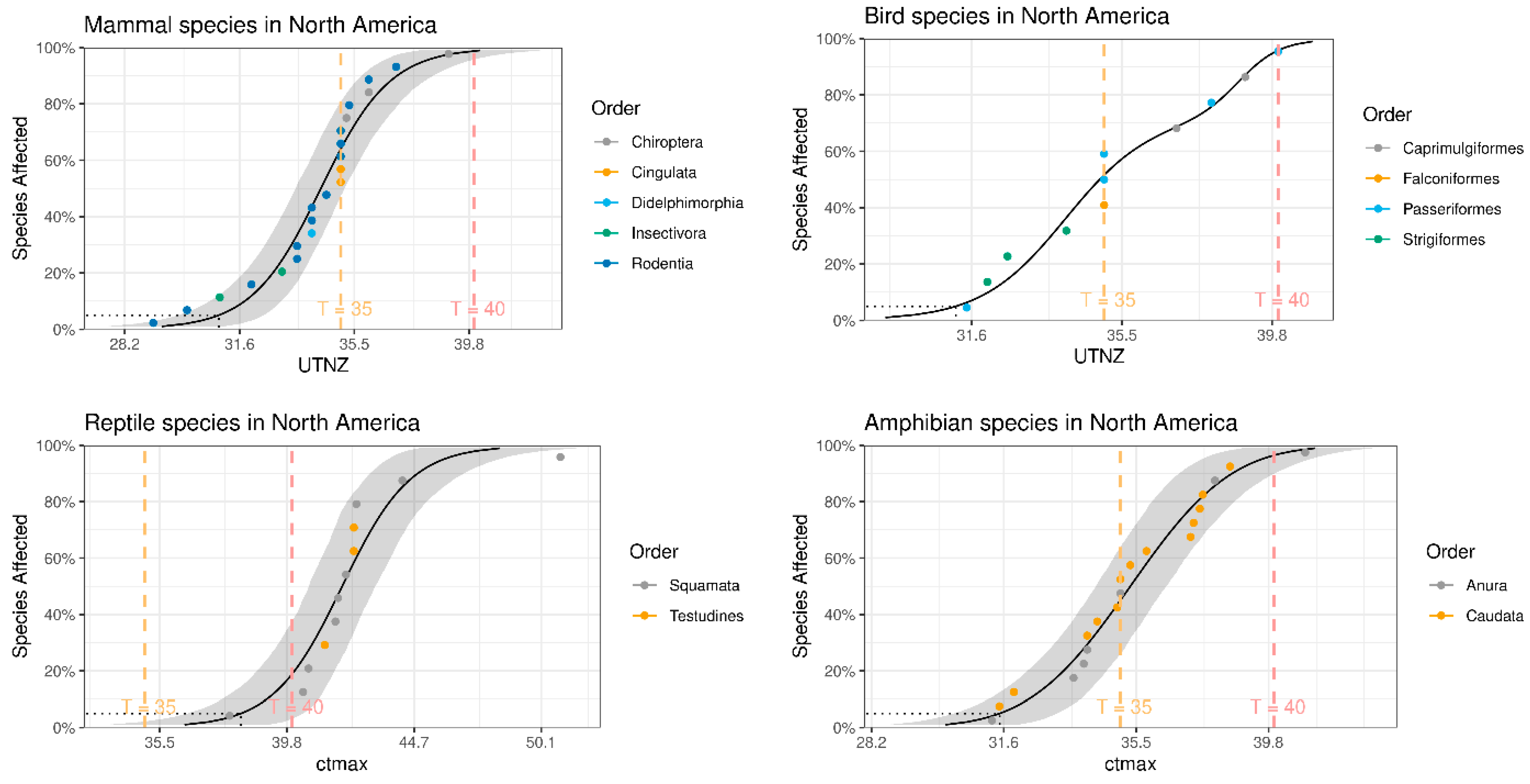
3. Results
3.1. Building Datasets
3.2. Bioclimatic Impact on Biodiversity
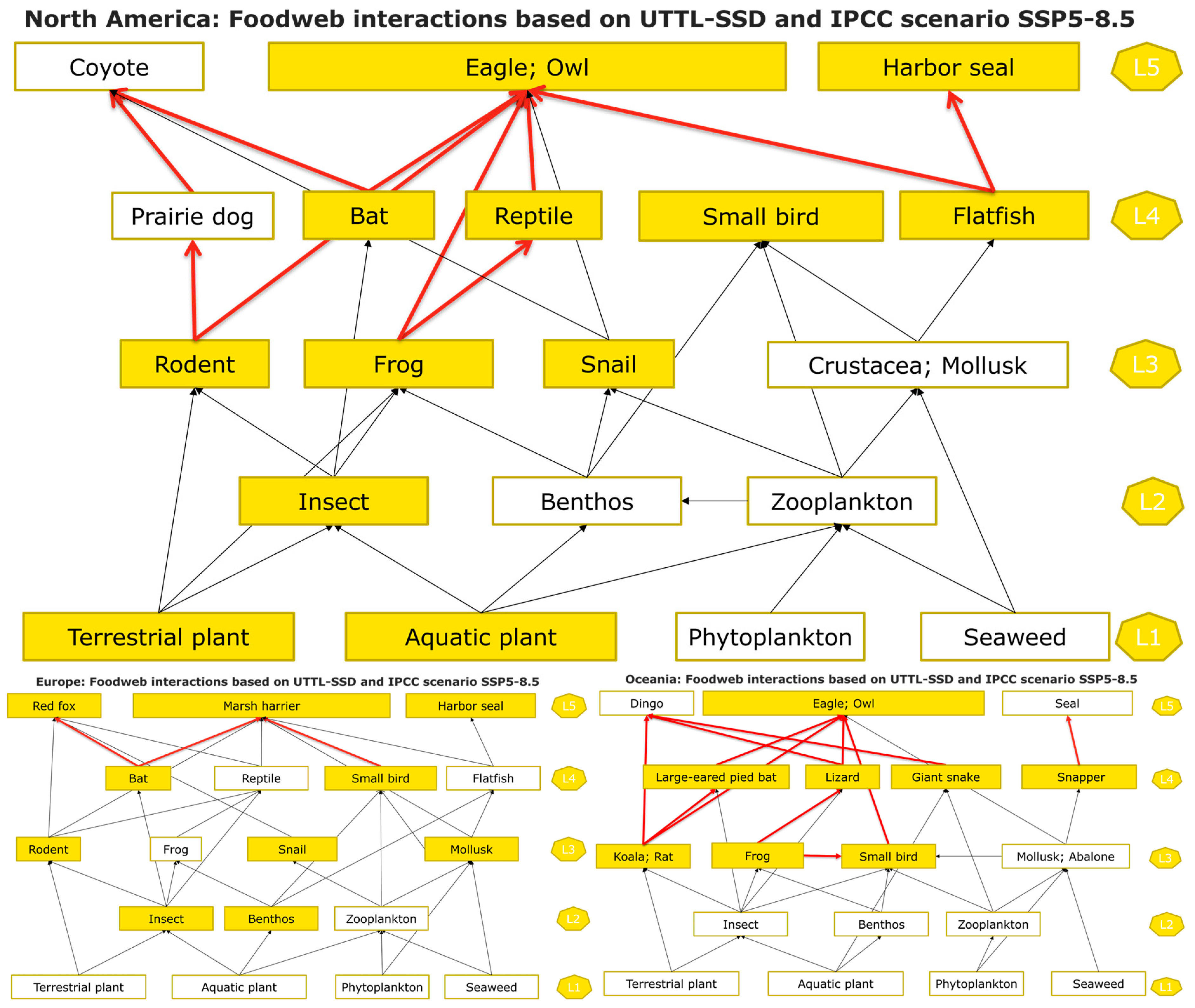
3.3. The Effect of Intensifying Climatic Conditions on Ecosystems as a Whole
4. Discussion
4.1. NbS Nature-Conservation Management Planning
4.2. Effects of Temperature Rise on Biodiversity
4.3. Cumulative Impacts on Biodiversity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | [7] | [8] | [75] | [76] | [9] | [10] | [11] | [12] |
|---|---|---|---|---|---|---|---|---|
| Geographical area | Europe | America | Europe, America, New Zealand, and Australia | Global | North America | Asia, Africa, Europe, America, and Australia | Global | Australia |
| Site | Mediterranean | Canada, North America, and Mexico | Global distribution of seaweed Undaria | Global oceans | Columbia river | Earth observation data and hydrodynamic modelling | Global | New South Wales, Australian Capital Territory, Victoria, Queensland, South, Western Australia, and Tasmania |
| Policy strategy | Intergovernmental Panel on Climate Change’s (IPCC) | RAMSAR Wetlands | Managing marine NIS | Consistency of fisheries and marine ecosystem models (MEMs) | National Climate Assessment (NCA), 4th National Climate Assessment | I Insight into the total potential cost reduction of these Nature-based Solutions | Global Climate change, biodiversity loss, and food security | Forest Fire Danger Index (FFDI) |
| Framework | AOGCM simulation model | Johnson wetland simulation model | NIS management plan | Empirical data simulation model | Ecosystems, ecosystem services, and biodiversity | Model study of wave–vegetation interaction | Review | Continuous Haines index model |
| Period studied | 1960–1989/2070–2099 | 1980–2004 | 1971–2016 | 1970–2005 | Literature review 1991–2019 | 1997– 2017 | None | 1930–2019 |
| Bioclimate indicators | Temperature and precipitation | Temperature and sea-level rise | Temperature | Increasing temperature | Warming stream temperature | Waves | Temperature | Temperature, rainfall, and soil moisture |
| Abiotic impact factors | Mediterranean climate extent (MCE) information | Coastal storm, changes in local precipitation and in humidity | Salinity, light, day length, nutrients, and wave exposure | No information | Temperature, acidification, drought, increasing CO2, N2 deposition, precipitation, water balance, sediment deposition, oxygen level, and stratification | Reduce wave impact | Reduce global cereal production | Including wind speed, humidity, fuel moisture deficit, and rainfall |
| Habitat loss and fragmentation | All terrestrial biomes | Coastal marshes | No information | No information | Climate change ecosystem impact | Data from salt marshes and mangrove map | Climate-change-driven migration, gene flow, and habitat fragmentation | Forest ecosystems |
| Biodiversity impact/changes in | Habitat loss | No information | May be driving ecosystem change in environments | Decreasing primary production; decrease of biomass fish stocks | Growth changes in invertebrates and vertebrates, genetic changes, changes in primary production, and changes in food web. | Salt marshes or mangrove vegetation | geographical distributions of species and ecosystems, and species range shifts | vegetation, biomass, and litter fractions of forested areas |
| Pollution, Nutrient loading and acidification | No information | water pollution from upstream | Growth of sporophyte and gametophyte stages is positively related to nutrients | No information | Affecting water quality | No information | Reduce the availability of water for crop | No information |
| Assessment | Quantitative of AOGCMs under multiple emissions scenarios | Quantitative | Qualitative | Quantitative | Qualitative literature review | Quantitative Sentinel-2 A and Landsat-8 images | None | Multivariate regression analysis |

| IUCN Direct Threats | Threat Description | Linked IPCC Bioclimatic Variable |
|---|---|---|
| Temperature extremes | Periods in which temperatures exceed or go below the normal range of variation due to heat waves, cold spells, oceanic temperature changes, etc. | Mean annual temperature |
| Storms and flooding | Extreme precipitation and/or wind events, due to multiple types of storms. | Max 5-day precipitation |
| Droughts | Periods in which rainfall falls below the normal range of variation due to severe lack of rain, loss of surface water sources, etc. | Consecutive dry days |
| Habitat shifting and alteration | Major changes in habitat composition and site due to sea level rise, desertification, etc. | Sea level rise |
| Europe | Europe | |||||||
|---|---|---|---|---|---|---|---|---|
| Het Zwin | Ringkøbing Fjord | Lower Saxony | Weijerswold | Klarälven Varmland | Medway Catchment | |||
| Temperature change | Temperature mean: | |||||||
| (annual) change in °C | ||||||||
| (rel. to baseline) | ||||||||
| 1850–1900 | Baseline | 10.13 | 8.31 | 8.73 | 9.06 | 3.53 | 10.21 | |
| 2081–2100 | SSP1-2.6 | 1.88 | 1.92 | 1.99 | 2.00 | 2.59 | 1.77 | |
| SSP5-8.5 | 4.59 | 4.53 | 4.77 | 4.89 | 6.14 | 4.44 | ||
| Temperature extremes: | ||||||||
| Number of days > 35 °C | ||||||||
| 2021–2040 | SSP1-2.6 | 0.04 | 0.00 | 0.29 | 0.75 | 0.10 | 0.13 | |
| SSP5-8.5 | 0.04 | 0.00 | 0.40 | 0.79 | 0.16 | 0.16 | ||
| 2081–2100 | SSP1-2.6 | 0.05 | 0.00 | 0.53 | 0.99 | 0.19 | 0.20 | |
| SSP5-8.5 | 1.17 | 0.00 | 3.98 | 9.00 | 3.99 | 3.91 | ||
| Temperature extremes: | ||||||||
| Number of days > 40 °C | ||||||||
| 2021–2040 | SSP1-2.6 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | |
| SSP5-8.5 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | ||
| 2081–2100 | SSP1-2.6 | 0.00 | 0.00 | 0.02 | 0.04 | 0.00 | 0.00 | |
| SSP5-8.5 | 0.03 | 0.00 | 0.42 | 1.53 | 0.42 | 0.16 | ||
| Change in water cycle | Precipitation (max 5 day): | |||||||
| in % (rel. to baseline) | ||||||||
| 1995–2014 | Baseline | 56.18 | 57.28 | 54.83 | 54.85 | 53.68 | 52.74 | |
| 2081–2100 | SSP1-2.6 | 3.40 | 5.43 | 7.47 | 4.68 | 6.48 | 6.70 | |
| SSP5-8.5 | 14.16 | 15.20 | 17.08 | 15.31 | 14.88 | 19.78 | ||
| Drought (CDD): | ||||||||
| in days (rel. to baseline) | ||||||||
| 1995–2014 | Baseline | 20.61 | 21.27 | 19.65 | 19.24 | 18.81 | 22.22 | |
| 2081–2100 | SSP1-2.6 | 0.69 | 0.69 | 0.56 | 0.28 | 0.95 | 2.08 | |
| SSP5-8.5 | 6.49 | 3.99 | 4.25 | 4.49 | 1.44 | 8.49 | ||
| Sea level rise (in meters) | ||||||||
| 2081–2100 | SSP1-2.6 | 0.43 | 0.42 | 0.43 | River areas | |||
| SSP5-8.5 | 0.68 | 0.67 | 0.68 | |||||
| North America & Oceania | Oceania | North America | ||||||
|---|---|---|---|---|---|---|---|---|
| Tomago Wetland | Taumanu Reserve | Long Beach Island | Springhouse Runs Str. | Upper Mississippi River | Mill River Taunton | |||
| Temperature change | Temperature mean: | |||||||
| (annual) change in °C | ||||||||
| (rel. to baseline) | ||||||||
| 1850–1900 | Baseline | 17.33 | 15.62 | 13.03 | 13.16 | 8.50 | 10.70 | |
| 2081–2100 | SSP1-2.6 | 1.89 | 1.61 | 2.29 | 2.37 | 2.78 | 2.44 | |
| SSP5-8.5 | 4.72 | 4.14 | 5.74 | 5.84 | 6.99 | 5.97 | ||
| Temperature extremes: | ||||||||
| Number of days > 35 °C | ||||||||
| 2021–2040 | SSP1-2.6 | 10.03 | 0.00 | 13.39 | 20.92 | 10.55 | 3.56 | |
| SSP5-8.5 | 11.19 | 0.00 | 14.72 | 23.60 | 11.81 | 3.95 | ||
| 2081–2100 | SSP1-2.6 | 14.47 | 0.00 | 16.31 | 24.92 | 13.82 | 5.19 | |
| SSP5-8.5 | 41.00 | 0.03 | 60.66 | 84.68 | 66.02 | 33.61 | ||
| Temperature extremes: | ||||||||
| Number of days > 40 °C | ||||||||
| 2021–2040 | SSP1-2.6 | 0.68 | 0.00 | 0.83 | 0.94 | 0.56 | 0.11 | |
| SSP5-8.5 | 0.65 | 0.00 | 0.91 | 1.30 | 0.70 | 0.18 | ||
| 2081–2100 | SSP1-2.6 | 1.02 | 0.00 | 1.14 | 1.57 | 0.99 | 0.25 | |
| SSP5-8.5 | 7.22 | 0.00 | 12.24 | 21.54 | 18.99 | 5.49 | ||
| Change in water cycle | Precipitation (max. 5 day): | |||||||
| in % (rel. to baseline) | ||||||||
| 1995–2014 | Baseline | 115.04 | 104.30 | 109.98 | 106.10 | 85.54 | 101.58 | |
| 2081–2100 | SSP1-2.6 | −0.21 | 6.00 | 5.83 | 5.23 | 5.95 | 7.52 | |
| SSP5-8.5 | 12.82 | 15.83 | 19.57 | 17.42 | 21.51 | 19.62 | ||
| Drought (CDD): | ||||||||
| in days (rel. to baseline) | ||||||||
| 1995–2014 | Baseline | 24.96 | 18.09 | 15.51 | 16.27 | 18.62 | 15.27 | |
| 2081–2100 | SSP1-2.6 | −1.51 | 0.15 | 0.12 | −0.20 | 0.36 | 0.61 | |
| SSP5-8.5 | 1.08 | 0.64 | 1.74 | 1.19 | 1.44 | 2.85 | ||
| Sea level rise (in meters) | ||||||||
| 2081–2100 | SSP1-2.6 | 0.39 | 0.39 | 0.69 | River areas | |||
| SSP5-8.5 | 0.70 | 0.70 | 0.97 | |||||


| IUCNxIPCC Correlation | Temperature | Precipitation | Drought | ||||
|---|---|---|---|---|---|---|---|
| SSP1 | SSP5 | SSP1 | SSP5 | SSP1 | SSP5 | ||
| All | Rho | −0.396 | −0.347 | −0.289 | 0.218 | 0.086 | 0.385 |
| n = 12 | p | 0.202 | 0.269 | 0.363 | 0.497 | 0.791 | 0.216 |
| Climate Zones (Present) | |||||||
| Cfb (n = 7) | Rho | 0.000 | 0.164 | 0.164 | 0.436 | 0.327 | 0.546 |
| p | 1.000 | 0.726 | 0.726 | 0.328 | 0.474 | 0.205 | |
| Dfa (n = 4) | Rho | −0.738 | −0.738 | −0.949 | −0.105 | −0.949 | −0.105 |
| p | 0.262 | 0.262 | 0.051 | 0.895 | 0.051 | 0.895 | |
| Dfb (n = 1) | |||||||
| Climate Zones (Future) | |||||||
| Cfa (n = 5) | Rho | 0.100 | 0.950 | −0.900 | 0.900 | −0.900 | 0.600 |
| p | 0.950 | 0.100 | 0.083 | 0.083 | 0.083 | 0.350 | |
| Cfb (n = 6) | Rho | 0.783 | −0.696 | 0.406 | 0.638 | 0.116 | 0.783 |
| p | 0.066 | 0.125 | 0.425 | 0.173 | 0.827 | 0.066 | |
| Dfa (n = 1) | |||||||
| Continents | Temperature | Precipitation | Drought | ||||
| EU (n = 6) | Rho | −0.783 | −0.696 | 0.406 | 0.638 | 0.116 | 0.783 |
| p | 0.066 | 0.125 | 0.425 | 0.173 | 0.827 | 0.066 | |
| NA (n = 4) | Rho | −0.738 | −0.738 | −0.949 | −0.105 | −0.949 | −0.105 |
| p | 0.262 | 0.262 | 0.051 | 0.895 | 0.051 | 0.089 | |
| OC (n = 2) | |||||||
| Type | |||||||
| Coastal (n = 6) | Rho | 0.265 | 0.530 | −0.265 | 0.088 | −0.177 | 0.618 |
| p | 0.312 | 0.280 | 0.612 | 0.868 | 0.738 | 0.191 | |
| Riverine (n = 6) | Rho | −0.841 | −0.841 | −0.232 | 0.058 | 0.058 | 0.551 |
| p | 0.036 | 0.036 | 0.658 | 0.913 | 0.913 | 0.257 | |
References
- Pearce-Higgins, J.; Antão, L.; Bates, R.; Bowgen, K.; Bradshaw, C.; Duffield, S.; Ffoulkes, C.; Franco, A.; Geschke, J.; Gregory, R.; et al. A framework for climate change adaptation indicators for the natural environment. Ecol. Indic. 2022, 136, 108690. [Google Scholar] [CrossRef]
- Pershing, A.J.; Alexander, M.A.; Hernandez, C.M.; Kerr, L.A.; Le Bris, A.; Mills, K.E.; Nye, J.A.; Record, N.R.; Scannell, H.A.; Scott, J.D.; et al. Response to Comments on “Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery”. Science 2016, 352, 423. [Google Scholar] [CrossRef] [PubMed]
- Jay, A.; Reidmiller, D.R.; Avery, C.W.; Barrie, D.; DeAngelo, B.J.; Dave, A.; Dzaugis, M.; Kolian, M.; Lewis, K.L.M.; Reeves, K.; et al. Overview. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; Reidmiller, D.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Lewis, K.L.M., Maycock, T.K., Stewart, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 33–71. [Google Scholar] [CrossRef]
- Vinagre, C.; Dias, M.; Cereja, R.; Abreu-Afonso, F.; Flores, A.A.; Mendonça, V. Upper thermal limits and warming safety margins of coastal marine species—Indicator baseline for future reference. Ecol. Indic. 2019, 102, 644–649. [Google Scholar] [CrossRef]
- Fausnacht, D.W.; Kroscher, K.A.; McMillan, R.P.; Martello, L.S.; Baumgard, L.H.; Selsby, J.T.; Hulver, M.W.; Rhoads, R.P. Heat Stress Reduces Metabolic Rate While Increasing Respiratory Exchange Ratio in Growing Pigs. Animals 2021, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Hallegatte, S.; Rogelj, J.; Allen, M.; Clarke, L.; Edenhofer, O.; Field, C.B.; Friedlingstein, P.; van Kesteren, L.; Knutti, R.; Mach, K.J.; et al. Mapping the climate change challenge. Nat. Clim. Change 2016, 6, 663–668. [Google Scholar] [CrossRef]
- Klausmeyer, K.R.; Shaw, M.R. Climate Change, Habitat Loss, Protected Areas and the Climate Adaptation Potential of Species in Mediterranean Ecosystems Worldwide. PLoS ONE 2009, 4, e6392. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Hernandez, M.E. Landscape and climate change threats to wetlands of North and Central America. Aquat. Sci. 2013, 75, 133–149. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total. Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- van Zelst, V.T.; Dijkstra, J.T.; van Wesenbeeck, B.K.; Eilander, D.; Morris, E.P.; Winsemius, H.C.; Ward, P.J.; de Vries, M.B. Cutting the costs of coastal protection by integrating vegetation in flood defences. Nat Commun. 2021, 12, 6533. [Google Scholar] [CrossRef]
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective—A review article. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- Canadell, J.G.; Meyer, C.P.; Cook, G.D.; Dowdy, A.; Briggs, P.R.; Knauer, J.; Pepler, A.; Haverd, V. Multi-decadal increase of forest burned area in Australia is linked to climate change. Nat. Commun. 2021, 12, 6921. [Google Scholar] [CrossRef] [PubMed]
- Trégarot, E.; D’Olivo, J.P.; Botelho, A.Z.; Cabrito, A.; Cardoso, G.O.; Casal, G.; Cornet, C.C.; Cragg, S.M.; Degia, A.K.; Fredriksen, S.; et al. Effects of climate change on marine coastal ecosystems—A review to guide research and management. Biol. Conserv. 2024, 289, 110394. [Google Scholar] [CrossRef]
- Rezaei, S.; Mohammadi, A.; Shadloo, S.; Ranaie, M.; Wan, H.Y. Climate change induces habitat shifts and overlaps among carnivores in an arid and semi-arid ecosystem. Ecol. Inform. 2023, 77, 102247. [Google Scholar] [CrossRef]
- Todgham, A.E.; Stillman, J.H. Physiological Responses to Shifts in Multiple Environmental Stressors: Relevance in a Changing World. Integr. Comp. Biol. 2013, 53, 539–544. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef]
- Tripathy, K.P.; Mukherjee, S.; Mishra, A.K.; Mann, M.E.; Williams, A.P. Climate change will accelerate the high-end risk of compound drought and heatwave events. Proc. Natl. Acad. Sci. USA 2023, 120, e2219825. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Sunday, J.; Calosi, P.; Villalobos, F.; Martínez, B.; Molina-Venegas, R.; Araújo, M.B.; Algar, A.C.; Clusella-Trullas, S.; Hawkins, B.A.; et al. The evolution of critical thermal limits of life on Earth. Nat. Commun. 2021, 12, 1198. [Google Scholar] [CrossRef]
- Moore, J.C. Predicting tipping points in complex environmental systems. Proc. Natl. Acad. Sci. USA 2018, 115, 635–636. [Google Scholar] [CrossRef]
- Sguotti, C.; Blöcker, A.M.; Färber, L.; Blanz, B.; Cormier, R.; Diekmann, R.; Letschert, J.; Rambo, H.; Stollberg, N.; Stelzenmüller, V.; et al. Irreversibility of regime shifts in the North Sea. Front. Mar. Sci. 2022, 9, 945204. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Stillman, J.H. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150401. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 2019, 201, 102953. [Google Scholar] [CrossRef]
- Dezetter, M.; Le Galliard, J.-F.; Leroux-Coyau, M.; Brischoux, F.; Angelier, F.; Lourdais, O. Two stressors are worse than one: Combined heatwave and drought affect hydration state and glucocorticoid levels in a temperate ectotherm. J. Exp. Biol. 2022, 225, 243777. [Google Scholar] [CrossRef] [PubMed]
- Clavero, M.; Villero, D.; Brotons, L. Climate Change or Land Use Dynamics: Do We Know What Climate Change Indicators Indicate? PLoS ONE 2011, 6, e18581. [Google Scholar] [CrossRef] [PubMed]
- Chausson, B.; Turner, D.; Seddon, N.; Chabaneix, C.A.J.; Girardin, V.; Kapos, I.; Key, D.; Roe, A.; Smith, S.; Woroniecki, N.; et al. Mapping the effectiveness of Nature-based Solutions for climate change adaptation. Glob Change Biol. 2020, 26, 6134–6155. [Google Scholar] [CrossRef]
- Aplet, G.H.; Mckinley, P.S. A portfolio approach to managing ecological risks of global change. Ecosyst. Health Sustain. 2017, 3, e01261. [Google Scholar] [CrossRef]
- Aurelle, D.; Thomas, S.; Albert, C.; Bally, M.; Bondeau, A.; Boudouresque, C.; Cahill, A.E.; Carlotti, F.; Chenuil, A.; Cramer, W.; et al. Biodiversity, climate change, and adaptation in the Mediterranean. Ecosphere 2022, 13, e3915. [Google Scholar] [CrossRef]
- Hoveka, L.N.; van der Bank, M.; Davies, T.J. Winners and losers in a changing climate: How will protected areas conserve red list species under climate change? Divers. Distrib. 2022, 28, 782–792. [Google Scholar] [CrossRef]
- Jan, S.A.; Shinwari, Z.K.; Habib, N.; Ali, S.; Afridi, M.S.; Khan, M. Impact of Climate Change on Marine Biodiversity: Current Challenges and Future Perspectives. Proc. Pak. Acad. Sci. B Pak. Acad. Sci. Life Environ. Sci. 2023, 60, 29–47. [Google Scholar]
- Hielkema, T.W.; Cor, A. Schipper, Berry Gersonius. Global nature conservation and the apparent ineffective adaptation to climate pressures. Aquat. Ecosyst. Health Manag. 2023, 26, 33–46. [Google Scholar] [CrossRef]
- Schipper, C.A.; Dekker, G.; Visser, B.D.; Bolman, B.; Lodder, Q. Characterization of SDGs towards climate resilient coastal infrastructure: Sustainability performance and cross-linking cumulative consequences. Sustainability 2020, 13, 1560. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture—Climate interactions in a changing climate: A review. Earth-Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Zscheischler, J.; Westra, S.; Van Den Hurk, B.J.J.M.; Seneviratne, S.I.; Ward, P.J.; Pitman, A.; AghaKouchak, A.; Bresch, D.N.; Leonard, M.; Wahl, T.; et al. Future climate risk from compound events. Nat. Clim. Change 2018, 8, 469–477. [Google Scholar] [CrossRef]
- Woolway, R.I.; Tong, Y.; Feng, L.; Zhao, G.; Dinh, D.A.; Shi, H.; Zhang, Y.; Shi, K. Multivariate extremes in lakes. Nat. Commun. 2024, 15, 4559. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Bridges, T.S.; Bourne, E.M.; King, J.K.; Kuzmitski, H.K.; Moynihan, E.B.; Suedel, B.C. Engineering with Nature: An Atlas; ERDC/EL SR-18-8; U.S. Army Engineer Research and Development Center: Vicksburg, MS, USA, 2018. [Google Scholar] [CrossRef]
- Bridges, T.S.; Bourne, E.M.; Suedel, B.C.; Moynihan, E.B.; King, J.K. Engineering with Nature: An Atlas, Volume 2; ERDC SR-21-2; U.S. Army Engineer Research and Development Center: Vicksburg, MS, USA, 2021. [Google Scholar] [CrossRef]
- Sayers, P.; Gersonius, B.; Özerol, G.; Nugraha, E.; Schipper, C.A. A Framework for Cloud to Coast Adaptation: Maturity and Experiences from across the North Sea. Land 2022, 11, 950. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson Delmotte, V., Zhai, A.P., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- IUCN, 2019. The International Union for Conservation of Nature (IUCN) Red List of Threatened Species: Spatial Data. Available online: https://www.iucnredlist.org/resources/spatial-data-download (accessed on 18 February 2022).
- Bennett, J.M.; Calosi, P.; Clusella-Trullas, S.; Martínez, B.; Sunday, J.; Algar, A.C.; Araújo, M.B.; Hawkins, B.A.; Keith, S.; Kühn, I.; et al. GlobTherm, a global database on thermal tolerances for aquatic and terrestrial organisms. Sci. Data 2018, 5, 180022. [Google Scholar] [CrossRef]
- Posthuma, L.; Suter, G.W., II; Traas, T.P.; Species Sensitivity Distributions in Ecotoxicology. CRC Press, 2001. Available online: https://www.routledge.com/Species-Sensitivity-Distributions-in-Ecotoxicology/Posthuma-II-Traas/p/book/9781566705783 (accessed on 18 July 2023).
- Schipper, C.A.; Rietjens, I.M.C.M.; Burgess, R.M.; Murk, A.J. Application of bioassays in toxicological hazard, risk and impact assessments of dredged sediments. Mar. Pollut. Bull. 2010, 60, 2026–2042. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; RG Jones, G.T.; Narisma, L.M.; Alves, M.; Amjad, I.V.; Gorodetskaya, M.; Grose, N.A.B.; Klutse, S.; Krakovska, J.; Li, D.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; In Press. Interactive Atlas; Available online: http://interactive-atlas.ipcc.ch/ (accessed on 8 March 2022).
- Lange, S. Trend-preserving bias adjustment and statistical downscaling with ISIMIP3BASD (v1.0). Geosci. Model Dev. 2019, 12, 3055–3070. [Google Scholar] [CrossRef]
- Bellard, C.; Leclerc, C.; Courchamp, F. Combined impacts of global changes on biodiversity across the USA. Sci. Rep. 2015, 5, 11828. [Google Scholar] [CrossRef]
- Bellard, C.; Leclerc, C.; Leroy, B.; Bakkenes, M.; Veloz, S.; Thuiller, W.; Courchamp, F. Vulnerability of biodiversity hotspots to global change. Glob. Ecol. Biogeogr. 2014, 23, 1376–1386. [Google Scholar] [CrossRef]
- Pottier, P.; Lin, H.Y.; Oh, R.R.; Pollo, P.; Rivera-Villanueva, A.N.; Valdebenito, J.O.; Yang, Y.; Amano, T.; Burke, S.; Drobniak, S.M.; et al. A comprehensive database of amphibian heat tolerance. Sci. Data 2022, 9, 600. [Google Scholar] [CrossRef]
- Khaliq, I.; Hof, C.; Prinzinger, R.; Böhning-Gaese, K.; Pfenninger, M. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141097. [Google Scholar] [CrossRef]
- Compton, T.J.; Rijkenberg, M.J.; Drent, J.; Piersma, T. Thermal tolerance ranges and climate variability: A comparison between bivalves from differing climates. J. Exp. Mar. Biol. Ecol. 2007, 352, 200–211. [Google Scholar] [CrossRef]
- Aldenberg, T.; Slob, W. Confidence Limits for Hazardous Concentrations Based on Logistically Distributed NOEC Toxicity Data. Ecotoxicol. Environ. Saf. 1993, 25, 48–63. [Google Scholar] [CrossRef]
- Aldenberg, T.; Jaworska, J.S.; Traas, T.P. Normal species sensitivity distributions and probabilistic ecological risk assessment. In Species Sensitivity, Distributions in Ecotoxicology; Posthuma, L., Suter, G.W., II, Traas, T.P., Eds.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 September 2024).
- Kemp, L.; Xu, C.; Depledge, J.; Ebi, K.L.; Gibbins, G.; Kohler, T.A.; Rockström, J.; Scheffer, M.; Schellnhuber, H.J.; Steffen, W.; et al. Climate Endgame: Exploring catastrophic climate change scenarios. Proc. Natl. Acad. Sci. USA 2022, 119, e2108146119. [Google Scholar] [CrossRef]
- Cox, N.; Young, B.E.; Bowles, P.; Fernandez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A global reptile assessment highlights shared conservation needs of tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef]
- Valdez, J.W.; Callaghan, C.T.; Junker, J.; Purvis, A.; Hill, S.L.L.; Pereira, H.M. The undetectability of global biodiversity trends using local species richness. Ecography 2023, 2023, e06604. [Google Scholar] [CrossRef]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Rayner, T.S.; Pusey, B.J.; Pearson, R.G.; Godfrey, P.C. Food web dynamics in an Australian Wet Tropics river. Mar. Freshw. Res. 2010, 61, 909–917. [Google Scholar] [CrossRef]
- Boonstra, R.; Andreassen, H.P.; Boutin, S.; Hušek, J.; Ims, R.A.; Krebs, C.J.; Skarpe, C.; Wabakken, P. Why Do the Boreal Forest Ecosystems of Northwestern Europe Differ from Those of Western North America? BioScience 2016, 66, 722–734. [Google Scholar] [CrossRef]
- Griggs, D.; Stafford-Smith, M.; Warrilow, D.; Street, R.; Vera, C.; Scobie, M.; Sokona, Y. Use of weather and climate information essential for SDG implementation. Nat. Rev. Earth Environ. 2021, 2, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]
- Mahecha, M.D.; Bastos, A.; Bohn, F.J.; Eisenhauer, N.; Feilhauer, H.; Hickler, T.; Kalesse-Los, H.; Migliavacca, M.; Otto, F.E.L.; Peng, J.; et al. Biodiversity and Climate Extremes: Known Interactions and Research Gaps. Earth’s Futur. 2023, 12, e2023EF003963. [Google Scholar] [CrossRef]
- Kearney, M. Habitat, environment and niche: What are we modelling? Oikos 2006, 115, 186–191. [Google Scholar] [CrossRef]
- Buckley, L.B.; Carrington, E.; Dillon, M.E.; García-Robledo, C.; Roberts, S.B.; Wegrzyn, J.L.; Urban, M.C. Characterizing biological responses to climate variability and extremes to improve biodiversity projections. PLOS Clim. 2023, 2, e0000226. [Google Scholar] [CrossRef]
- UN (2017a). The Sustainable Development Goals Report; United Nations: New York, NY, USA, 2017; Available online: https://unstats.un.org/sdgs/files/report/2017/TheSustainableDevelopmentGoalsReport2017.pdf (accessed on 20 January 2021).
- IPCC. Climate Change 2023: Synthesis Report Contribution of Working Groups I., II. and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Noce, S.; Caporaso, L.; Santini, M. A new global dataset of bioclimatic indicators. Sci. Data 2020, 7, 398. [Google Scholar] [CrossRef]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef]
- Hillebrand, H.; Kuczynski, L.; Kunze, C.; Rillo, M.C.; Dajka, J.-C. Thresholds and tipping points are tempting but not necessarily suitable concepts to address anthropogenic biodiversity change—An intervention. Mar. Biodivers. 2023, 53, 43. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, Y.; Ouyang, L.; Li, Y.; Sun, F.; Liu, Y.; Zhu, J. Drought-heatwave compound events are stronger in drylands. Weather. Clim. Extrem. 2023, 42, 100632. [Google Scholar] [CrossRef]
- Biber, M.F.; Voskamp, A.; Hof, C. Potential effects of future climate change on global reptile distributions and diversity. Glob. Ecol. Biogeogr. 2023, 32, 519–534. [Google Scholar] [CrossRef]
- Lebreton, J.-D. The impact of global change on terrestrial Vertebrates. Comptes Rendus Biol. 2011, 334, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Okello, M.M.; Kenana, L.; Maliti, H.; Kiringe, J.W.; Kanga, E.; Warinwa, F.; Bakari, S.; Ndambuki, S.; Massawe, E.; Sitati, N.; et al. Population density of elephants and other key large herbivores in the Amboseli ecosystem of Kenya in relation to droughts. J. Arid. Environ. 2016, 135, 64–74. [Google Scholar] [CrossRef]
- Vorste, R.V.; Obedzinski, M.; Pierce, S.N.; Carlson, S.M.; Grantham, T.E. Refuges and ecological traps: Extreme drought threatens persistence of an endangered fish in intermittent streams. Glob. Change Biol. 2020, 26, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Epstein, G.; Smale, D.A. Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecol. Evol. 2017, 7, 8624–8642. [Google Scholar] [CrossRef]
- Lotze, H.K.; Tittensor, D.P.; Bryndum-Buchholz, A.; Eddy, T.D.; Cheung, W.W.L.; Galbraith, E.D.; Barange, M.; Barrier, N.; Bianchi, D.; Blanchard, J.; et al. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl. Acad. Sci. USA 2019, 116, 12907–12912. [Google Scholar] [CrossRef]




| Köppen–Geiger Classification | |||
|---|---|---|---|
| Continent | NbS Sites | 1980–2016 | 2081–2100 |
| North America | Long Beach Island | Dfa | Cfa |
| Springhouse Runs | Dfa/Cfa | Cfa | |
| Mill River Taunton | Dfa | Cfa | |
| Upper Mississippi River | Dfa/Dfb | Cfa/Dfa | |
| Oceania | Tomago Wetland AS | Cfb/Dfa | Cfa/Cfb |
| Taumanu NZ | Cfb | Cfa/Cfb | |
| Europe | Zwin | Cfb | Cfb/Cfa |
| Weijerswold | Cfb | Cfb | |
| Lower Saxony | Cfb | Cfb | |
| Medway | Cfb | Cfb | |
| Ringkobing fjord | Cfb | Cfb | |
| Klarälven Värmland | Dfb | Cfb/Dfb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipper, C.A.; Hielkema, T.W.; Ziemba, A. Impact of Climate Change on Biodiversity and Implications for Nature-Based Solutions. Climate 2024, 12, 179. https://doi.org/10.3390/cli12110179
Schipper CA, Hielkema TW, Ziemba A. Impact of Climate Change on Biodiversity and Implications for Nature-Based Solutions. Climate. 2024; 12(11):179. https://doi.org/10.3390/cli12110179
Chicago/Turabian StyleSchipper, Cor A., Titus W. Hielkema, and Alexander Ziemba. 2024. "Impact of Climate Change on Biodiversity and Implications for Nature-Based Solutions" Climate 12, no. 11: 179. https://doi.org/10.3390/cli12110179
APA StyleSchipper, C. A., Hielkema, T. W., & Ziemba, A. (2024). Impact of Climate Change on Biodiversity and Implications for Nature-Based Solutions. Climate, 12(11), 179. https://doi.org/10.3390/cli12110179











