Abstract
As a new type of green propellant, ammonium dinitramide (ADN)-based energetic propellants have wide application value and development potential in the field of space propulsion. This paper delves into the intricate impact of varying initial temperatures, pressures, and propellant component ratios on critical parameters, including temperature, combustion rate, and heat release, in the straight nozzle of an ADN-based propellant. The findings indicate that an elevation in both initial temperature and ADN ratio expedites the thermal decomposition rate of ADN, thereby elevating the average temperature in the nozzle. However, the elevation in initial temperature has a negative effect on the overall rise amplitude of average temperature. Furthermore, the initial pressure setting is crucial in determining whether the oxidation reaction of the fuel CH3OH occurs in ADN propellants. When the initial pressure is greater than 10 atm, CH3OH is completely consumed, and the final average temperature is about 2650 K, which increases by 558.89% compared with that at 1 atm. Our work aims to provide theoretical guidance and practical optimization strategies for enhancing propellant performance and optimizing thruster structure design.
1. Introduction
In recent years, ammonium dinitramide (ADN)-based liquid propellants have become a substitute for traditional propellants, such as hydrazine and ammonium perchlorate (AP), due to the characteristics of the high energy, green, and low characteristic signal [1,2], which has attracted extensive attention in the field of aerospace propulsion. In 1971, the former Soviet Union successfully synthesized solid ADN [3,4] with its molecular formula (NH4N(NO2)2) for the first time, which can be used as a high-energy oxidant in rocket propellants [5,6,7]. Owing to the strong water absorption and high solubility characteristics of solid ADN [8], the Swedish Defense Research developed ADN-based liquid propellants [9] at the beginning of the 21st century, which was mainly composed of ADN, fuel, and water. Subsequently, they revealed that ADN-based liquid propellants have better performance when methanol (CH3OH) is used as fuel. After that, many researchers carried out a series of experimental and theoretical studies on ADN-based liquid propellants in the form of the ADN-CH3OH-H2O combination [10,11,12]. Lei Li et al. successfully conducted ignition experiments on ADN-based propellant droplets using contact electric heating ignition, investigating the effects of ambient pressure, droplet size, and gas atmosphere [13,14,15] on ignition performance. Yangyang Hou et al. [16] employed non-contact microwave ignition to study the sequential evolution processes of swelling, micro-explosion, and combustion exhibited by ADN-based propellant droplets during microwave loading. Michele Negri et al. [17] utilized pulsed laser irradiation on ADN-based propellant droplets, observing explosive phenomena in the ADN droplets but lacking direct evidence of successful ignition. In another study, Michele Negri et al. [18] reported that ADN-based liquid propellants, upon undergoing vaporization treatment, become more susceptible to successful ignition. Within our research group, Baosheng Du et al. [19] applied high-energy pulsed laser ablation to ADN-based propellant droplets placed within micron-scale pits, achieving an impulse of 9.8 μN·s and a specific impulse of 234.9 s, thereby demonstrating the feasibility of generating micro-thrust through the pulsed laser ablation of micro-droplets.
Simultaneously, significant progress and new breakthroughs have been made in related theoretical modeling efforts. Gustavo F. Velardez et al. [20] performed molecular dynamics calculations on the melting point and liquid properties of ADN. Weihua Zhu et al. [21] employed the density functional theory to conduct a comparative analysis of the electronic structure, absorption spectra, and thermodynamic changes of crystalline AP and ADN, finding that ADN exhibits a greater number of vibrational frequency modes than AP across the low-to-high frequency range and that ADN is more prone to decomposition than AP as temperature increases. The Yusong Yu team from Beijing Jiaotong University [22] investigated the influence of catalyst bed length, diameter, and wall thickness on the thrust performance of ADN thrusters, revealing that wall thickness plays the most crucial role among these factors, with optimal parameters yielding a 5.9% increase in specific impulse. Researchers led by Xiaoqing You at Tsinghua University proposed a detailed reaction mechanism for the combustion process of ADN-based propellants, encompassing 48 species and 242 chemical reactions [23]. Subsequently, the team simplified this intricate mechanism (comprising 18 components and 40 reactions) and, based on the simplified model, conducted a study on the catalytic combustion process of ADN-based propellants in space thrusters. They discovered that ADN does not directly react with CH3OH; rather, it is the interaction between N-containing small molecules resulting from ADN pyrolysis and C-bearing small molecules produced by the dehydrogenation of CH3OH that drives the reaction [24]. The team led by Liu Xuhui [25] at the Beijing Institute of Control Engineering employed three-dimensional numerical simulations to investigate the flow and phase change characteristics of the propellant at the microscale of capillaries within the ADN-based micro thruster. In summary, prior research on ADN has primarily focused on its physicochemical properties and combustion behavior. However, the thermal decomposition and combustion mechanisms of ADN-based propellants at the microscale in nozzles remain in the early stages of investigation. Moreover, studies examining the influence of varying initial conditions on the combustion and flow dynamics of ADN-based propellants are scarce. To address these gaps, the present work focuses on gaseous ADN-based propellants, employing a two-dimensional numerical simulation approach utilizing the reactingFoam solver. This study investigates the effects of different initial temperatures, pressures, and component ratios within the propellant on the thermal decomposition and combustion processes in the straight nozzle. The aim is to provide theoretical guidance for enhancing thruster performance and optimizing operating conditions in the thruster design.
2. Numerical Modeling
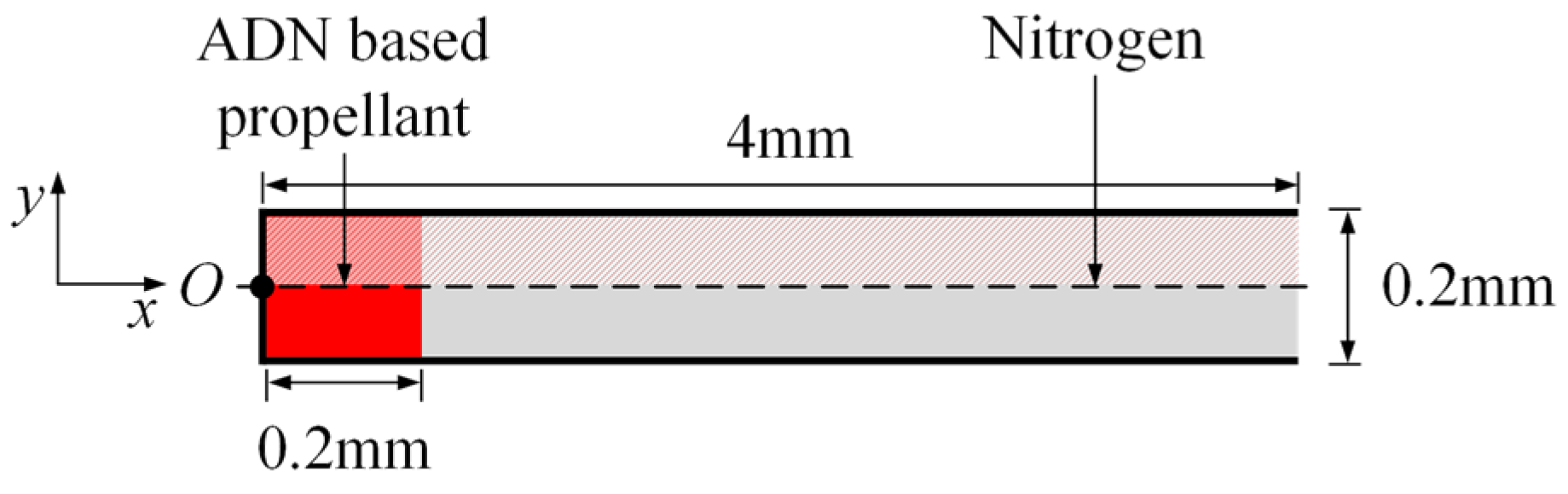
In this paper, the two-dimensional transient simulation of the combustion process of a gaseous ADN-based propellant in a straight nozzle is carried out. The nozzle with an ADN-based propellant is shown in Figure 1, with a length of 4 mm, a bottom width of 0.2 mm, and an expansion angle of 0°. The propellant filling length is 0.2 mm, and the rest of the nozzle is filled with nitrogen. Considering the symmetry of geometry and flow, the upper part is taken for meshing and numerical calculation.
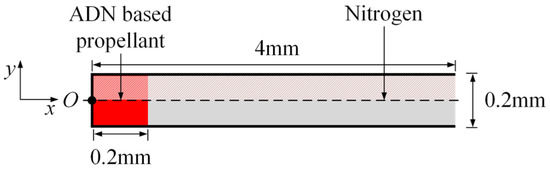
Figure 1.
Geometric model of the straight nozzle with an ammonium dinitramide (ADN)-based propellant.
OpenFOAM is a mature CFD calculation software. As an open-source framework, it can successfully calculate the combustion reaction [26,27,28]. In this paper, the reactingFoam solver of OpenFOAM (V.6) was used to carry out the numerical simulation of an ADN-based propellant. The relevant mass conservation, momentum conservation, component conservation, and energy conservation equations are shown in Equation (1) [29]. The control equation is solved by the built-in finite volume method solver, and the pressure implicit operator splitting (PISO) algorithm [30] is used to correct the velocity field using the output of the pressure equation.
where is the viscous stress tensor; is the i-component of the diffusion velocity for the kth component; is the mass fraction of the kth component; is the reaction rate of the kth component; is the heat release rate of the combustion process; is the heat flux, including the thermal diffusion term caused by the temperature gradient and the thermal diffusion term caused by the diffusion of components with different specific enthalpies; and is the deviatoric stress tensor [31].
The gaseous ADN-based propellant mainly involved an ADN decomposition reaction and a CH3OH oxidation reaction. In this paper, the simplified reaction mechanism in reference [24] is used for the calculation. The working conditions involved in the ADN-based propellant on the left side of the nozzle in the study are shown in Table 1. Case 1 is the control group; the control variable of cases 1–3 is the initial temperature T0; the control variable of cases 1, 4, 5, and 6 is the initial pressure p0; and the control variable of cases 1, 7, 8, and 9 is the initial component ratio of propellant: YADN:YCH3OH:YH2O. For the remaining part of the nozzle, the initial temperature is uniformly set at 300 K, and the initial pressure is maintained at 1 atm.

Table 1.
Initial state of the gaseous ADN propellant in different working conditions.
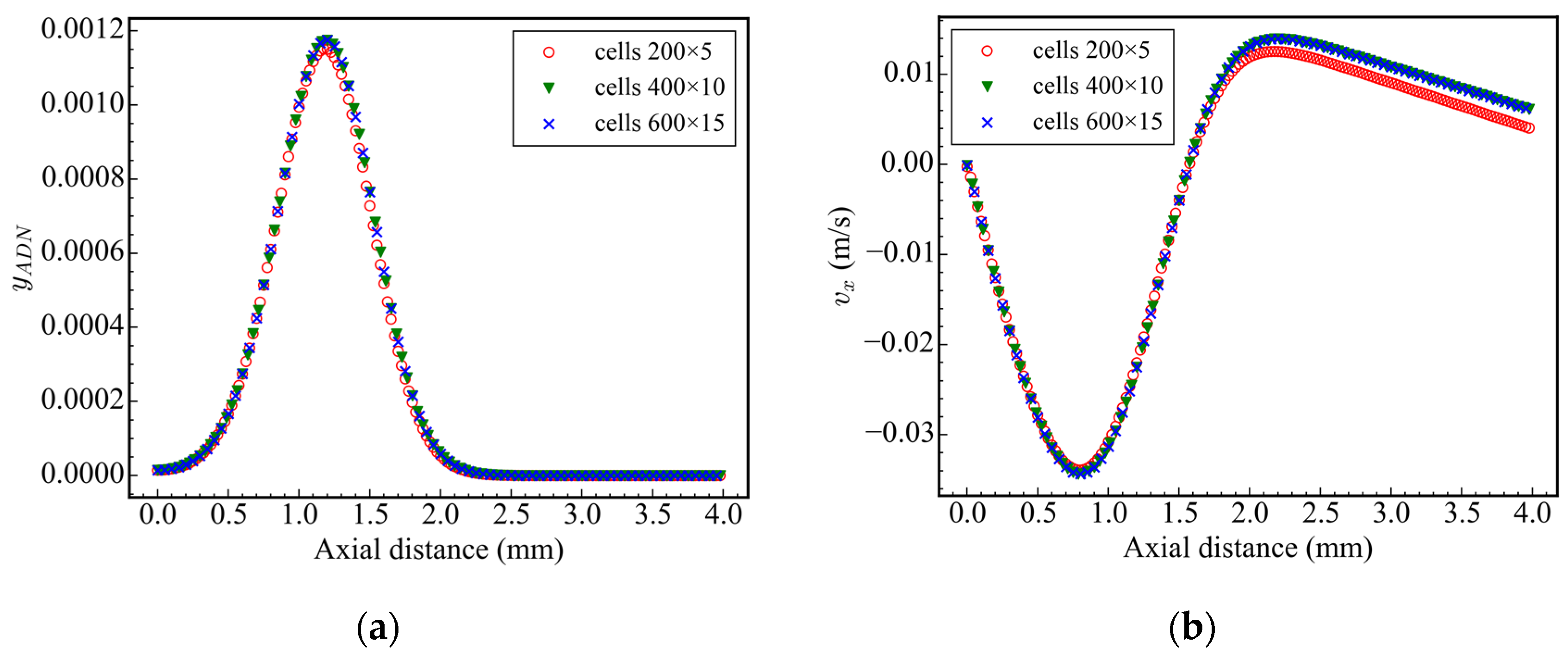
In computational geometry, the symmetrical boundary condition is used at the axis of symmetry, the zero gradient boundary condition is used at the outlet, and the other boundaries are set as no-slip walls. In order to ensure the accuracy of the calculation results and improve the calculation efficiency, the grid independence test is carried out in case 1. The number of grids is 200 × 5, 400 × 10, and 600 × 15, respectively (corresponding to the X and Y directions, structured grid). The distribution of ADN mass fraction and X-direction velocity on the central axis at 5 ms is surveyed. The results are shown in Figure 2, and the corresponding maximum values of ADN mass fraction and X-direction velocity are shown in Table 2. It was found that the calculation results of the 200 × 5 grid are different from those of the latter two grids, while the calculation results of the 400 × 10 grid and 600 × 15 grid are consistent, indicating that few grid numbers can cause the loss of effective information. At the same time, considering the calculation efficiency, the calculation time of the 400 × 10 grid is faster than that of the 600 × 15 grid, so the 400 × 10 grid distribution is adopted for all subsequent calculations. In order to ensure the accuracy of the calculation and minimize the diffusion caused by time discretization, the initial time step is set to 1 × 10−11 s, the maximum Courant–Friedrichs–Lewy (CFL) number is set to 0.2, and the maximum time step is set to 1 × 10−6 s.
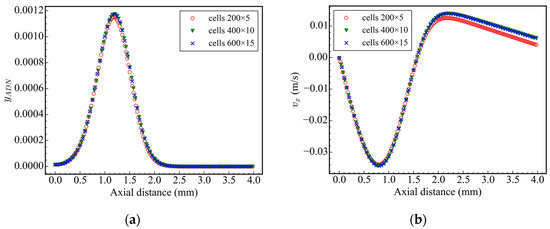
Figure 2.
Axial ADN mass fraction and velocity at 5 ms: (a) ADN mass fraction; (b) axial velocity.

Table 2.
Maximum values of ADN mass fraction and axial velocity.
3. Results and Discussion
3.1. Analysis of the Combustion Process of a Gaseous ADN-Based Propellant
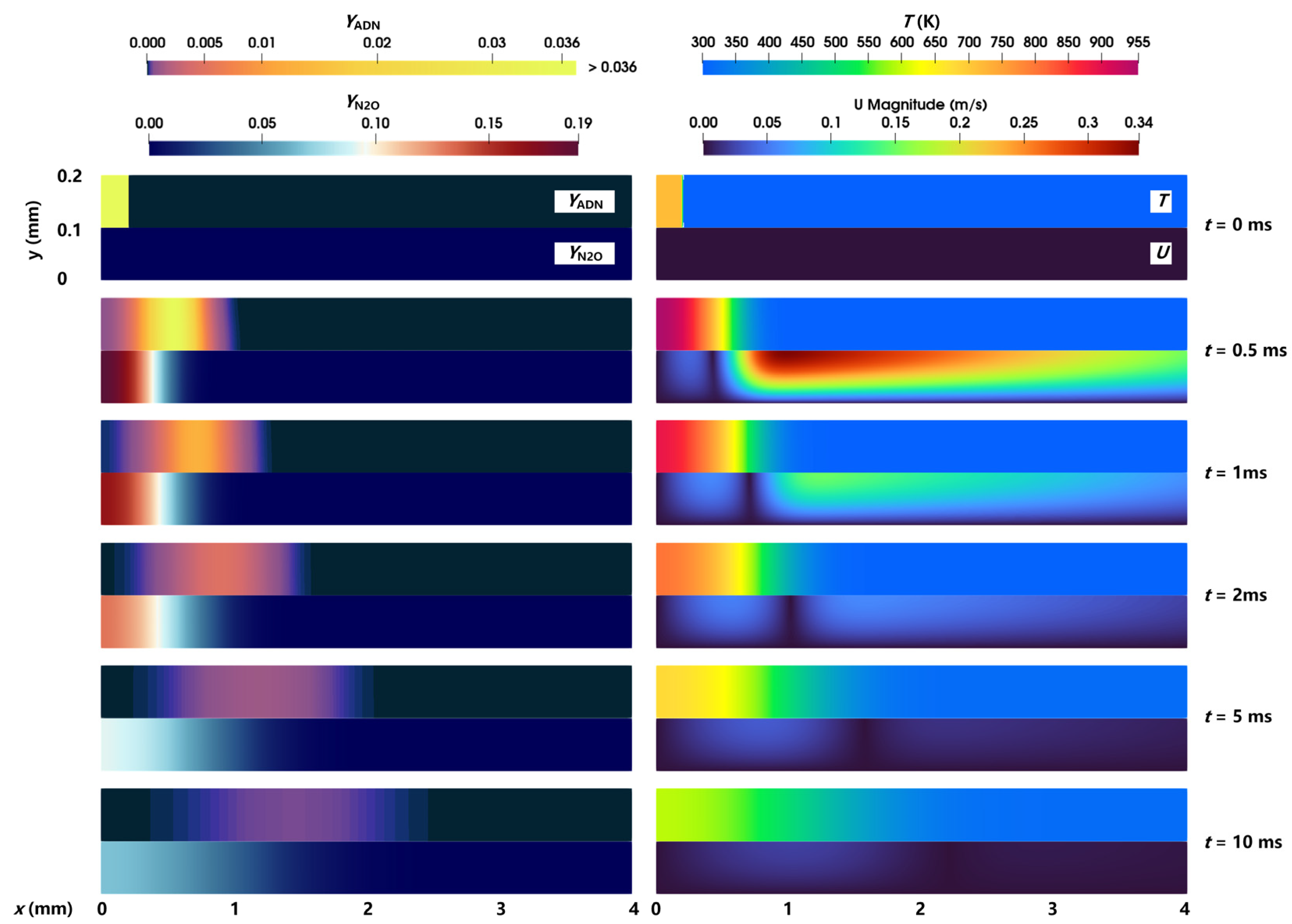
The evolution process of the combustion and flow of a gaseous ADN-based propellant in the nozzle in case 1 is analyzed. The distribution of the reactant ADN mass fraction, intermediate product N2O mass fraction, temperature, and velocity is shown in Figure 3. In general, the chromaticity bar of the ADN mass fraction (YADN) exhibited a color transition from dark cyan → orange → blue over time, indicating that the combustion pyrolysis reaction of reactant ADN occurred and content continued to decrease. Correspondingly, the mass fraction of the intermediate product N2O (YN2O) produced from the combustion pyrolysis reaction of ADN experienced a change from blue → dark red → cyan, respectively, representing the stages of N2O formation → increase → diffusion. In addition, the content of ADN in the nozzle showed an uneven distribution of high in the middle and low on both sides, indicating that, compared with the middle position of the nozzle, the closer to the bottom wall of the nozzle, the faster the reaction of ADN, and the less the residual content. The decrease in ADN away from the wall was due to the concentration difference between the right side of ADN and the ambient gas, which led to the right diffusion of ADN. Compared with the reactant ADN, the product N2O had a higher content near the bottom wall and a lower content far away from the bottom wall.
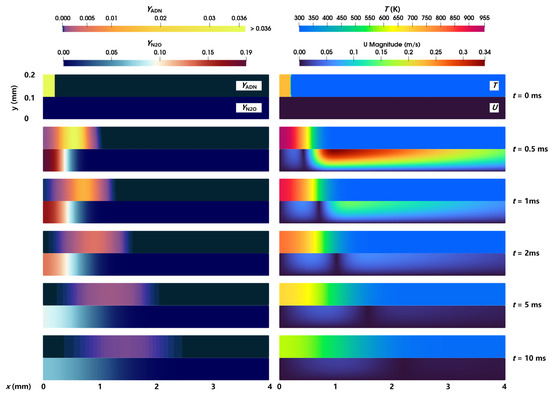
Figure 3.
Distribution of the ADN mass fraction, N2O mass fraction, temperature, and velocity magnitude at different times.
The temperature in the nozzle rose rapidly due to the occurrence of the ADN pyrolysis reaction, and the temperature rise range reached 200 K in 0–0.5 ms. Under the convection and diffusion caused by the temperature difference and concentration difference, a large velocity was generated on the right side of ADN at 0.5 ms; the velocity inside the reactant was low due to the limitation of the left wall; and a region with virtually zero velocity appeared near the interface between high and low temperatures. Due to the influence of wall viscosity, the closer the position was to the central axis, the higher the velocity was, which led to the uneven distribution of ADN. The movement speed of ADN at the upper wall was slower than that at the central axis. In contrast, due to the low velocity near the bottom wall, no similar uneven distribution of N2O could be observed from the figure. Over time, the components diffused to the right, and the high temperature-low temperature interface moved to the right, but the movement speed gradually decreased. After 5 ms, the maximum temperature was lower than the initial temperature (718 K). On the one hand, the temperature was reduced due to convection and component diffusion. On the other hand, the combustion pyrolysis reaction was slowed down and no more heat was generated. At 10 ms, ADN was almost completely consumed, indicating that the pyrolysis reaction of ADN almost stopped. However, because the local temperature was not high enough, N2O could not react further and, hence, still maintained a high concentration.
3.2. Effects of Different Initial Temperatures on the Combustion Process of an ADN-Based Propellant
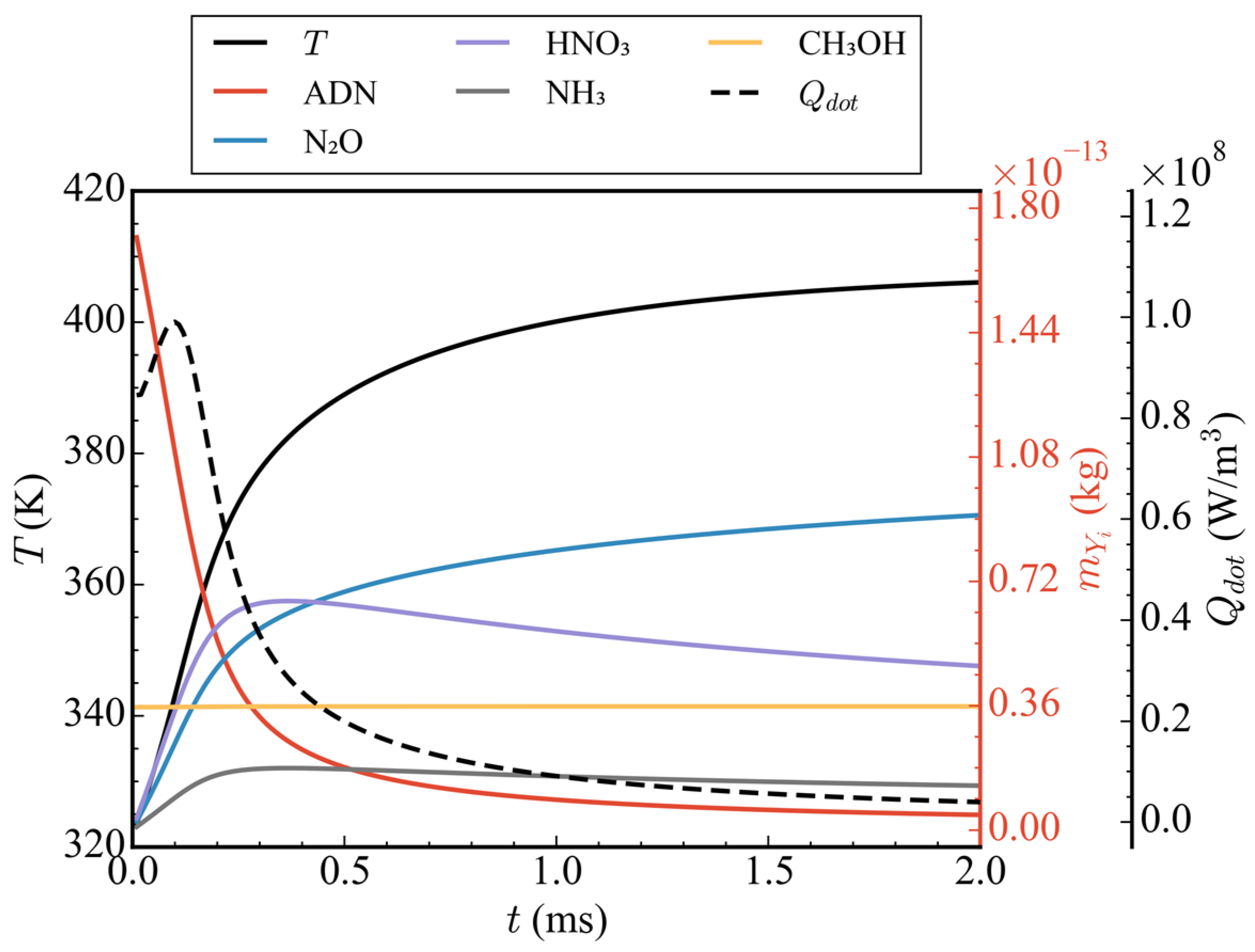
Figure 4 shows the variation of average temperature, total mass of five components (ADN, N2O, HNO3, NH3, and CH3OH), and average heat release rate in the straight nozzle with time in case 1. As shown in Figure 4, the average heat release first increased within 0–2 ms, reached its peak at 0.1 ms, then rapidly decreased and approached 0. Correspondingly, the average temperature inside the nozzle first rose rapidly and then tended to flatten, rising from 323 K to 406 K. By analyzing the total mass curves of the five components, it can be seen that the total mass of ADN decreased rapidly from time 0, and the total mass of the ADN pyrolysis products, N2O, HNO3, and NH3, increased continuously before 0.25 ms, resulting in a rapid rise in the temperature inside the nozzle. After 0.25 ms, the mass of NH3 began to decrease slowly, and HNO3 decreased faster. This is because HNO3 continued to decompose to produce N2O. Under the combined influence of ADN pyrolysis and HNO3 decomposition, N2O kept an upward trend after 0.25 ms, but, due to the rapid reduction in ADN content, the upward trend of N2O also gradually slowed down. It is worth mentioning that, as one of the main components of propellants, the total mass of CH3OH had remained unchanged, indicating that its oxidation reaction did not start, which can be attributed to the CH3OH aggregation region not reaching its reaction temperature.
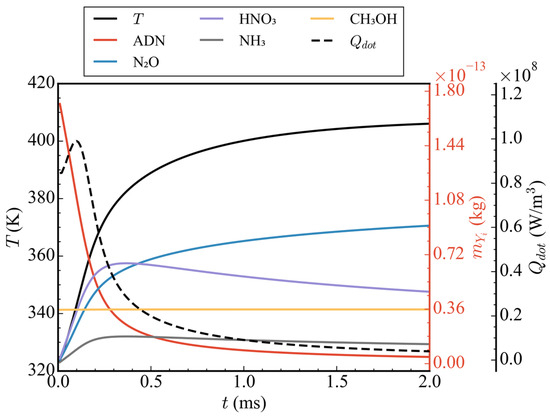
Figure 4.
Time-varying curves of average temperature, total mass of ADN/N2O/HNO3/NH3/CH3OH, and average heat release rate in case 1.
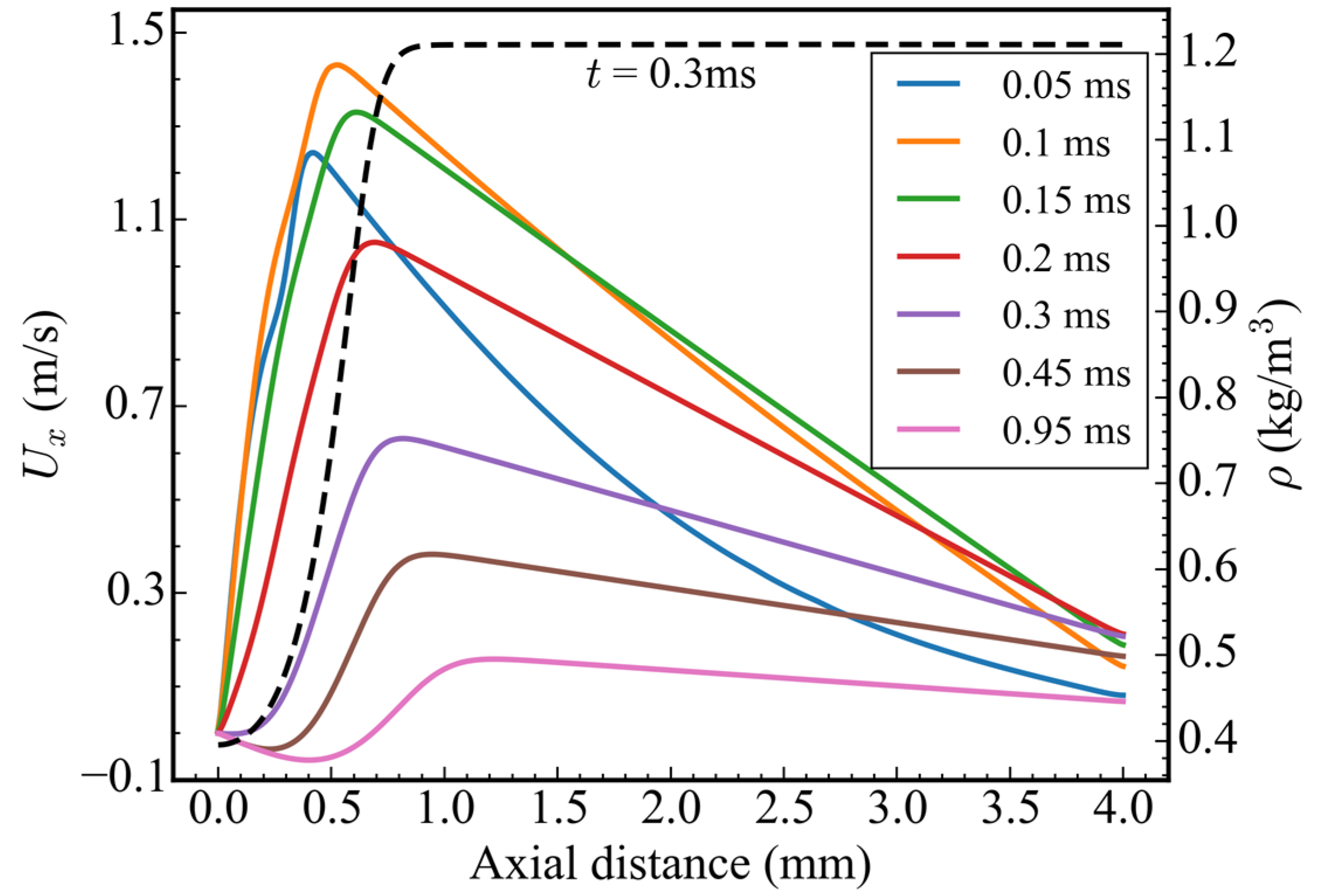
Figure 5 shows the velocity distribution in the x-direction along the central axis at different times. Before 0.3 ms, the velocity exhibited a profile of initially increasing and then decreasing along the x-direction. Under the action of the temperature gradient, the maximum velocity of 1.24 m/s appeared at x = 0.42 mm, i.e., in front of the ADN-based propellant at 0.05 ms. With the pyrolysis of ADN, the temperature in this area increased, and, hence, the flow velocity also gradually increased. At 0.1 ms, the peak velocity of 1.43 m/s appeared at x = 0.535 mm, and the peak moving velocity was 2.3 m/s. Then, the peak velocity continued to decrease, and the position of peak velocity also continued to move forward, but the moving velocity continued to decrease. After 0.3 ms, the velocity presented an “S” shape distribution along the x-direction. By investigating the density distribution at 0.3 ms, it can be concluded that the negative velocity near the bottom wall was due to the negative flow caused by the density difference.
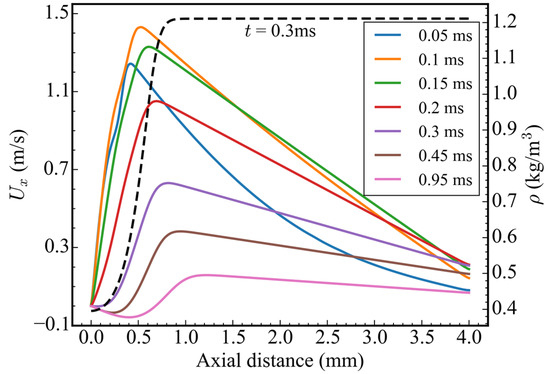
Figure 5.
Velocity distribution in the x-direction along the central axis and density distribution at 0.3 ms.
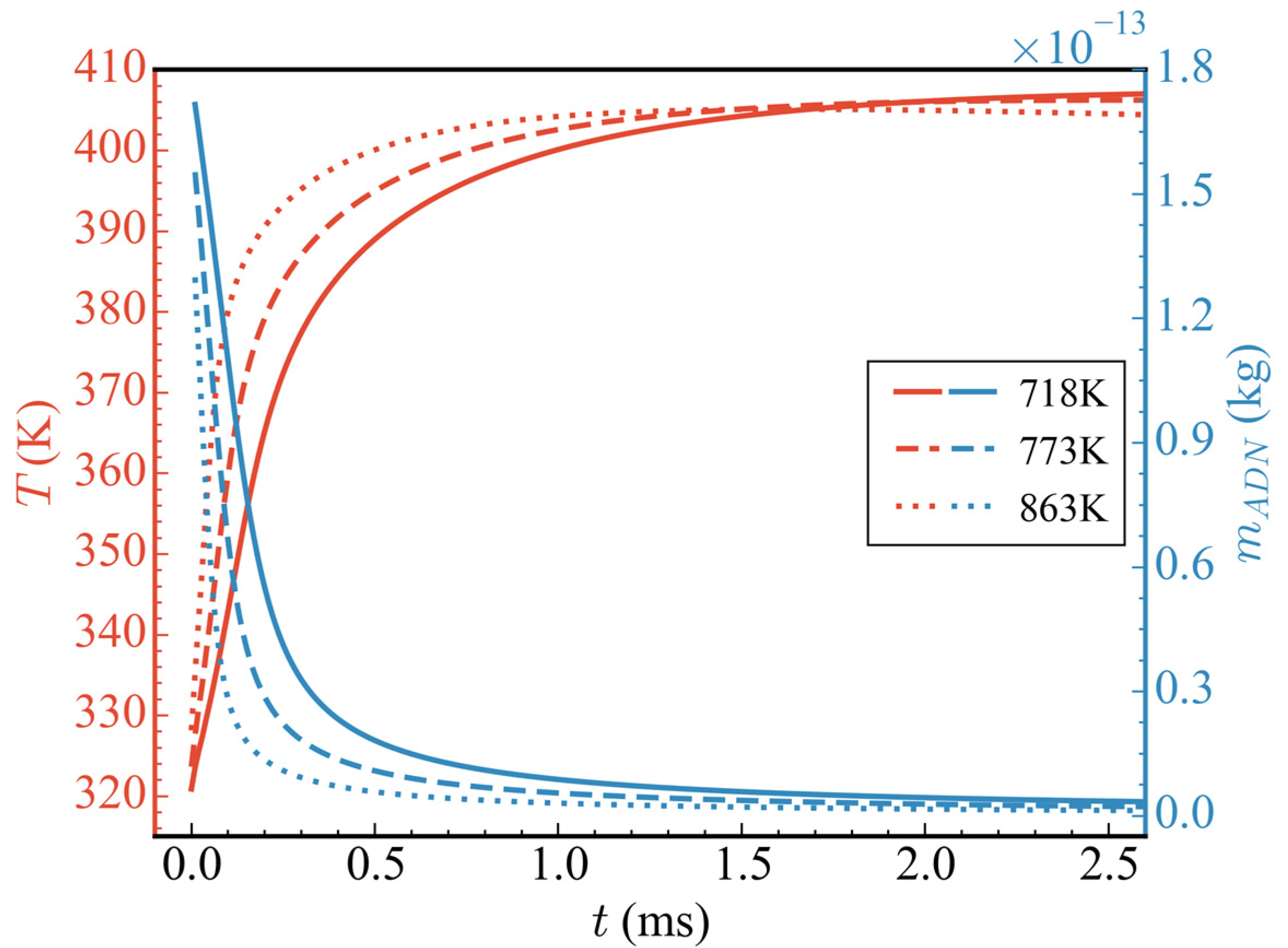
Figure 6 shows the evolution of the influence of different initial temperatures (cases 1/2/3) on the average temperature in the nozzle and the total mass of ADN. In the range of 0–0.3 ms, with the increase in initial temperature, the consumption rate of ADN increased, and the heat release increased, which accelerated the rise rate of the average temperature in the nozzle. After 0.5 ms, the average temperature of case 3 (the initial temperature was 863 K) gradually stabilized, and the temperature in the nozzle gradually began to decrease after 1.5 ms. This is because the initial temperature of case 3 was higher than that of cases 1 and 2, which made ADN reach the average temperature peak in a shorter time. However, due to the lack of subsequent CH3OH participation in the reaction and the continuous outflow of substances in the nozzle, the average temperature in the nozzle decreased. Although the higher the initial temperature of the propellant the faster it reached the average temperature peak, the increase in average temperature became less due to the reduction in initial ADN mass, which in turn led to the reduction in heat release. Specifically, the average temperature increments corresponding to the initial temperatures of 718 K, 773 K, and 863 K are 86.38 K, 82.60 K, and 76.97 K, respectively, with increases of 26.92%, 25.52%, and 23.46%.
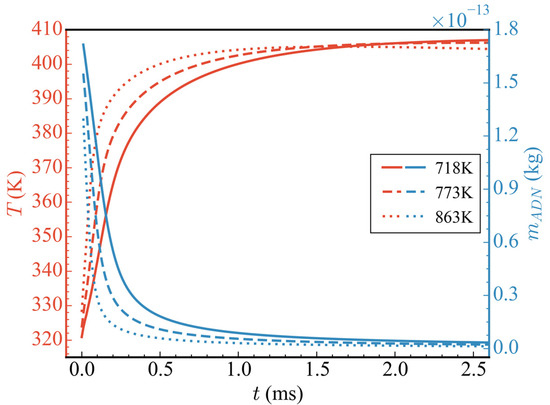
Figure 6.
Temporal evolution of average temperatures and the total mass of ADN at different initial propellant temperatures.
3.3. Effects of Different Initial Pressures on the Combustion Process of an ADN-Based Propellant
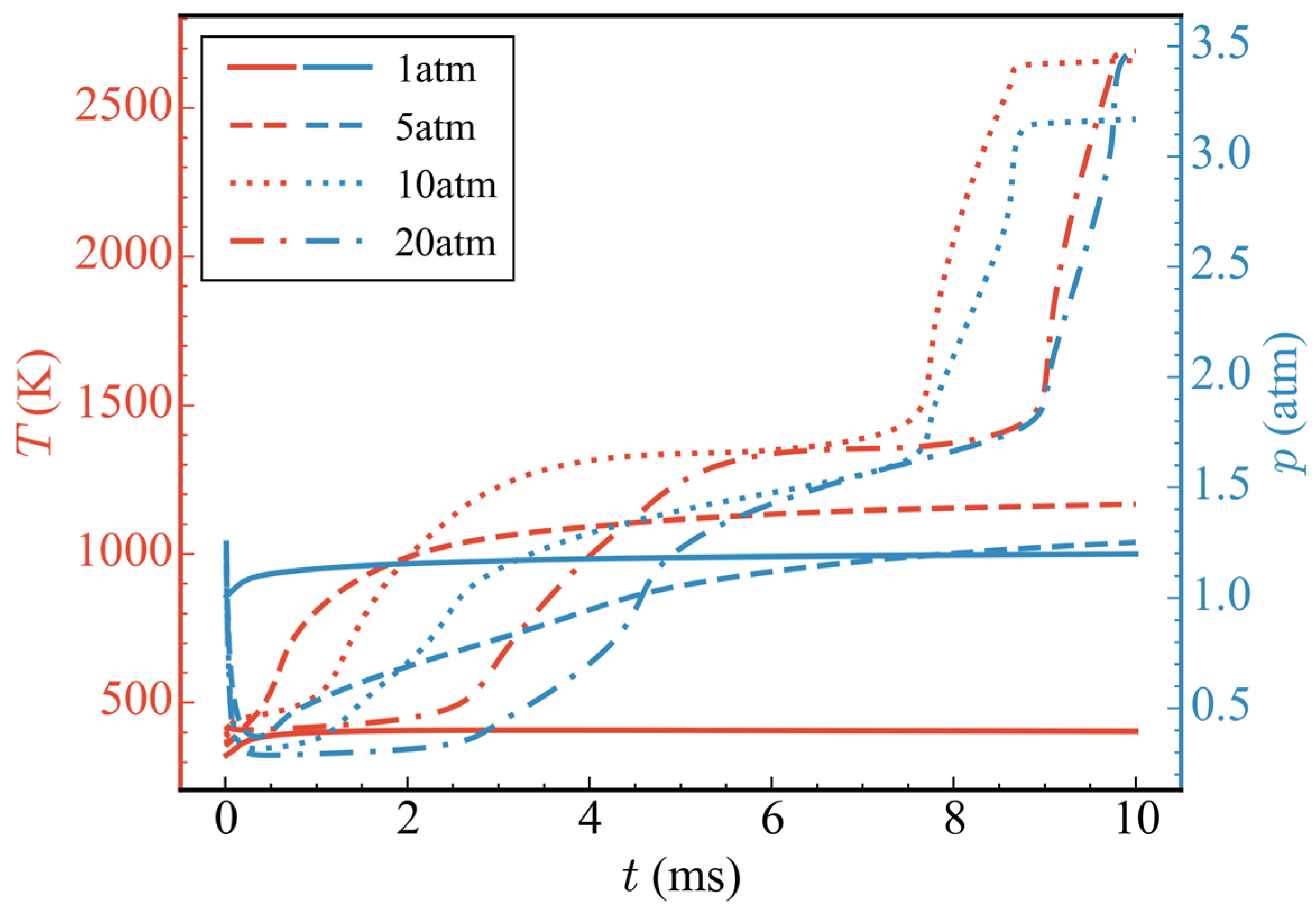
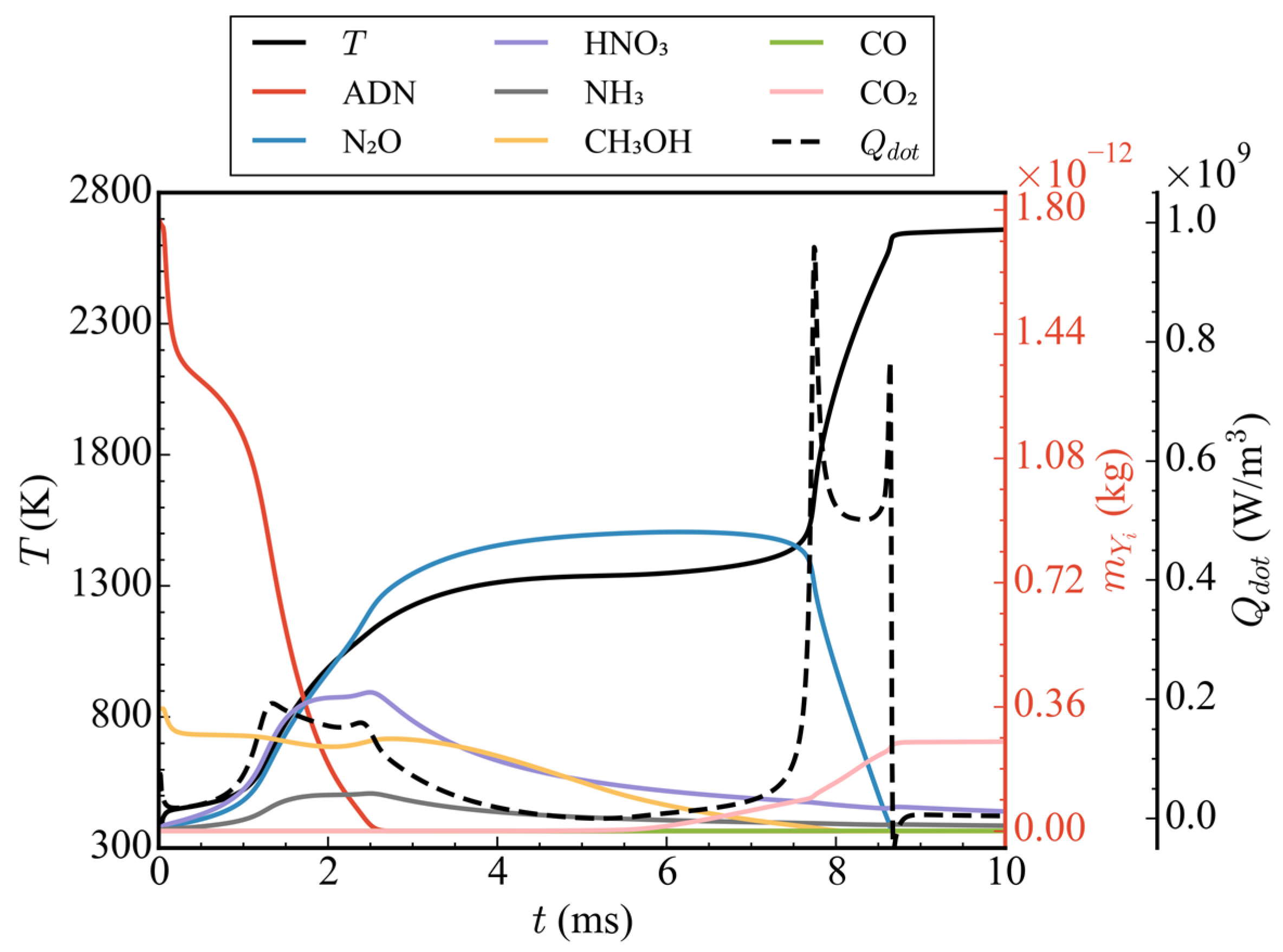
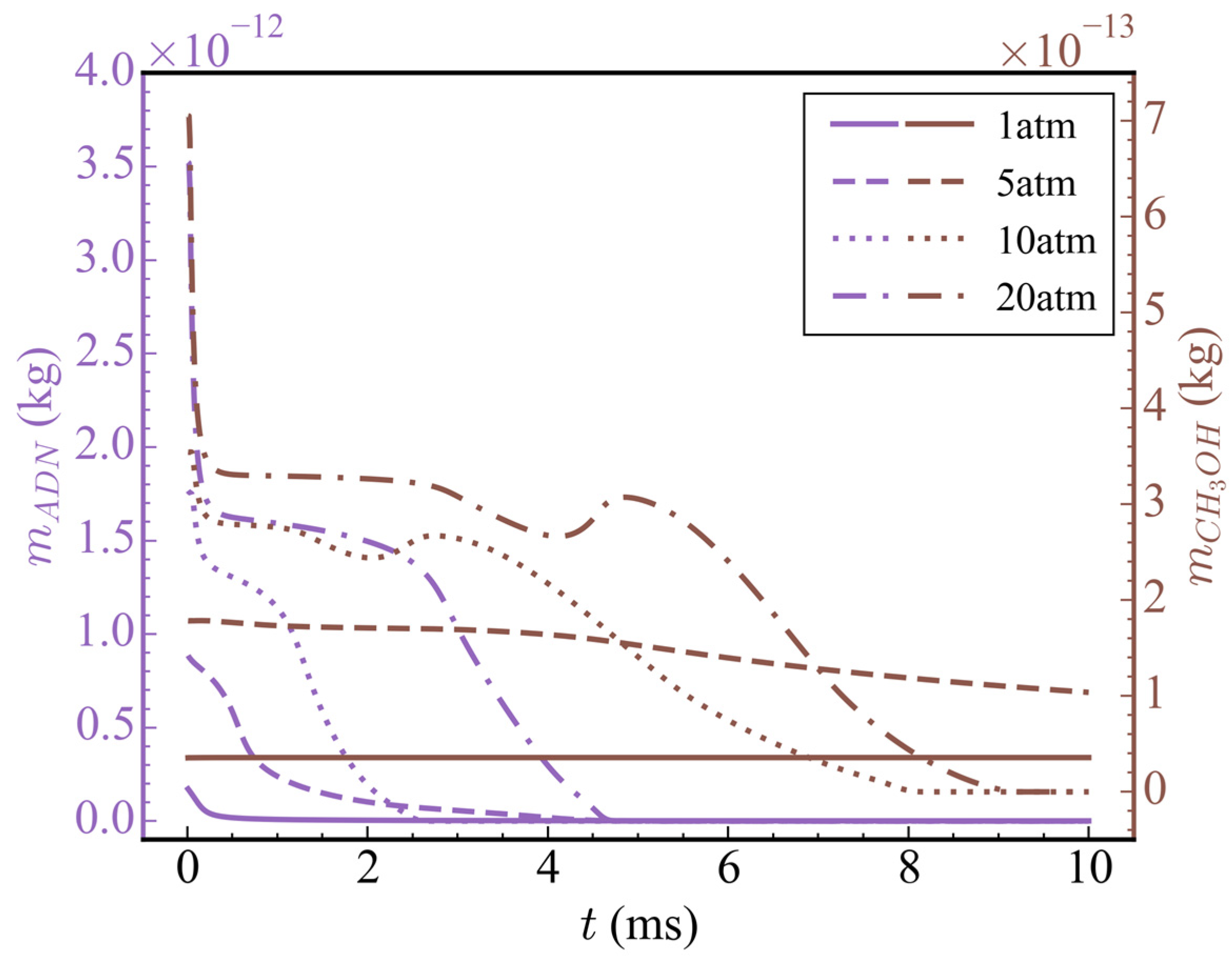
Figure 7 shows the temporal evolution of average temperatures and average pressures in the nozzle at different initial pressures (cases 1/4/5/6). It can be seen from the figure that, when the initial pressure was 1 atm (case 1), because only the ADN pyrolysis reaction took effect and CH3OH did not participate in the subsequent reaction, the average temperature and average pressure increased only slightly before 0.5 ms, and then remained basically unchanged. At 10 ms, the average temperature and average pressure in the nozzle were 403.66 K and 1.20 atm, respectively. When the initial pressure rose to 5 atm (case 4), according to the ideal gas equation, because the initial ADN concentration increased by 4 times and the heat released by the pyrolysis reaction also increased, the final average temperature was higher than that of the initial pressure 1 atm. The average temperature in the nozzle was 1166.64 K at 10 ms. The average pressure decreased rapidly before 0.5 ms, which was due to pressure conduction from the high pressure zone to the low pressure zone, and, finally, the pressure was released at the nozzle outlet. After 0.5 ms, the average pressure gradually increased due to the heat release of the pyrolysis reaction of ADN. As observed in the temperature-pressure curve, there was no intense oxidation reaction of CH3OH at the initial pressure of 5 atm. When the initial pressure was increased to 10 atm (case 5), an obvious three-stage heat release could be observed. The variations in average temperature, average heat release rate, and total mass of ADN/N2O/HNO3/NH3/CH3OH/CO/CO2 with time at the initial pressure is shown in Figure 8. The first stage occurred from 0 to 0.3 ms, during which the partial pyrolysis reaction of ADN took place, resulting in a slight increase in the average temperature inside the nozzle, which then stabilized at around 500 K. The second stage spanned from 1 to 3 ms, during which extensive decomposition of ADN occurred, yielding thermal decomposition products such as N2O, HNO3, and NH3. The average temperature rose to approximately 1300 K, remaining relatively constant from 3 to 7.5 ms. However, during this period, the total mass of CH3OH decreased steadily at a stable rate, indicating oxidation and dehydrogenation reactions with relatively low heat release, as inferred from the average heat release rate. The third exothermic stage spanned from 7.5 to 8.6 ms, during which other nitrogen-containing small molecules, like N2O, were extensively consumed, while CO2 was generated in large quantities through the oxidation of CO. Although CO2 was derived from CO oxidation, the total mass of CO remained relatively low throughout, suggesting rapid consumption upon generation, thus maintaining a low concentration. The average temperature ultimately rose to around 2650 K, with an increase of 558.89% compared to 1 atm. There was a noticeable decrease in average pressure before 1 ms due to the pressure release through conduction, followed by two distinct increases, corresponding to the rise in average temperature. Overall, the consumption of nitrogenous small molecules and the oxidation of CO contributed significantly to the high average heat release rate, playing a crucial role in the elevation of average temperature. It was noteworthy that, when the initial pressure was increased to 20 atm, compared to 10 atm, the secondary heating and pressurization processes occurred later at 7.5 ms and 9 ms, respectively. Although there was no difference in the temperature achieved at each stage, the initial pressure impacted the heat release of the reactions to some extent. This indicated that a higher initial pressure was not necessarily advantageous. When the initial pressure was excessively high, pressure transmission and release at the initial moment led to a lower mean pressure, thereby slowing down the progression of the reaction.
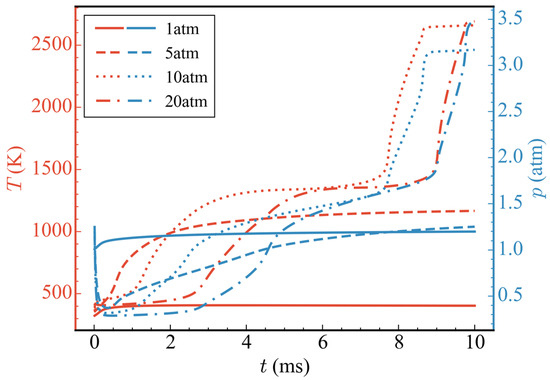
Figure 7.
Temporal evolution of average temperatures and average pressures at different initial propellant pressures.
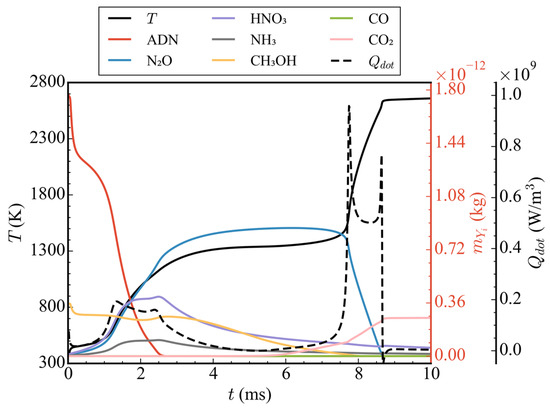
Figure 8.
Time-varying curves of average temperatures, average heat release rates, and the total mass of ADN/N2O/HNO3/NH3/CH3OH/CO/CO2 at the initial propellant pressure of 10 atm.
Figure 9 shows the temporal evolution of the total mass of ADN and CH3OH at different initial pressures. With the increase in initial pressure, the initial total mass of ADN and CH3OH also increased accordingly. Although high pressure was conducive to the reaction near t = 0, the rapid release of high initial pressure created a low-pressure environment in the nozzle, which would slow down the reaction rate after the low-pressure environment was generated. Therefore, the consumption rate of ADN and CH3OH did not increase proportionally with the increase in initial pressure. When the initial pressure was 1 atm, the time for ADN to decompose completely was 0.5 ms; when the initial pressure was 5 atm, the time for ADN to decompose completely was 4.5 ms; and when the initial pressure was 10 atm, the time for ADN to decompose completely was 2.5 ms, which was nearly twice as fast as 5 atm. At an initial pressure of 20 atm, the time for ADN to decompose completely was similar to that at 5 atm. Among the four cases mentioned above, only when the initial pressure was 10 atm or 20 atm, CH3OH was completely consumed, while at 5 atm, it was partially consumed. Based on the changes in average temperature at different initial pressures mentioned earlier, it can be speculated that CH3OH did not undergo a drastic oxidation reaction due to insufficient temperatures or reaction times.
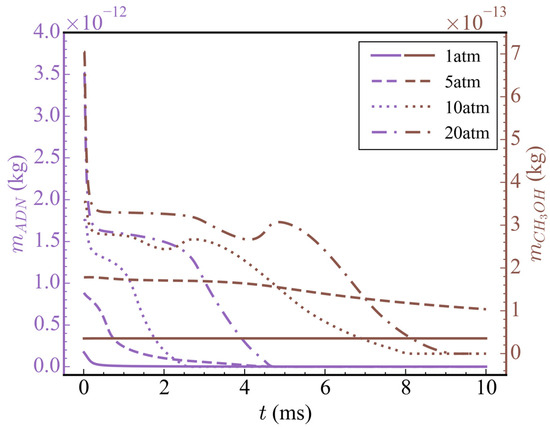
Figure 9.
Temporal evolution of the total mass of ADN and CH3OH at different initial propellant pressures.
3.4. Effects of Different Initial Component Ratios on the Combustion Process of an ADN-Based Propellant
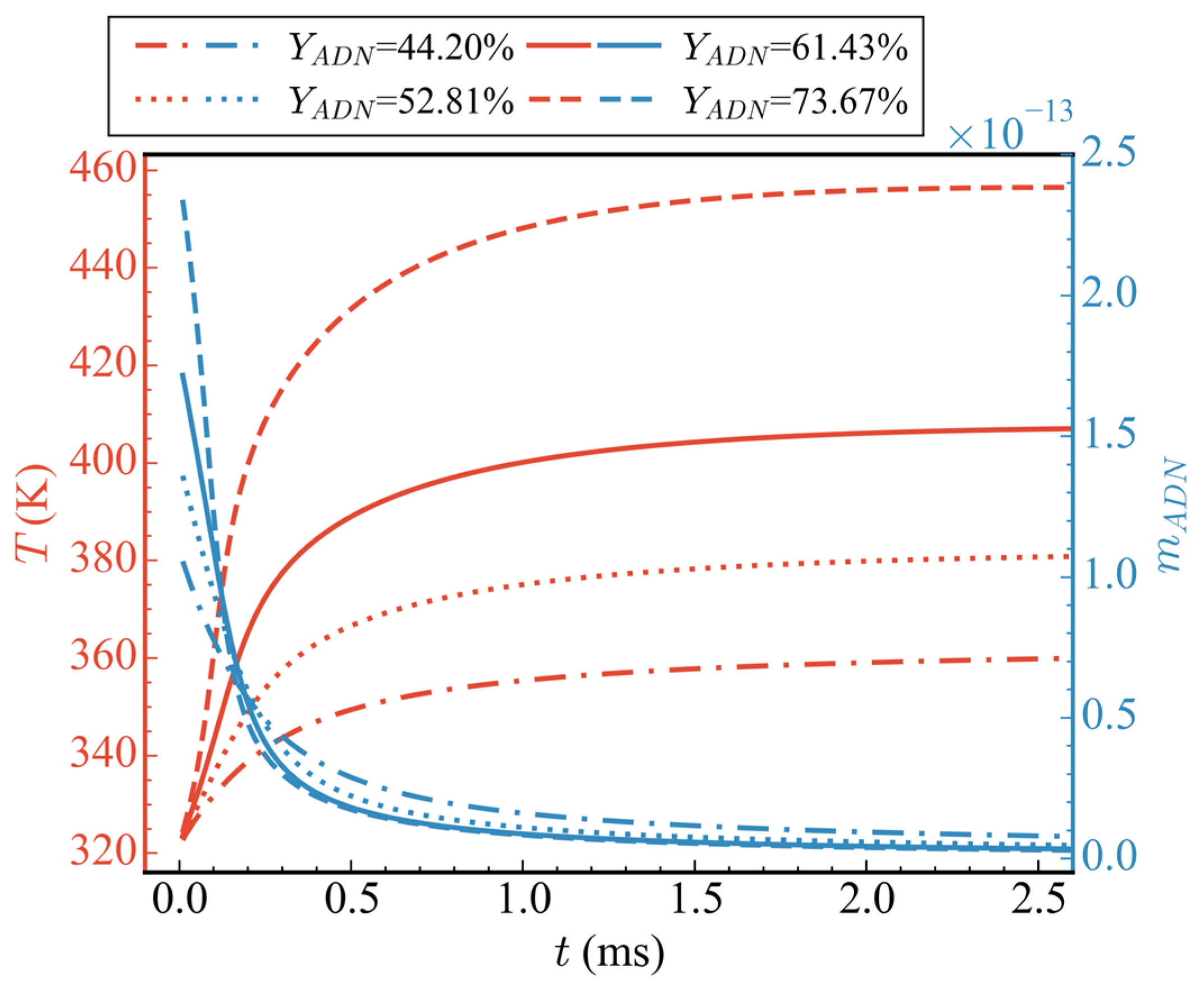
Figure 10 shows the changes in average temperature and total mass of ADN over time for different initial component ratios (cases 1/7/8/9), where the proportion of H2O remained constant and only the proportions of ADN and CH3OH were altered. It can be observed from the figure that, as the proportion of ADN increased, its total mass also increased, the consumption rate was faster, and the rate of increase in average temperature was also faster. The final stable temperature reached was higher as well. At 2.5 ms, the average temperatures corresponding to ADN proportions of 44.20%, 52.81%, 61.43%, and 73.67% in the propellant were 359.83 K, 380.71 K, 406.92 K, and 456.48 K, respectively, with increases of 12.13%, 18.64%, 26.81%, and 42.25%. Inspection of the average temperature curve revealed that, regardless of whether ADN proportion was high or low, no intense oxidation of CH3OH occurred. Consequently, there was no secondary rise in temperature.
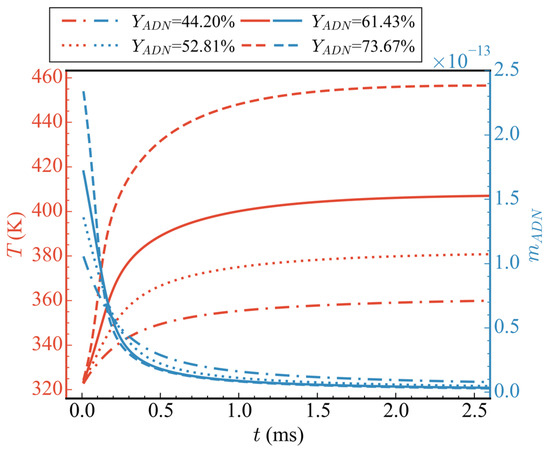
Figure 10.
Temporal evolution of average temperatures and total ADN mass at different initial propellant ratios.
4. Conclusions
In this paper, the combustion of gaseous ADN-based propellants in the nozzle at different initial temperatures, pressures, and propellant ratios was compared and analyzed. In all cases, ADN first decomposed and released heat to generate small nitrogen-containing molecules, such as N2O and NH3. In this process, the average temperature and pressure rose, and the flow in the nozzle accelerated. Through the comparison of different initial conditions, the main conclusions were as follows:
- Both the increase in initial temperature and the increase in the ADN component ratio had a positive effect on the pyrolysis of ADN, which led to the increase in average temperature in the nozzle, but the elevation in initial temperature had a negative effect on the overall rise amplitude of average temperature.
- The initial pressure of propellant was an important factor affecting whether CH3OH reacted. When the initial pressure was 1 atm, CH3OH did not react. When the initial pressure was 5 atm, part of CH3OH reacted, but there was no intense oxidation or heat release. When the initial pressure was 10 atm and 20 atm, CH3OH reacted violently, producing CO2 with a large amount of heat release, resulting in an average temperature of approximately 2650 K, which increased by 558.89% compared with that at 1 atm.
- In the cases of this study, setting a higher initial pressure of the propellant did not necessarily result in faster reaction rates of ADN and CH3OH. In the cases where CH3OH was completely consumed, the reaction time at an initial pressure of 10 atm was shorter than at 20 atm. For situations with higher pressures, the influence of shock waves needs to be taken into consideration.
Author Contributions
Conceptualization, L.J. and J.H.; methodology, B.D.; validation, C.M.; formal analysis, Y.Z.; investigation, L.J.; resources, J.Y.; data curation, H.C.; writing—original draft preparation, L.J.; writing—review and editing, J.H.; visualization, L.J. and C.M.; supervision, J.Y.; project administration, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Li, Y.; Xie, W.; Wang, H.; Yang, H.; Huang, H.; Liu, Y.; Fan, X. Investigation on the Thermal Behavior of Ammonium Dinitramide with Different Copper-Based Catalysts. Prop. Explos. Pyrotech. 2020, 45, 1607–1613. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Bulakh, N.R.; Wagh, R.M.; Talawar, M.B. Synthesis, Characterization and Purity Determination of Ammonium Dinitramide (ADN) and its Precursors. Sci. Technol. Energetic Mater. 2016, 77, 59–64. [Google Scholar]
- Golden, D.M.; McMillen, D.F.; Rossi, M.J. Low Pressure Thermal Decomposition Studies Selected Nitramine and Dinitramine Energetic Materials; ADA247972; SRI International: Menlo Park, CA, USA, 1992. [Google Scholar]
- Tartakovsky, V.A.; Lukyanov, O.A. Synthesis of Dinitramide Salts. In Proceedings of the 25th International Annual Conference of ICT, Karlsruhe, Germany, 28 June–1 July 1994; p. 13. [Google Scholar]
- Bottaro, J.C.; Penwell, P.E.; Schmitt, R.J. 1,1,3,3-Tetraoxo-1,2,3-triazapropene Anion, a New Oxy Anion of Nitrogen: The Dinitramide Anion and Its Salts. J. Am. Chem. Soc. 1997, 119, 9405–9410. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W.; Petrie, M.A.; Michels, H.H.; Bottaro, J.C.; Gilardi, R. The Dinitramide Anion, N(NO2)2−. Inorg. Chem. 1996, 35, 5068–5071. [Google Scholar] [CrossRef] [PubMed]
- Östmark, H.; Bemm, U.; Langlet, A.; Sandén, R.; Wingborg, N. The Properties of Ammonium Dinitramide (ADN): Part 1, Basic Properties and Spectroscopic Data. J. Energetic Mater. 2000, 18, 123–138. [Google Scholar] [CrossRef]
- Wingborg, N. Ammonium Dinitramide−Water: Interaction and Properties. J. Chem. Eng. Data 2006, 51, 1582–1586. [Google Scholar] [CrossRef]
- Wingborg, N.; Eldsater, C.; Skifs, H. Formulation and Characterization of ADN-Based Liquid Monopropellants. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion, Sardinia, Italy, 7–8 June 2004. [Google Scholar]
- Ide, Y.; Takahashi, T.; Iwai, K.; Nozoe, K.; Habu, H.; Tokudome, S. Potential of ADN-based Ionic Liquid Propellant for Spacecraft Propulsion. Procedia Eng. 2015, 99, 332–337. [Google Scholar] [CrossRef]
- Jing, L.; Huo, J.; Yao, Z.; You, X.; Zhu, M. Numerical Investigation of an Aerospace Thruster with ADN-Based Liquid Propellant. J. Tsinghua Univ. 2016, 56, 1085–1090. [Google Scholar] [CrossRef]
- Zhang, T.; Li, G.; Yu, Y.; Sun, Z.; Wang, M.; Chen, J. Numerical Simulation of Ammonium Dinitramide (ADN)-Based Non-Toxic Aerospace Propellant Decomposition and Combustion in a Monopropellant Thruster. Energy Conv. Manag. 2014, 87, 965–974. [Google Scholar] [CrossRef]
- Li, L.; Li, G.-X.; Li, H.-M.; Yao, Z.-P. Effect of Voltage and Droplet Size on Electrical Ignition Characteristics of ADN-Based Liquid Propellant Droplet. Aerosp. Sci. Technol. 2019, 93, 105314. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Li, H.; Yao, Z. Experimental Study on Electrical Ignition Characteristics of Ammonium Dinitramide based Propellant under Different Environmental Pressures. Prop. Explos. Pyrotech. 2020, 45, 1056–1065. [Google Scholar] [CrossRef]
- Li, L.; Li, G.-X.; Li, H.-M.; Yao, Z.-P.; Zhang, T. Study on Electrical Ignition Characteristics of Ammonium Dinitramide (ADN)-Based Liquid Propellant Droplet in Nitrous Oxide Environment. Sci. Technol. Energetic Mater. 2023, 84, 72–79. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, Y.; Li, Y.; Liu, X.; Wang, X. Experimental Study on Microwave-Induced Puffing, Micro-Explosion, and Combustion Characteristics of Ammonium Dinitramide-Based Liquid Propellant Droplets. Phys. Fluids 2023, 35, 117122. [Google Scholar] [CrossRef]
- Negri, M.; Hendrich, C.; Wilhelm, M.; Freudenmann, D.; Ciezki, H.K.; Gediminas, L.; Adelöw, L. Thermal Ignition of ADN-based Propellants. In Proceedings of the Space Propulsion 2016, Rome, Italy, 2–6 May 2016; p. SP2016_3125004. [Google Scholar]
- Negri, M.; Wilhelm, M.; Ciezki, H.K. Thermal Ignition of ADN-Based Propellants. Propellants Explos. Pyrotech. 2019, 44, 1096–1106. [Google Scholar] [CrossRef]
- Du, B.; Zheng, Y.; Mao, C.; Cui, H.; Han, J.; Jiang, L.; Ye, J.; Hong, Y. Transmissive Mode Laser Micro-Ablation Performance of Ammonium Dinitramide-Based Liquid Propellant for Laser Micro-Thruster. Micromachines 2023, 14, 1219. [Google Scholar] [CrossRef] [PubMed]
- Velardez, G.F.; Alavi, S.; Thompson, D.L. Molecular Dynamics Studies of Melting and Liquid Properties of Ammonium Dinitramide. J. Chem. Phys. 2003, 119, 6698–6708. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, T.; Zhu, W.; Xiao, H. Comparative DFT Study of Crystalline Ammonium Perchlorate and Ammonium Dinitramide. J. Phys. Chem. A 2008, 112, 4688–4693. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-S.; Li, G.-X.; Zhang, T.; Chen, J.; Wang, M. Effects of Catalyst-Bed’s Structure Parameters on Decomposition and Combustion Characteristics of an Ammonium Dinitramide (ADN)-Based Thruster. Energy Conv. Manag. 2015, 106, 566–575. [Google Scholar] [CrossRef]
- Jing, L.; Huo, J.; Wang, H.; You, X.; Zhu, M.; Yang, Y.; Yao, Z. Experimental Investigation on the Evaporation and Combustion Processes of Ammonium-Dinitramide-Based Liquid Propellant. J. Propul. Power 2017, 33, 343–349. [Google Scholar] [CrossRef]
- Jing, L.; You, X.; Huo, J.; Zhu, M.; Yao, Z. Experimental and Numerical Studies of Ammonium Dinitramide Based Liquid Propellant Combustion in Space Thruster. Aerosp. Sci. Technol. 2017, 69, 161–170. [Google Scholar] [CrossRef]
- Liu, X.; Su, G.; Yao, Z.; Yan, Z.; Yu, Y. Numerical Study of Flow Boiling of ADN-Based Liquid Propellant in a Capillary. Materials 2023, 16, 1858. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Gonzalez-Juez, E.; Haworth, D.C. Flame Simulations with an Open-source Code. Comput. Phys. Commun. 2019, 237, 219–229. [Google Scholar] [CrossRef]
- Gutiérrez Marcantoni, L.F.; Tamagno, J.; Elaskar, S. Rhocentralrffoam: An Openfoam Solver for High Speed Chemically Active Flows—Simulation of Planar Detonations. Comput. Phys. Commun. 2017, 219, 209–222. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, M.; Xu, Y.; Li, G.; Zhang, H. Eulerian-Lagrangian Modelling of Detonative Combustion in Two-phase Gas-droplet Mixtures with OpenFOAM: Validations and Verifications. Fuel 2021, 286, 119402. [Google Scholar] [CrossRef]
- Rizvi, M.S. Detailed Numerical Comparison of Laminar Burning Speed of Stratified Hydrogen–Air and Methane–Air Mixture with Corresponding Homogeneous Mixture Using Open-Source Code. J. Energy Resour. Technol. 2021, 143, 102303. [Google Scholar] [CrossRef]
- Issa, R.I. Solution of the Implicitly Discretised Fluid Flow Equations by Operator-Splitting. J. Comput. Phys. 1986, 62, 40–65. [Google Scholar] [CrossRef]
- Poinsot, T.; Veynante, D. Theoretical and Numerical Combustion, 3rd ed.; R.T. Edwards, Inc.: Philadelphia, PA, USA, 2012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).