Demonstration of Polyethylene Nitrous Oxide Catalytic Decomposition Hybrid Thruster with Dual-Catalyst Bed Preheated by Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAQ | Data Acquisition |
| HDPE | High-Density Polyethylene |
| MFC | Mass Flow Controller |
| MFM | Mass Flow Meter |
| PE | Polyethylene |
| PLC | Programmable Logic Controller |
Nomenclature
| area | |
| characteristic velocity | |
| diameter | |
| length | |
| length to diameter ratio | |
| mass flow rate | |
| quantity | |
| pressure | |
| regression rate | |
| burning time | |
| volume | |
| fluctuation | |
| decomposition capacity | |
| efficiency | |
| density | |
| Superscript | |
| average | |
| Subscript | |
| catalyst bed | |
| combustion chamber | |
| experimental | |
| fuel | |
| maximum | |
| minimum | |
| nozzle throat | |
| preheatant | |
| port | |
| post | |
| theoretical |
References
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements, 9th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zakirov, V.; Sweeting, M.; Lawrence, T.; Sellers, J. Nitrous Oxide as a Rocket Propellant. Acta Astronaut. 2001, 48, 353–362. [Google Scholar] [CrossRef]
- Nosseir, A.E.S.; Cervone, A.; Pasini, A. Review of State-of-the-Art Green Monopropellants: For Propulsion Systems Analysts and Designers. Aerospace 2021, 8, 20. [Google Scholar] [CrossRef]
- Gallo, G.; Di Martino, G.D.; Festa, G.; Savino, R. Experimental Investigation of N2O Decomposition with Pd/Al2O3 Cylindrical Pellets Catalyst. J. Spacecr. Rocket. 2020, 57, 720–727. [Google Scholar] [CrossRef]
- Gallo, G.; Kamps, L.; Hirai, S.; Carmicino, C.; Harunori, N. Prediction of the fuel regression-rate in a HDPE single port hybrid rocket fed by liquid nitrous oxide. Combust. Flame 2024, 259, 113160. [Google Scholar] [CrossRef]
- Okuda, R.; Komizu, K.; Tsuji, A.; Miwa, T.; Fukada, M.; Yokobori, S.; Soeda, K.; Kamps, L.; Nagata, H. Fuel Regression Characteristics of Axial-Injection End-Burning Hybrid Rocket Using Nitrous Oxide. J. Propuls. Power 2022, 38, 759–770. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T. Study on Endurance and Performance of Impregnated Ruthenium Catalyst for Thruster System. J. Nanosci. Nanotechnol. 2018, 18, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Zakirov, V.; Zhang, H.-Y. A model for the operation of nitrous oxide monopropellant. Aerosp. Sci. Technol. 2008, 12, 318–323. [Google Scholar] [CrossRef]
- D’Elia, R.; Bernhart, G.; Hijlkema, J.; Cutard, T. Experimental analysis of SiC-based refractory concrete in hybrid rocket nozzles. Acta Astronaut. 2016, 126, 168–177. [Google Scholar] [CrossRef]
- Tokudome, S.; Goto, K.; Yagishita, T.; Suzuki, N.; Yamamoto, T. An Experimental Study of a Nitrous Oxide/Ethanol (NOEL) Propulsion System. In Proceedings of the AIAA Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019; pp. 1–12. [Google Scholar] [CrossRef]
- Jung, E.S.; Kwon, S. Autoignitable and Restartable Hybrid Rockets Using Catalytic Decomposition of an Oxidizer. J. Propuls. Power 2014, 30, 514–518. [Google Scholar] [CrossRef]
- Hendley, C.T.; Connell, T.L., Jr.; Wilson, D.; Young, G. Catalytic Decomposition of Nitrous Oxide for Use in Hybrid Rocket Motors. J. Propuls. Power 2021, 37, 474–478. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.; Kwon, S. High performance microthruster with ammonium-dinitramide-based monopropellant. Sens. Actuators A Phys. 2018, 283, 211–219. [Google Scholar] [CrossRef]
- Heo, S.; Jo, S.; Yun, Y.; Kwon, S. Effect of dual-catalytic bed using two different catalyst sizes for hydrogen peroxide thruster. Aerosp. Sci. Technol. 2018, 78, 26–32. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, J.; Jung, E.; Kwon, S. Measurement of fuel port pressure distribution by exposed solid fuel hydrogen peroxide catalytic decomposition hybrid thruster. Acta Astronaut. 2024, 219, 71–78. [Google Scholar] [CrossRef]
- Available online: https://commonchemistry.cas.org/ (accessed on 20 December 2024).
- Gordon, S.; McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications I. Analysis; NASA RP-1311; NASA Lewis Research Center: Cleveland, OH, USA, 1994. [Google Scholar]
- McBride, B.J.; Gordon, S. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications II; Users Manual and Program Description; NASA RP-1311; NASA Lewis Research Center: Cleveland, OH, USA, 1996. [Google Scholar]
- Mechentel, F.S.; Cantwell, B.J. Experimental Findings on Pre- and Post-combustion Chamber Effects in a Laboratory-scale Motor. In Proceedings of the AIAA Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Gontijo, M.S.; Filho, R.B.N.; Domingos, C.H.F.L. Design of Pre-Combustion Chambers for Hybrid Propellant Rocket Motors and Related Aspects. In Proceedings of the AIAA SciTech 2023 Forum, National Harbor, MD, USA, 23–27 January 2023; pp. 1–16. [Google Scholar] [CrossRef]
- Ghadiri, S.; Miller, J.C.; Nalim, M.R. 3-D Simulation of Combustion in a Pre-Chamber for Jet Ignition of a Constant-Volume Combustor. In Proceedings of the AIAA Aviation Forum and ASCEND 2024, Las Vegas, NV, USA, 29 July–2 August 2024; Volume 2024, pp. 1–21. [Google Scholar] [CrossRef]
- Swami, R.D.; Gany, A. Analysis and testing of similarity and scale effects in hybrid rocket motors. Acta Astronaut. 2003, 52, 619–628. [Google Scholar] [CrossRef]
- Cai, G.; Zeng, P.; Li, X.; Tian, H.; Yu, N. Scale effect of fuel regression rate in hybrid rocket motor. Aerosp. Sci. Technol. 2013, 24, 141–146. [Google Scholar] [CrossRef]
- Shan, F.; Hou, L.; Piao, Y. Combustion performance and scale effect from N2O/HTPB hybrid rocket motor simulations. Acta Astronaut. 2013, 85, 1–11. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, Y.-Y.; Liu, W.-L.; Xu, B.-J.; Ge, J.-R.; Xuan, X.-C.; Jen, T.-C. Numerical investigation of scale effect of various injection diameters on interaction in cold kerosene-fueled supersonic flow. Acta Astronaut. 2016, 129, 111–120. [Google Scholar] [CrossRef]
- Liang, C.; Sun, M.; Huang, Y.; Liu, Y.; Zhao, G.; Yang, Y.; Zhu, J.; Wang, H.; Li, F.; Ma, G. Scale effect of gas injection into a supersonic crossflow. Aerosp. Sci. Technol. 2021, 117, 106947. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, C.; Li, F.; Ma, G.; Liu, M.; Yang, Y.; Wang, H.; Sun, M. On scale effect of supersonic combustion in axisymmetric combustor. Aerosp. Sci. Technol. 2024, 115, 109566. [Google Scholar] [CrossRef]

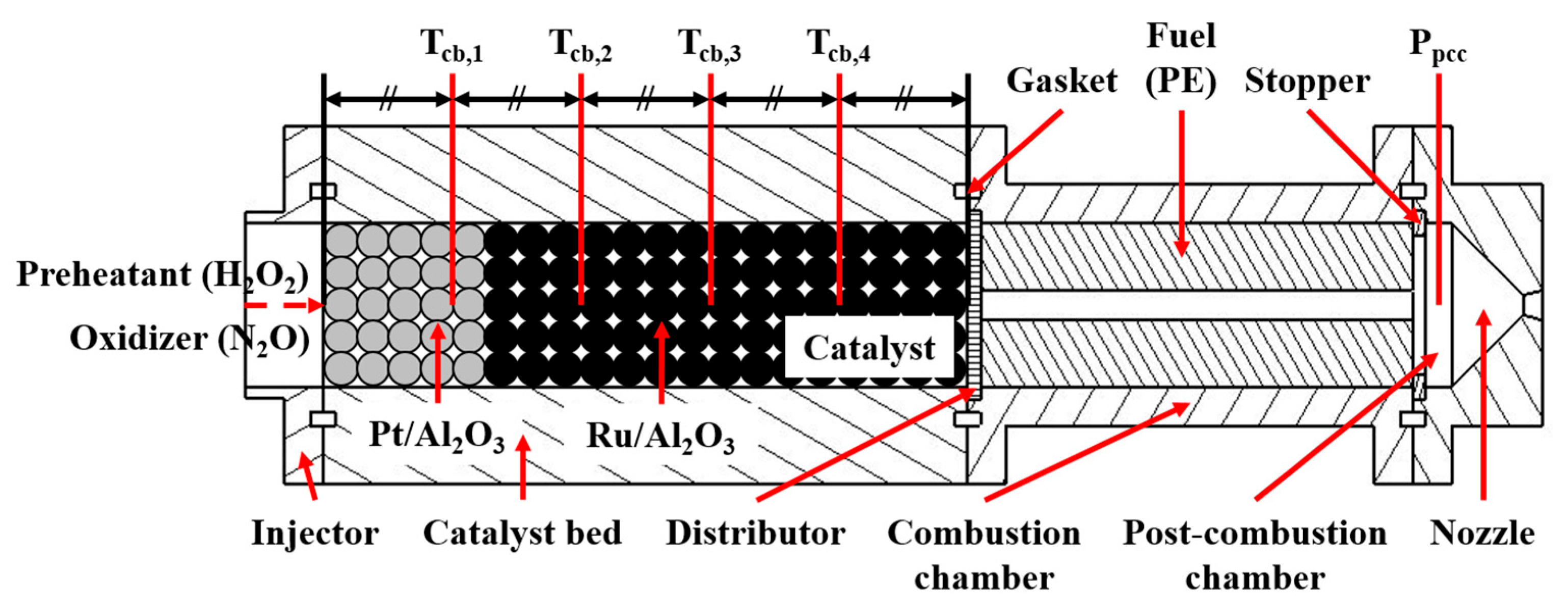
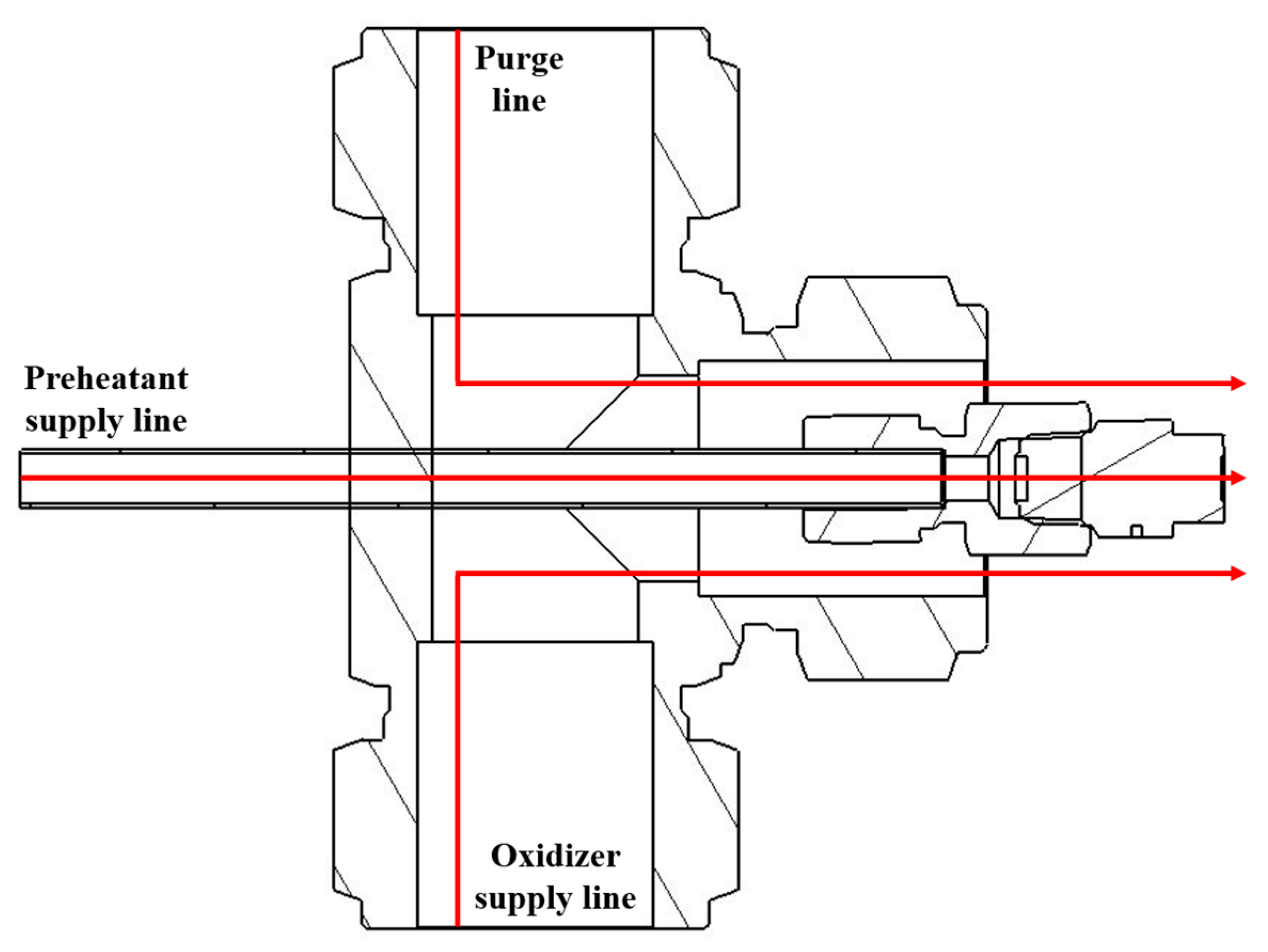
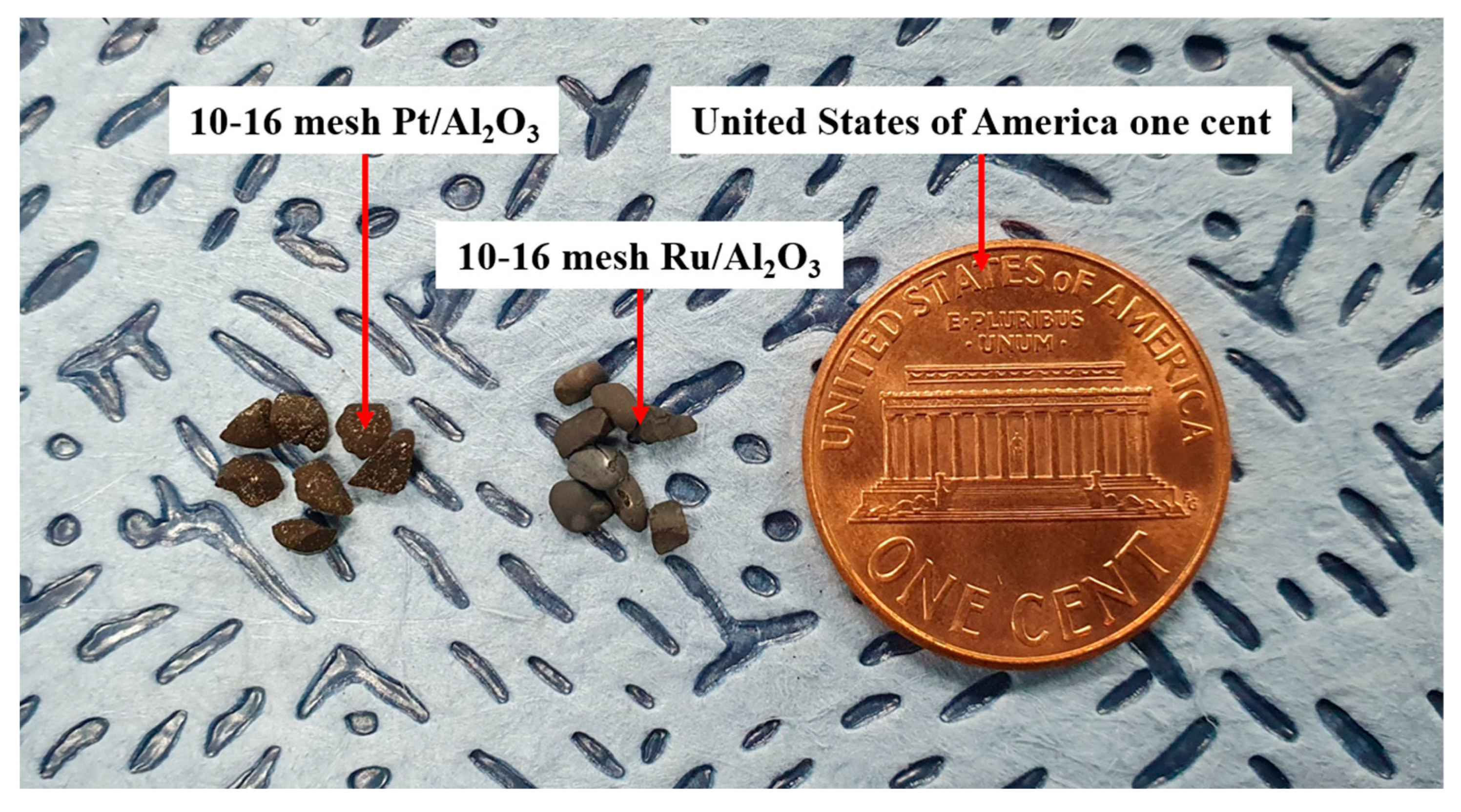
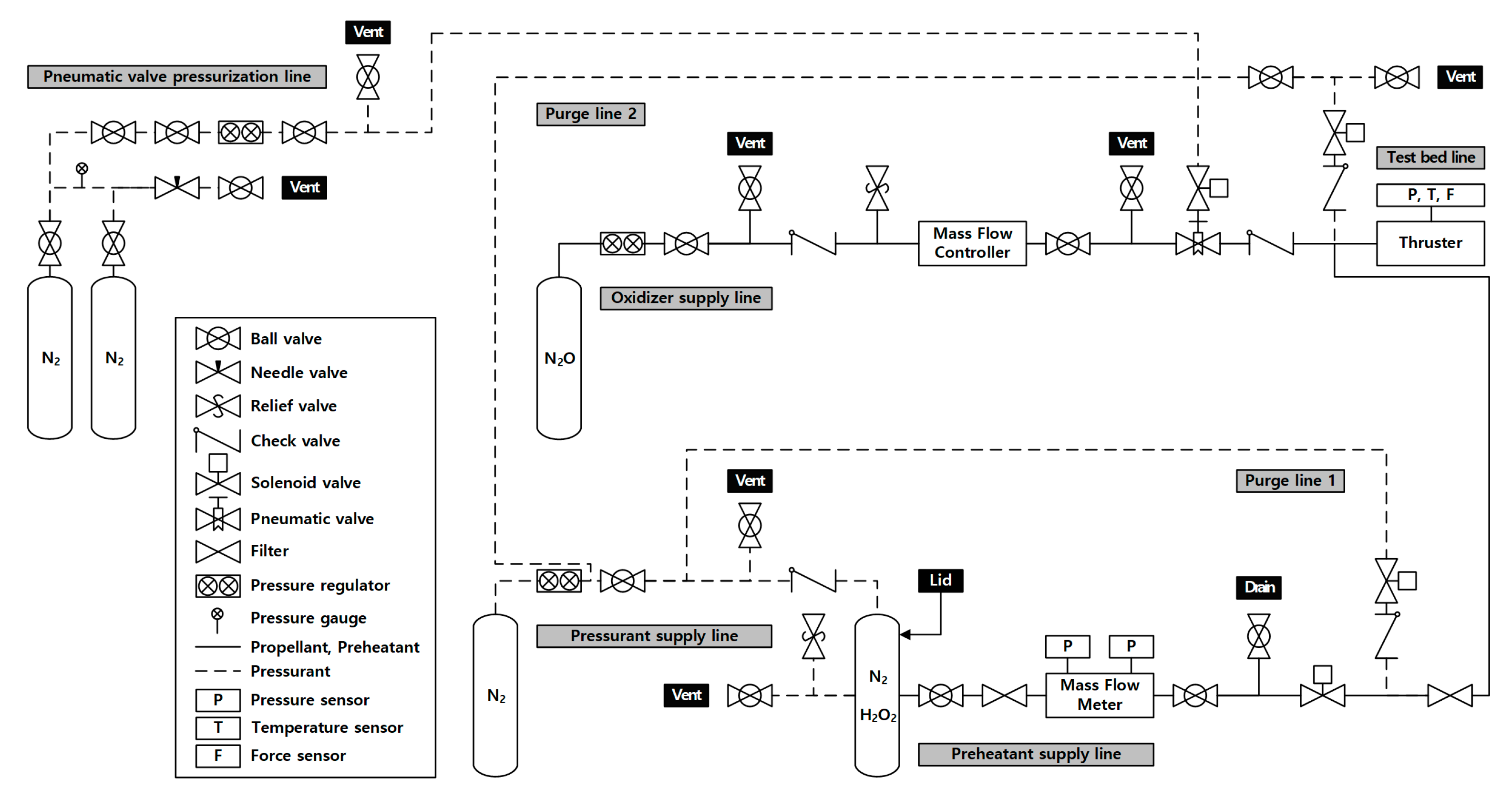
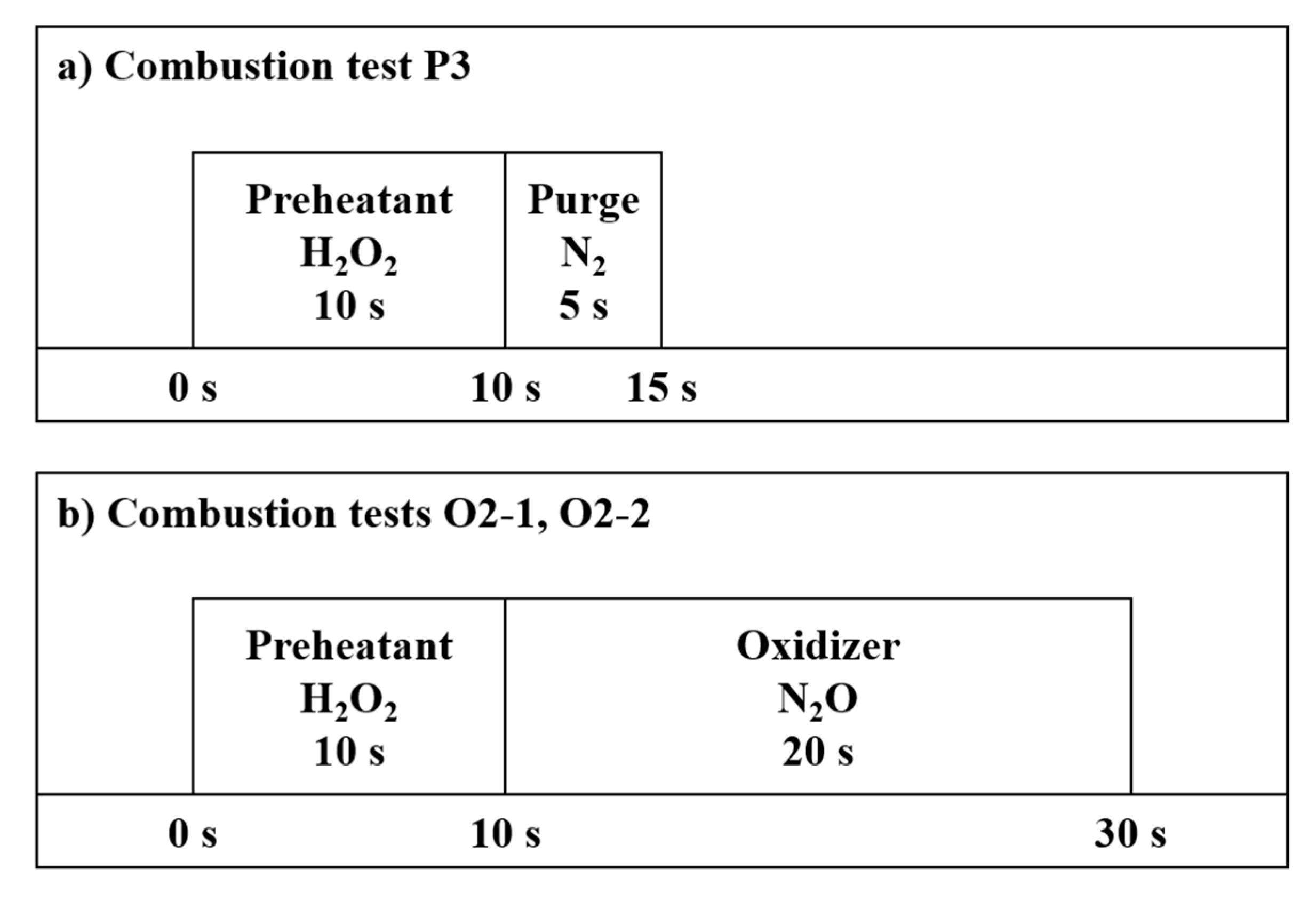
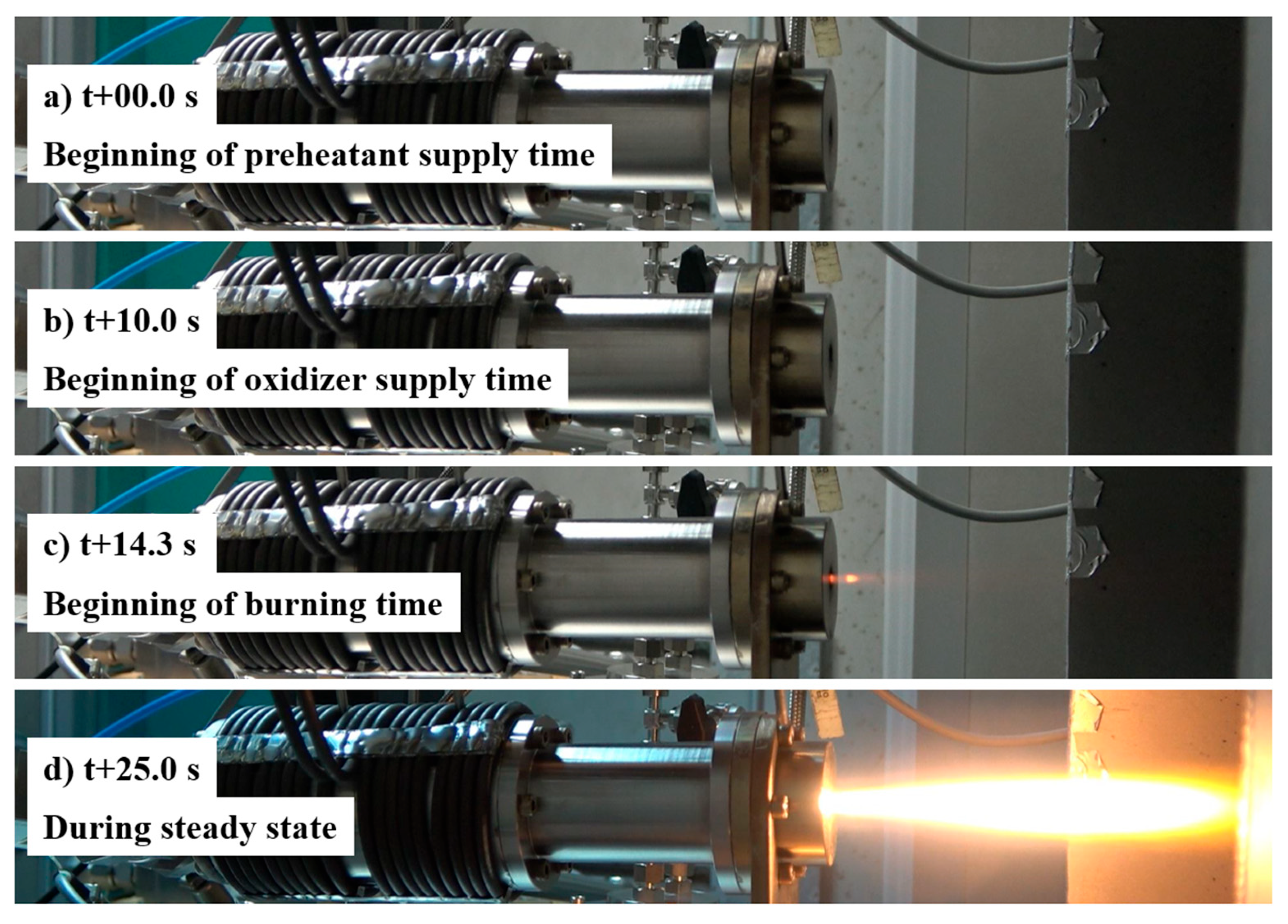
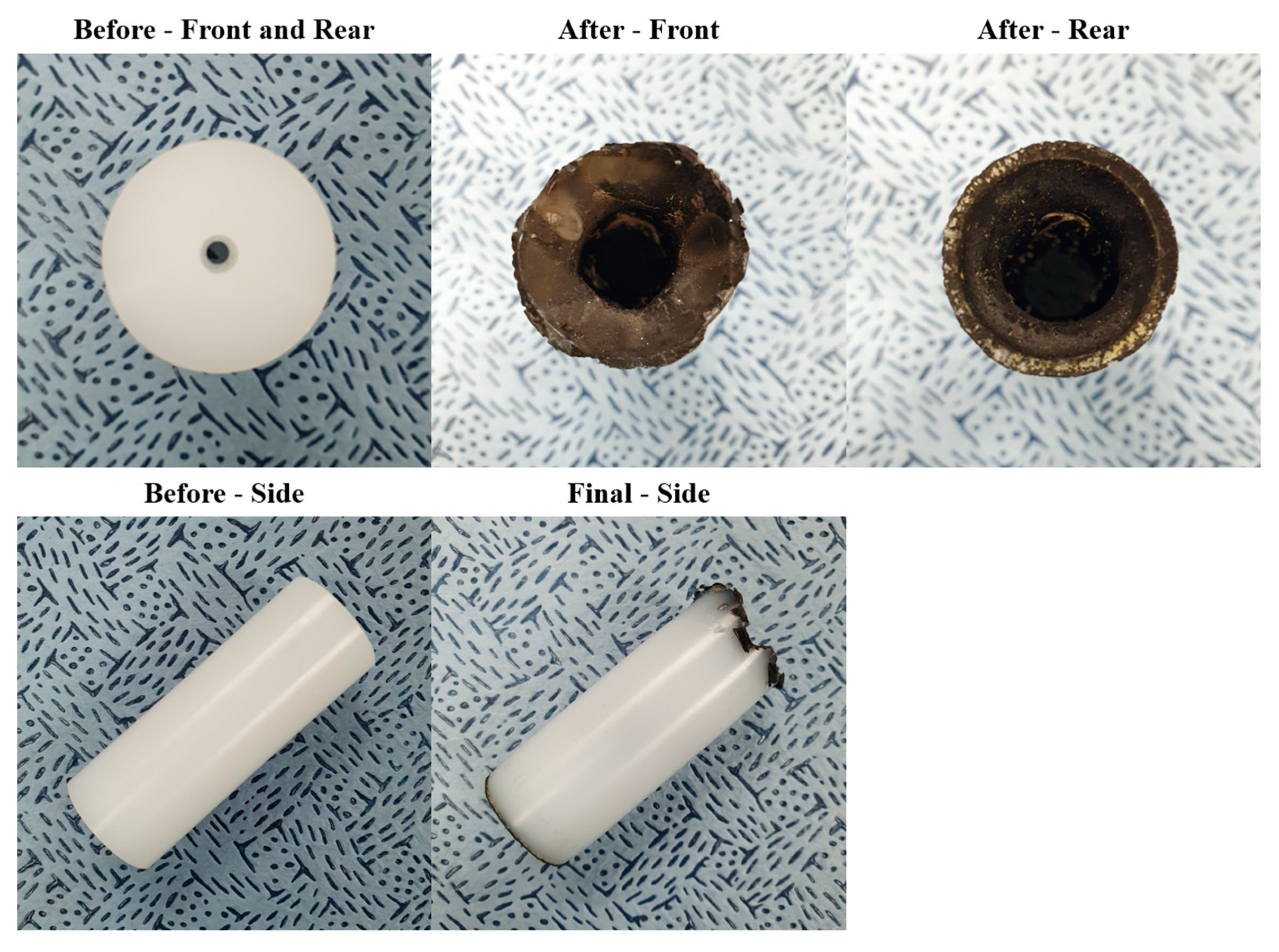
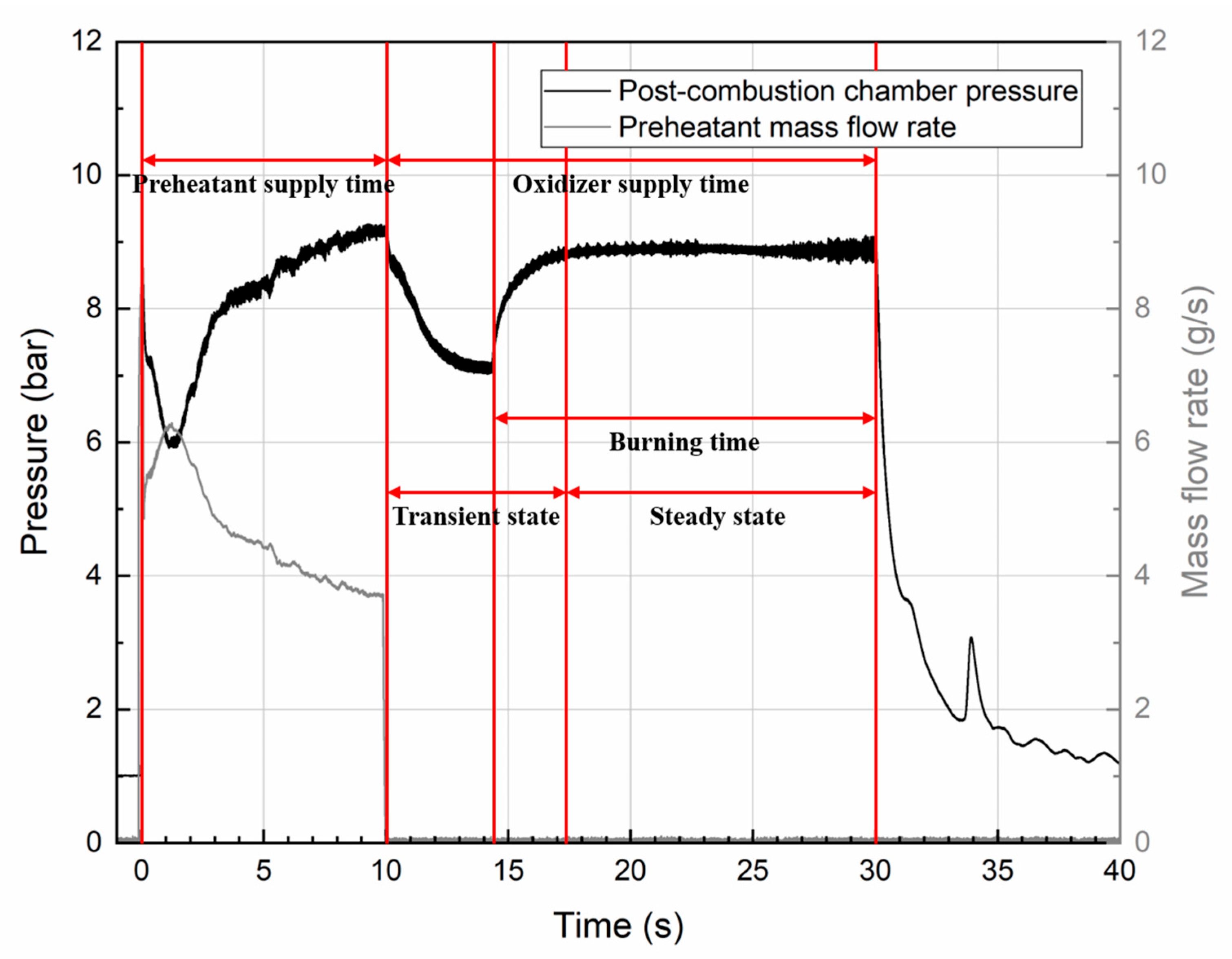
| Parameter | Value |
|---|---|
| Fuel type | PE |
| Oxidizer type | N2O |
| Preheatant type | H2O2 (+H2O) |
| Preheatant concentration | 90 wt.% |
| Oxidizer to fuel mass ratio | 6 |
| Combustion chamber pressure | 10 bar |
| Specific impulse (Sea level) | 2070.4 m/s, 211.1 s |
| Characteristic velocity | 1633.3 m/s |
| Nozzle expansion ratio | 2.3 |
| Thrust | 10 N |
| Fuel mass flow rate | 0.69 g/s |
| Oxidizer mass flow rate | 4.14 g/s |
| Characteristic velocity efficiency | 0.95 |
| Nozzle throat diameter | 3.1 mm |
| Nozzle exit diameter | 4.7 mm |
| Nozzle type | Cone |
| Nozzle convergence half-angle | 45 deg |
| Nozzle divergence half-angle | 15 deg |
| Catalyst bed diameter | 25.4 mm |
| Catalyst bed length | 100 mm |
| Preheatant catalyst bed decomposition capacity | 1.27 g/s/cm3 |
| Preheatant catalyst bed length to diameter ratio | 1 |
| Preheatant catalyst bed length | 25.4 mm |
| Maximum decomposable preheatant mass flow rate | 16.3 g/s |
| Nozzle throat to fuel port total area ratio | 0.5 |
| Fuel port quantity | 1 |
| Fuel port diameter | 4.4 mm |
| Combustion chamber diameter | 25.4 mm |
| Average fuel regression rate | 0.7 mm/s |
| Burning time | 15 s |
| Fuel density | 974.5 kg/m3 |
| Fuel port length | 66.9 mm |
| Parameter | Value | |
|---|---|---|
| Catalyst type | Pt/Al2O3 | Ru/Al2O3 |
| Catalyst target | Preheatant, H2O2 | Oxidizer, N2O |
| Catalyst volume fraction | 25 vol.% | 75 vol.% |
| Catalyst form | Pellet | Pellet |
| Catalyst size | 10–16 mesh, 1.19–2.00 mm | 10–16 mesh, 1.19–2.00 mm |
| Catalyst active material mass fraction | 20.4 wt.% | 21.1 wt.% |
| Parameter | Value | |||||
|---|---|---|---|---|---|---|
| Combustion test name | P1 | P2 | P3 | O1 | O2-1 | O2-2 |
| Preheatant supply time (s) | 2 | 5 | 10 | 10 | 10 | 10 |
| Purge time (s) | 5 | 5 | 5 | 0 | 0 | 0 |
| Oxidizer supply time (s) | 0 | 0 | 0 | 15 | 20 | 20 |
| Parameter | Value | ||
|---|---|---|---|
| Combustion test name | P1 | P2 | P3 |
| Preheatant mass flow rate (g/s) | 5.16 | 4.22 | 4.24 |
| Maximum catalyst bed temperature during preheatant supply time (°C) | 166 ± 0.32 | 273 ± 0.52 | 655 ± 1.24 |
| Combustion during preheatant supply time | No | No | No |
| Parameter | Value | ||
|---|---|---|---|
| Combustion test name | O1 | O2-1 | O2-2 |
| Preheatant mass flow rate (g/s) | 4.57 | 4.63 | 4.27 |
| Maximum catalyst bed temperature during preheatant supply time (°C) | 557 ± 1.06 | 583 ± 1.11 | 521 ± 0.99 |
| Combustion during preheatant supply time | No | No | No |
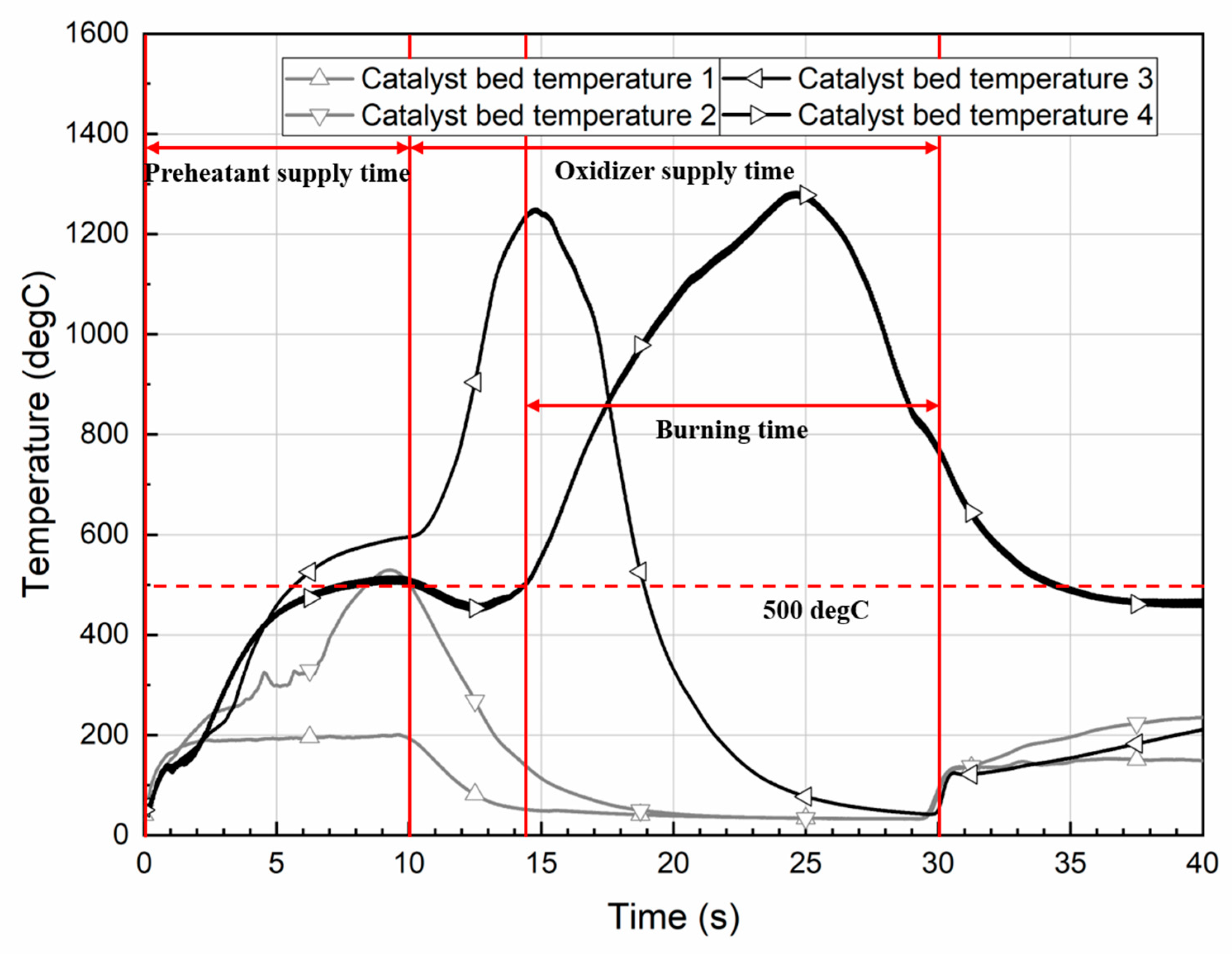
| Burning time (s) | 10.3 | 15.7 | 15.8 |
| Post-combustion chamber pressure 1 (bar) | 8.99 ± 0.01 | 8.88 ± 0.01 | 10.80 ± 0.02 |
| Post-combustion chamber pressure fluctuation 1 | 0.01 | 0.05 | 0.03 |
| Oxidizer mass flow rate 2 (g/s) | 3.97 ± 0.01 | 4.05 ± 0.01 | 4.68 ± 0.01 |
| Fuel mass flow rate 2 (g/s) | 0.80 | 0.94 | 1.09 |
| Average fuel regression rate 2 (mm/s) | 0.44 | 0.42 | 0.46 |
| Characteristic velocity efficiency | 0.87 | 0.82 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Ugolini, V.M.P.; Jung, E.; Kwon, S. Demonstration of Polyethylene Nitrous Oxide Catalytic Decomposition Hybrid Thruster with Dual-Catalyst Bed Preheated by Hydrogen Peroxide. Aerospace 2025, 12, 158. https://doi.org/10.3390/aerospace12020158
Lee S, Ugolini VMP, Jung E, Kwon S. Demonstration of Polyethylene Nitrous Oxide Catalytic Decomposition Hybrid Thruster with Dual-Catalyst Bed Preheated by Hydrogen Peroxide. Aerospace. 2025; 12(2):158. https://doi.org/10.3390/aerospace12020158
Chicago/Turabian StyleLee, Seungho, Vincent Mario Pierre Ugolini, Eunsang Jung, and Sejin Kwon. 2025. "Demonstration of Polyethylene Nitrous Oxide Catalytic Decomposition Hybrid Thruster with Dual-Catalyst Bed Preheated by Hydrogen Peroxide" Aerospace 12, no. 2: 158. https://doi.org/10.3390/aerospace12020158
APA StyleLee, S., Ugolini, V. M. P., Jung, E., & Kwon, S. (2025). Demonstration of Polyethylene Nitrous Oxide Catalytic Decomposition Hybrid Thruster with Dual-Catalyst Bed Preheated by Hydrogen Peroxide. Aerospace, 12(2), 158. https://doi.org/10.3390/aerospace12020158






