The Modulatory Effect of Inhibitors on the Thermal Decomposition Performance of Graded Al@AP Composites
Abstract
1. Introduction
2. Experiment
2.1. Materials
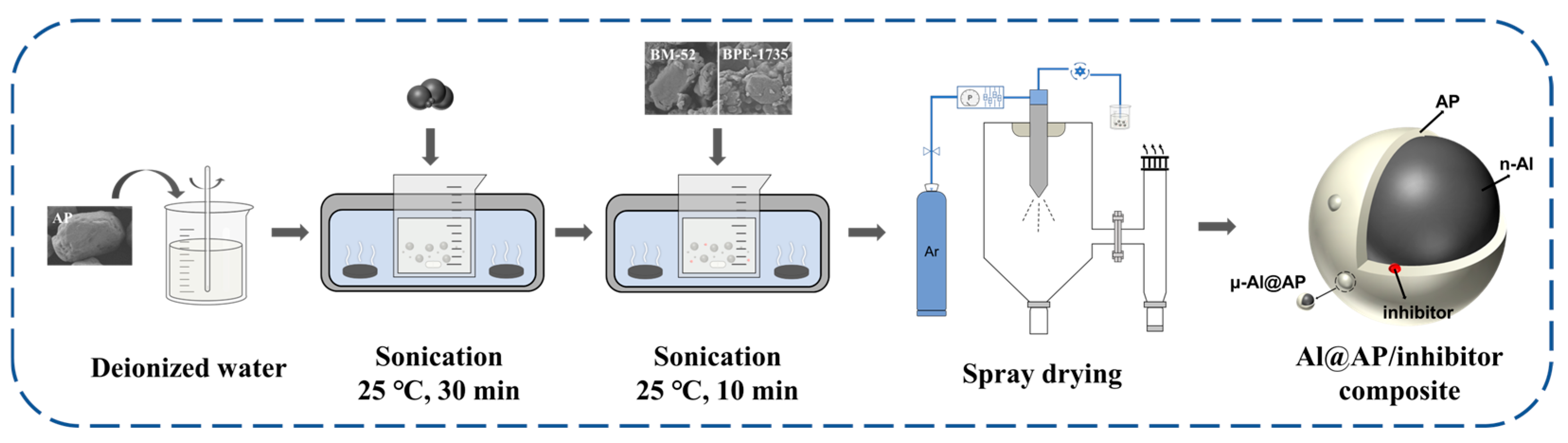
2.2. Preparation Methods
2.3. Characterization Technique
3. Results and Discussions
3.1. Morphology and Composition Characterization
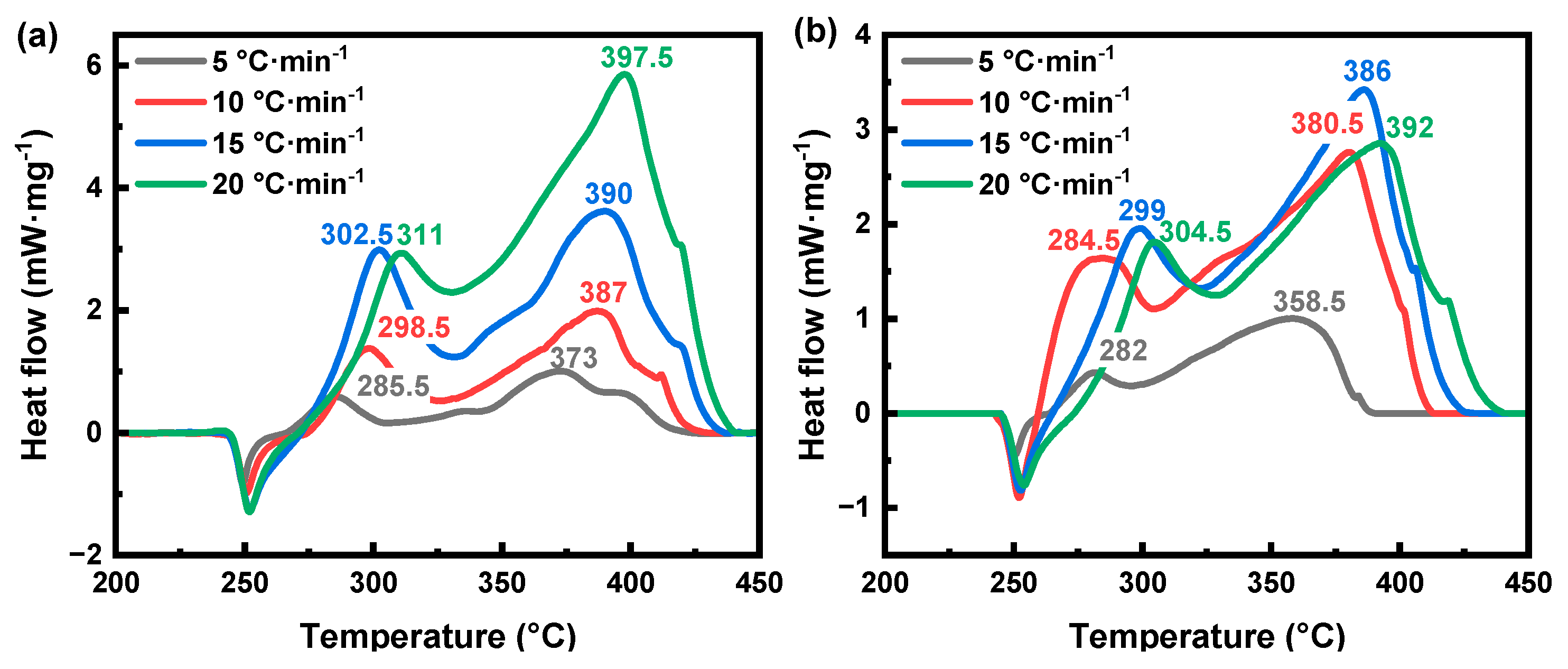
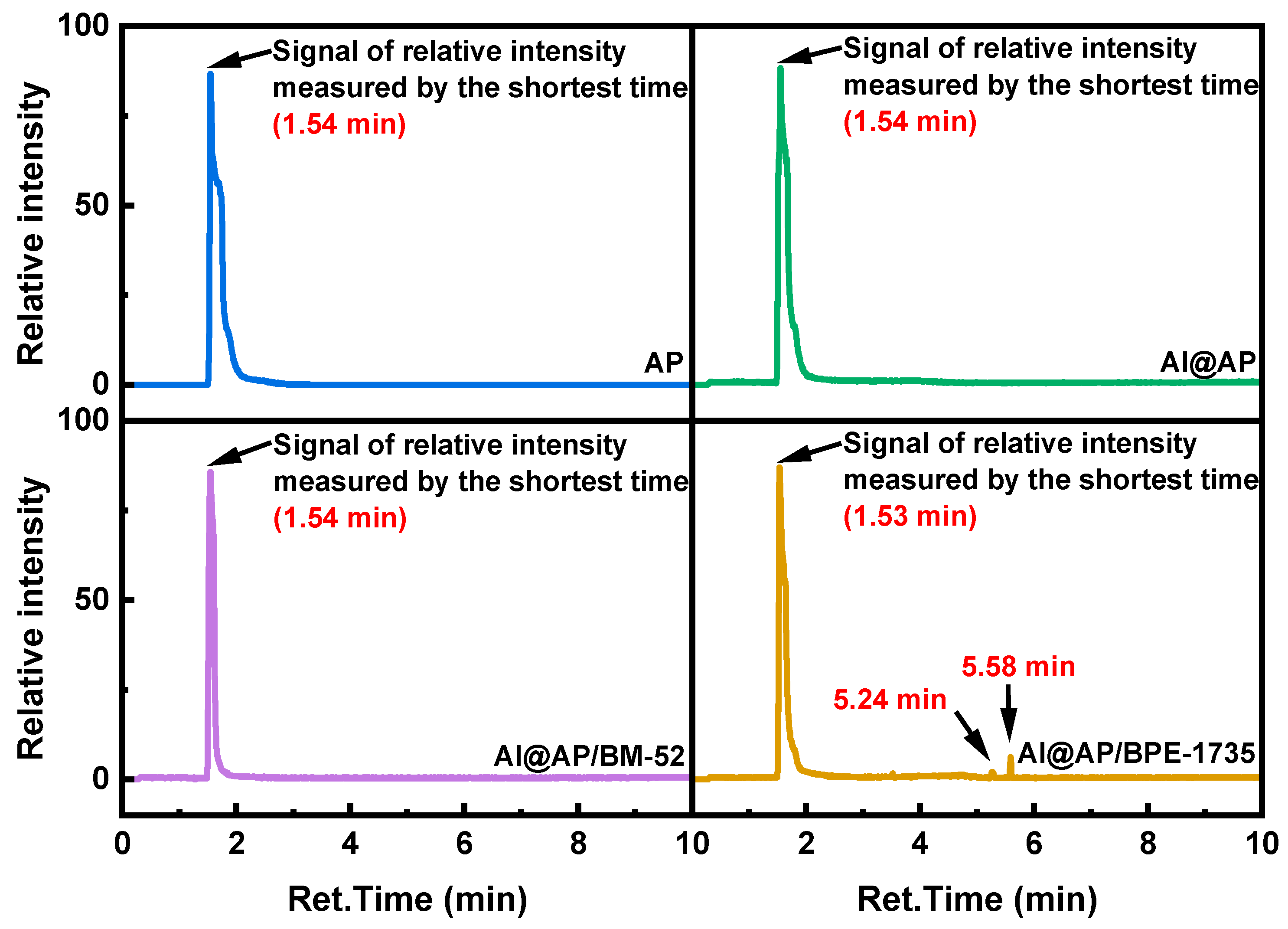
3.2. Thermal Decomposition Behavior of AP
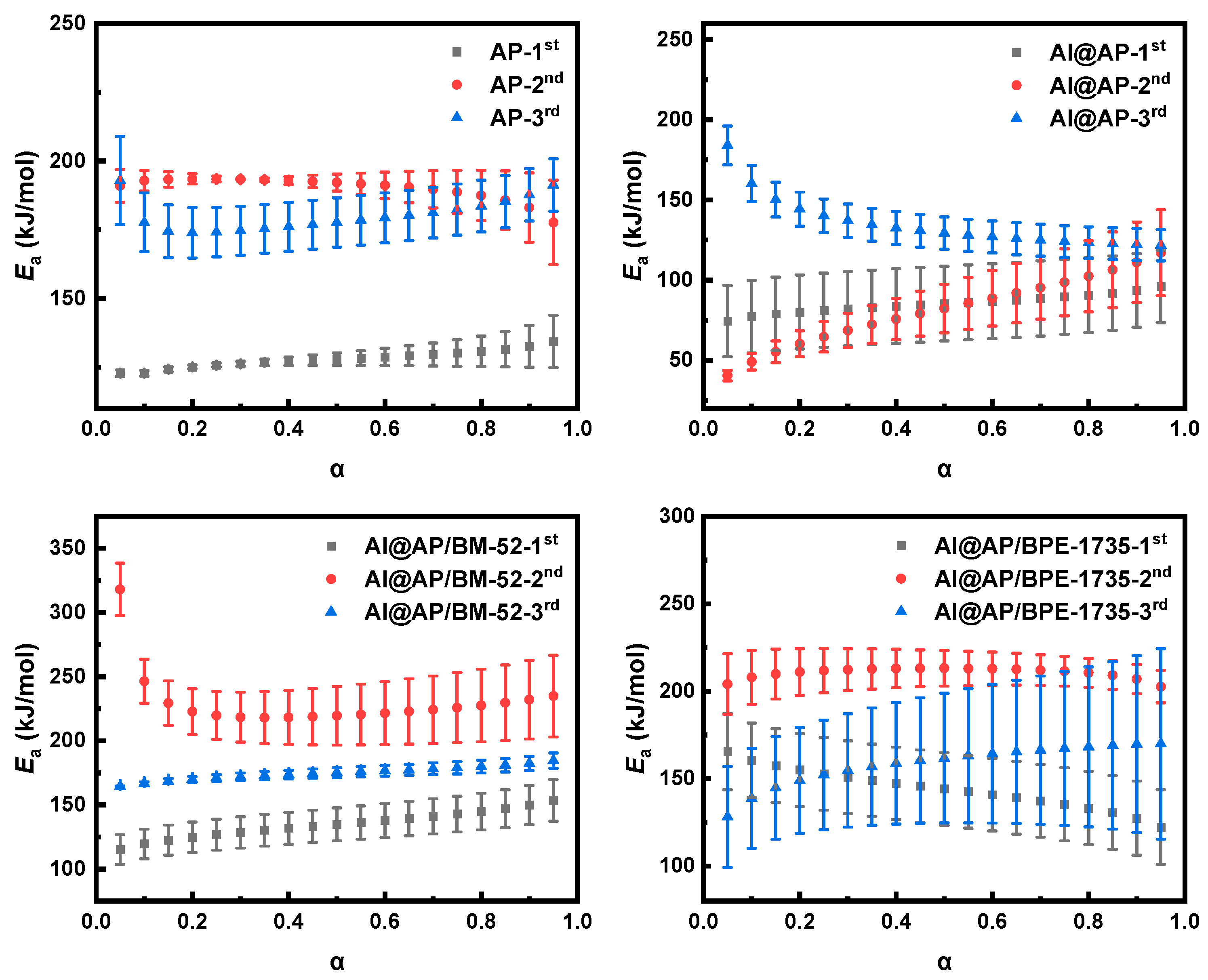
3.3. Non-Isothermal Dynamics Analysis of the Thermal Decomposition of AP
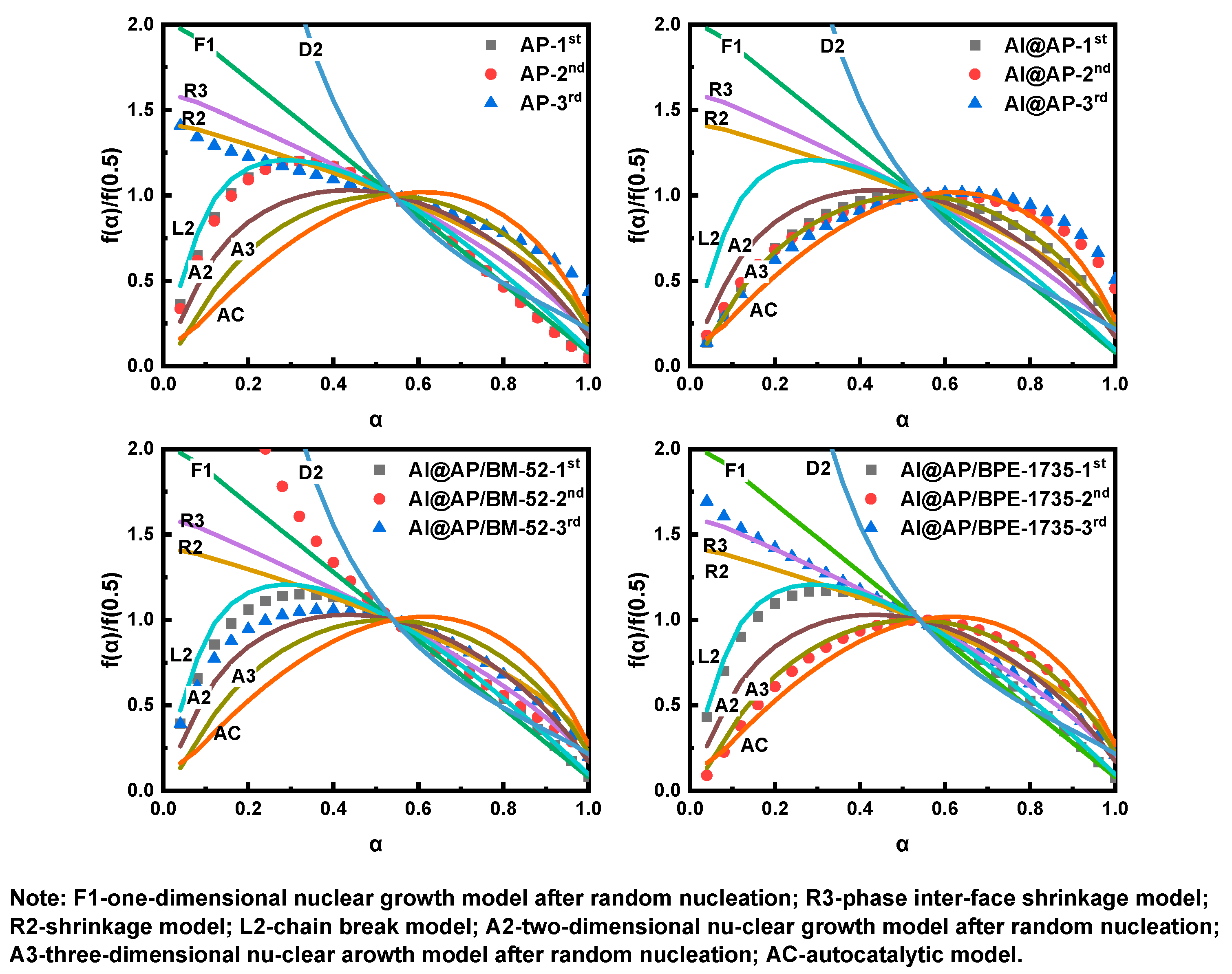
3.4. Thermal Decomposition Mechanism
4. Conclusions
- (1)
- The reactivity of AP is capable of being effectively enhanced by both BM−52 and BPE−1735 inhibitors. The incorporation of BM−52 facilitates a more comprehensive AP decomposition reaction. The total heat release is elevated from 365.4 J·g−1 to 714.0 J·g−1 with the introduction of BPE−1735.
- (2)
- The Ea of AP thermal decomposition can be remarkably enhanced by both BM−52 and BPE-735, thereby increasing the difficulty of the HTD of AP. Under the influence of BM−52, the physical model of HTD of AP is transformed from the chain-breaking model (L2) and the phase boundary-controlled reaction model (R2) into a model that lies between F1 and D2, as well as the two-dimensional nucleation and nuclei growth model (A2). Under the effect of BPE−1735, the physical model of HTD of AP is converted into the three-dimensional nucleation and nuclei growth model (A3) and the phase boundary-controlled reaction model (R3).
- (3)
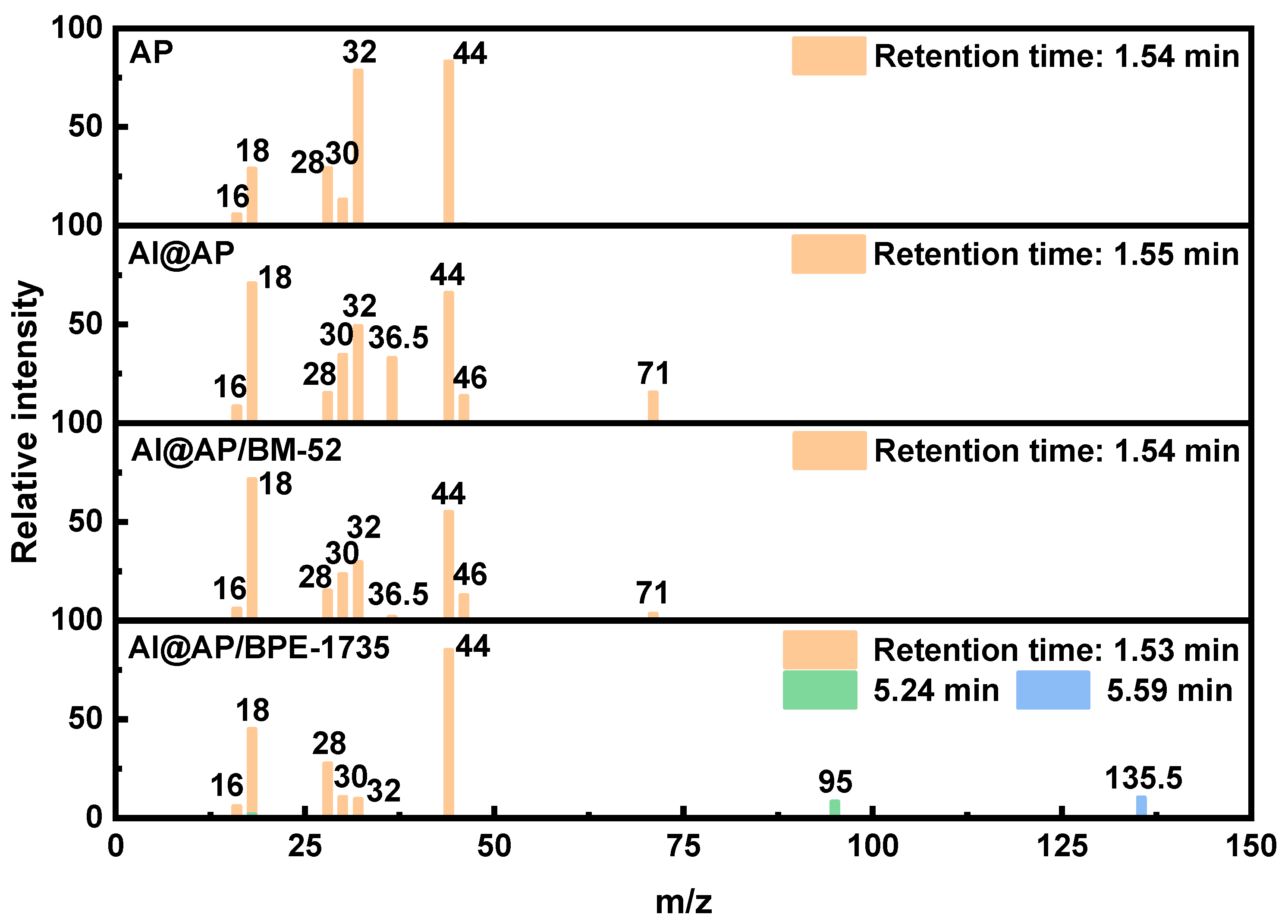
- After the addition of BM−52 and BPE−1735, the proportion of oxygen-containing compound products in the Pyro-GC/MS products of AP is reduced. Evidently, a more complete decomposition of AP can be achieved with the aid of these two inhibitors. Additionally, specific chemical reactions are induced by BPE−1735 during the thermal decomposition process, leading to the generation of unique chemical species.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Yadav, N.; Srivastava, P.K.; Varma, M. Recent Advances in Catalytic Combustion of AP-Based Composite Solid Propellants. Def. Technol. 2021, 17, 1013–1031. [Google Scholar] [CrossRef]
- Manash, A.; Kumar, P. Comparison of Burn Rate and Thermal Decomposition of AP as Oxidizer and PVC and HTPB as Fuel Binder Based Composite Solid Propellants. Def. Technol. 2019, 15, 227–232. [Google Scholar] [CrossRef]
- Wang, Z.; Qiang, H. Mechanical Properties of Thermal Aged HTPB Composite Solid Propellant under Confining Pressure. Def. Technol. 2022, 18, 618–625. [Google Scholar] [CrossRef]
- Xu, S.; Pang, A.-M.; Wang, Y.; Pan, X.-Z.; Li, S.-W.; Li, H.-T.; Kong, J. A Review on the Use of Burning Rate Suppressants in AP-Based Composite Propellants. Propellants Explos. Pyrotech. 2022, 47, e202000327. [Google Scholar] [CrossRef]
- Jacobs, P.W.M.; Whitehead, H.M. Decomposition and Combustion of Ammonium Perchlorate. Chem. Rev. 1969, 69, 551–590. [Google Scholar] [CrossRef]
- Poulter, L.W.; Nelson, R.W.; Smalley, R.B., Jr.; Hawkins, M.C. Robust Propellant Liner and Interfacial Propellant Burn Rate Control 1998. U.S. Patent No. 5,767,221, 16 June 1998. [Google Scholar]
- Luo, Y.; Ma, T.; Zhao, H.; Yang, N.; Xu, S.; Zheng, W. Effect of amine-based burning rate suppressants on the HTPB propellants: Thermal decomposition and combustion characteristics. Acta Astronaut. 2024, 219, 497–505. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Li, S.-F.; Ding, D.-H. Effect of Ammonium Oxalate/Strontium Carbonate on the Burning Rate Characteristics of Composite Propellants. J. Therm. Anal. Calorim 2006, 86, 497–503. [Google Scholar] [CrossRef]
- Dey, A.; Ghorpade, V.G.; Kumar, A.; Gupta, M. Biuret: A Potential Burning Rate Suppressant in Ammonium Chlorate(VII) Based Composite Propellants. Cent. Eur. J. Energetic Mater. 2014, 11, 3–13. [Google Scholar]
- Ghorpade, V.G.; Dey, A.; Jawale, L.S.; Kotbagi, A.M.; Kumar, A.; Gupta, M. Study of Burn Rate Suppressants in AP-Based Composite Propellants. Propellants Explo Pyrotec 2010, 35, 53–56. [Google Scholar] [CrossRef]
- Glaskova, A.P. Three Possible Ways to Inhibit the Ammonium Perchlorate Combustion Process. AIAA J. 1975, 13, 438–442. [Google Scholar] [CrossRef]
- Stoner, C.E.; Brill, T.B. Thermal Decomposition of Energetic Materials 46. The Formation of Melamine-like Cyclic Azines as a Mechanism for Ballistic Modification of Composite Propellants by DCD, DAG, and DAF. Combust. Flame 1991, 83, 302–308. [Google Scholar] [CrossRef]
- Pang, W.Q.; Li, J.Q.; DeLuca, L.; Wang, K.; Fu, X.; Fan, X.Z.; Li, H. Effects of different deceleration agents on the properties of hydroxyl terminated polyether (htpe)-based composite solid propellants. Int. J. Energetic Mater. Chem. Propuls. 2017, 16, 125–138. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S.; Cao, C.-Y.; Liu, C.-C.; Gong, L.-L.; Zhang, H.-P. Impacts on Combustion Behavior of Adding Nanosized Metal Oxide to CH3N5-Sr(NO3)2 Propellant. Fuel 2017, 191, 371–382. [Google Scholar] [CrossRef]
- Chalghoum, F.; Trache, D.; Benziane, M.; Chelouche, S. Effect of Complex Metal Hydride on the Thermal Decomposition Behavior of AP/HTPB-Based Aluminized Solid Rocket Propellant. J. Therm. Anal. Calorim. 2022, 147, 11507–11534. [Google Scholar] [CrossRef]
- Oberth, A.E.; Bruenner, R.S. Solid Propellants Containing Burning Rate Depressants 1971. U.S. Patent 3779825, 18 December 1973. [Google Scholar]
- Li, R. Application of Novel Energetic Quaternary Ammonium Salt Burning-Rate Inhibitor. Master’s Thesis, National University of Defense Technology, Changsha, China, 2019. [Google Scholar]
- Xu, R.-X.; Xue, Z.-H.; Zhang, H.-R.; Shl, L.-W.; Lǔ, L.; Yan, Q.-L. Effects of burning rate inhibitors and their location on combustion performance of three-component propellants. J. Propuls. Technol. 2024, 45, 180–188. [Google Scholar] [CrossRef]
- Xu, R.-X.; Zhang, H.-R.; Xue, Z.-H.; Shl, L.-W.; Lǔ, L.; Yan, Q.-L. Effeet of Burning Rate Inhibitors and Their Location on the Combustion Performance of Four-component HTPB Propellants. Chin. J. Explos. Propellants 2024, 47, 229–236. [Google Scholar]
- Galfetti, L.; DeLuca, L.T.; Severini, F.; Colombo, G.; Meda, L.; Marra, G. Pre and Post-Burning Analysis of Nano-Aluminized Solid Rocket Propellants. Aerosp. Sci. Technol. 2007, 11, 26–32. [Google Scholar] [CrossRef]
- Yang, S.-L.; Xie, K.; Wang, J.; An, B.; Tian, B.; Nie, H.; Lyu, J.-Y.; Yan, Q.-L. Enhancing RDX Thermal Decomposition in Al@RDX Composites with Co Transition Metal Interfacial Layer. Aerospace 2024, 11, 81. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, J.; Zhou, Y.; Wang, J.; Xv, T. Aluminum Agglomeration of AP/HTPB Composite Propellant. Acta Astronaut. 2019, 156, 14–22. [Google Scholar] [CrossRef]
- Jiang, A.-F. Preparation and Combustion Performance of Micro/Nano-Structured Aluminum-Based Energy Fuel. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- De Luca, L.T. Chapter 6—Nanoenergetic Ingredients to Augment Solid Rocket Propulsion. In Nanomaterials in Rocket Propulsion Systems; Micro and Nano Technologies; Yan, Q.-L., He, G.-Q., Liu, P.-J., Gozin, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–261. ISBN 978-0-12-813908-0. [Google Scholar]
- Lyu, J.-Y.; Xu, G.; Zhang, H.; Yang, W.; Yan, Q.-L. Thermal Decomposition and Combustion Behavior of the Core-Shell Al@AP Composite Embedded with CuO as a Catalyst. Fuel 2024, 356, 129587. [Google Scholar] [CrossRef]
- Starink, M.J. The Determination of Activation Energy from Linear Heating Rate Experiments: A Comparison of the Accuracy of Isoconversion Methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Budrugeac, P. A Simple and Precise Differential Incremental Isoconversional Method to Kinetic Analysis of Heterogeneous Processes under Arbitrary Temperature Programs. Thermochim. Acta 2018, 661, 116–123. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined Kinetic Analysis of Solid-State Reactions: A Powerful Tool for the Simultaneous Determination of Kinetic Parameters and the Kinetic Model without Previous Assumptions on the Reaction Mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef]
- Yang, S.-L.; Meng, K.-J.; Xie, W.; Nie, H.; Yan, Q.-L. Thermal Reactivity of Metastable Metal-Based Fuel al/Co/AP: Mutual Interaction Mechanisms of the Components. Fuel 2022, 315, 123203. [Google Scholar] [CrossRef]
- An, T.; He, W.; Chen, S.-W.; Zuo, B.-L.; Qi, X.-F.; Zhao, F.-Q.; Luo, Y.; Yan, Q.-L. Thermal Behavior and Thermolysis Mechanisms of Ammonium Perchlorate under the Effects of Graphene Oxide-Doped Complexes of Triaminoguanidine. J. Phys. Chem. C 2018, 122, 26956–26964. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Zhang, X.-X.; Fu, X.; Liu, J.; Qi, X.; Yan, Q.-L. Decomposition Mechanisms of Insensitive 2D Energetic Polymer TAGP Using ReaxFF Molecular Dynamics Simulation Combined with Pyro-GC/MS Experiments. J. Anal. Appl. Pyrolysis 2022, 162, 105453. [Google Scholar] [CrossRef]
- ZHU, X.-F.; HU, C.-B.; YANG, J.-G.; Ll, Y.; LlU, S.-N.; DENG, Z. Research of Filling Ratio and Fluidization Performance of Dense-Packing Aluminum Powder. J. Northwestern Polytech. Univ. 2019, 37, 13–20. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Jiao, Q.-J.; Huang, H.; Ren, H. Effect of Aluminum Particle Size on Thermal Decomposition of AP. Chem. J. Chin. Univ. 2013, 34, 662. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Thermal Decomposition of Ammonium Perchlorate. Thermochim. Acta 2006, 443, 1–36. [Google Scholar] [CrossRef]
- Gromov, A.; DeLuca, L.T.; Il’in, A.P.; Teipel, U.; Petrova, A.; Prokopiev, D. Nanometals in energetic systems: Achievements and future. Int. J. Energetic Mater. Chem. Prop. 2014, 13, 399–419. [Google Scholar] [CrossRef]
- Ismail, I.M.K.; Hawkins, T. Kinetics of Thermal Decomposition of Aluminium Hydride: I-Non-Isothermal Decomposition under Vacuum and in Inert Atmosphere (Argon). Thermochim. Acta 2005, 439, 32–43. [Google Scholar] [CrossRef]
- Predel, M.; Kaminsky, W. Pyrolysis of Mixed polyoleÆns in a Øuidised-Bed Reactor and on a Pyro-GC/MS to Yield Aliphatic Waxes. Polym. Degrad. Stab. 2000, 70, 373–385. [Google Scholar]
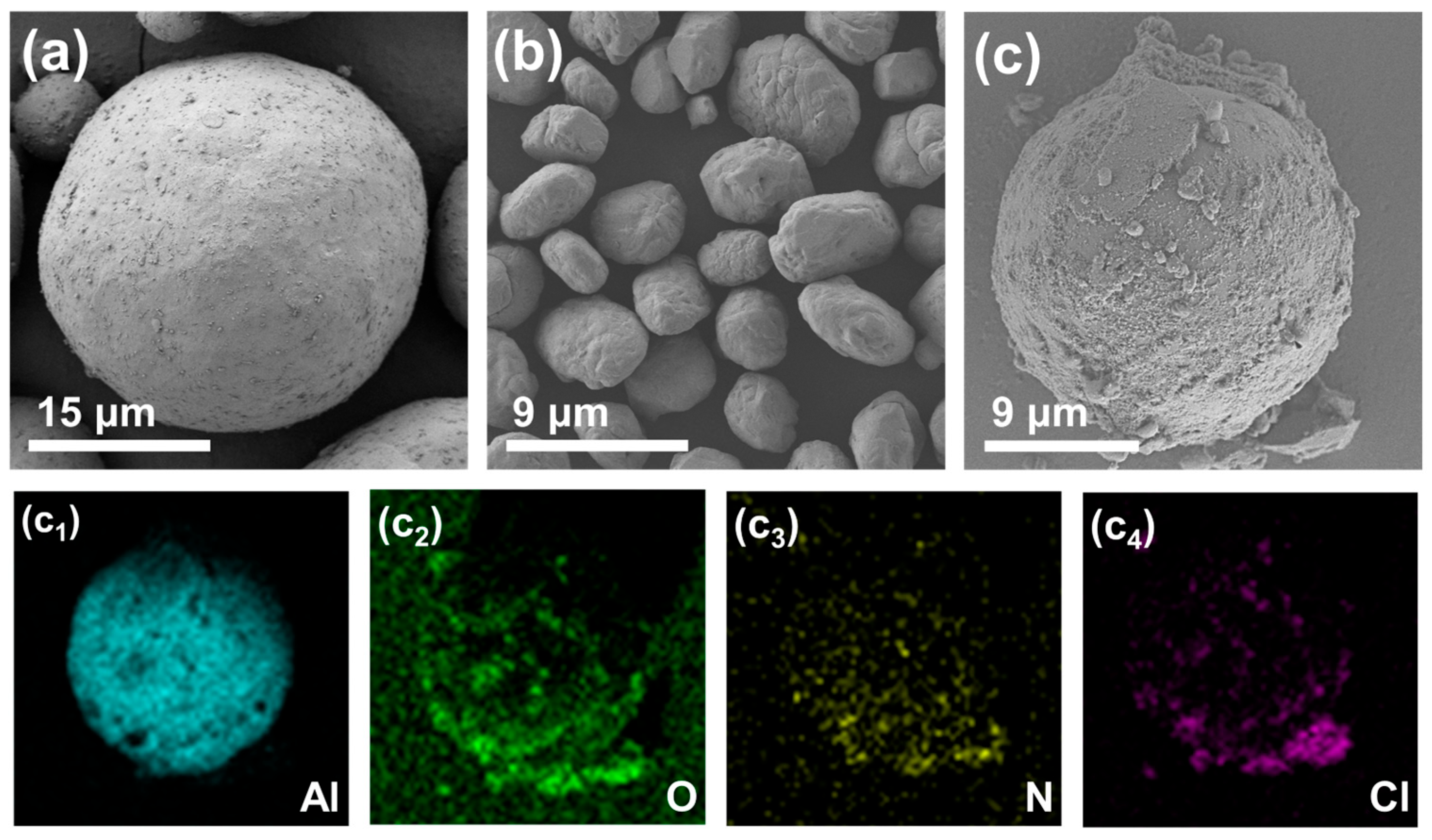

| Sample | TG | DTG | ||||
|---|---|---|---|---|---|---|
| Ti (°C) | To (°C) | Te (°C) | Mass Loss (%) | Tp (°C) | Lmax (%·min−1) | |
| AP-1st | 289.4 | 295.8 | 303.6 | 9.54 | 295.8 | 15.06 |
| AP-2nd | 392.5 | 421.7 | 430.9 | 81.51 | 421.7 | 18.90 |
| Al@AP | 376.4 | 408.8 | 416.4 | 49.08 | 408.8 | 13.87 |
| Al@AP/BM−52-1st | 258.5 | 260.6 | 298.1 | 9.93 | 293.0 | 1.74 |
| Al@AP/BM−52-2nd | 374.7 | 405.7 | 411.6 | 54.89 | 405.7 | 12.53 |
| Al@AP/BPE−1735-1st | 267.4 | 269.0 | 297.2 | 4.54 | 293.5 | 1.99 |
| Al@AP/BPE−1735-2nd | 366.0 | 395.4 | 404.0 | 50.79 | 395.4 | 12.31 |
| Sample | Ti (°C) | Tp (°C) | Te (°C) | Width (°C) | ∆H (J·g−1) |
|---|---|---|---|---|---|
| AP-1st | 287.6 | 298.9 | 312.6 | 21.0 | 323.0 |
| AP-2nd | 364.4 | 394.0 | 431.3 | 65.8 | 502.0 |
| Al@AP | 364.2 | 389.0 | 426.2 | 47.8 | 365.4 |
| Al@AP/BM−52-1st | 279.7 | 297.7 | 319.1 | 29.0 | 184.4 |
| Al@AP/BM−52-2nd | 267.4 | 388.6 | 410.6 | 50.2 | 333.5 |
| Al@AP/BPE−1735-1st | 257.6 | 276.3 | 300.2 | 35.1 | 252.5 |
| Al@AP/BPE−1735-2nd | 348.3 | 381.1 | 401.1 | 47.9 | 461.5 |
| Sample | Ea/kJ mol−1 | ln A/s−1 | r |
|---|---|---|---|
| AP-1st | 116.7 | 11.26 | 0.9981 |
| AP-2nd | 152.0 | 14.68 | 0.9934 |
| AP-3rd | 165.8 | 14.90 | 0.9944 |
| Al@AP-1st | 191.4 | 24.42 | 0.9804 |
| Al@AP-2nd | 178.7 | 19.64 | 0.9902 |
| Al@AP-3rd | 146.7 | 12.38 | 0.9804 |
| Al@AP/BM−52-1st | 131.7 | 14.59 | 0.9999 |
| Al@AP/BM−52-2nd | 193.9 | 22.11 | 0.9920 |
| Al@AP/BM−52-3rd | 214.5 | 24.55 | 0.9808 |
| Al@AP/BPE−1735-1st | 144.3 | 17.36 | 0.9951 |
| Al@AP/BPE−1735-2nd | 121.8 | 8.88 | 0.9909 |
| Al@AP/BPE−1735-3rd | 166.6 | 16.48 | 0.9630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Wang, J.; Zhang, Z.-Y.; Tian, B.; Yang, S.-L.; Lei, J.; Yu, M.-H. The Modulatory Effect of Inhibitors on the Thermal Decomposition Performance of Graded Al@AP Composites. Aerospace 2025, 12, 298. https://doi.org/10.3390/aerospace12040298
Xie K, Wang J, Zhang Z-Y, Tian B, Yang S-L, Lei J, Yu M-H. The Modulatory Effect of Inhibitors on the Thermal Decomposition Performance of Graded Al@AP Composites. Aerospace. 2025; 12(4):298. https://doi.org/10.3390/aerospace12040298
Chicago/Turabian StyleXie, Kan, Jing Wang, Zhi-Yu Zhang, Bin Tian, Su-Lan Yang, Jingyu Lei, and Ming-Hui Yu. 2025. "The Modulatory Effect of Inhibitors on the Thermal Decomposition Performance of Graded Al@AP Composites" Aerospace 12, no. 4: 298. https://doi.org/10.3390/aerospace12040298
APA StyleXie, K., Wang, J., Zhang, Z.-Y., Tian, B., Yang, S.-L., Lei, J., & Yu, M.-H. (2025). The Modulatory Effect of Inhibitors on the Thermal Decomposition Performance of Graded Al@AP Composites. Aerospace, 12(4), 298. https://doi.org/10.3390/aerospace12040298





