1. Introduction
Next generation small satellites, also known as nanosats, have masses significantly lower than standard satellites, often in the range of 1–10 kg. These satellites are being developed for numerous applications related to research, defense and industry. An important subcategory of nanosats are those built on the CubeSat specification, which allows for cube-shaped satellites, as small as 10 cm per side, with masses as low as 1.33 kg [
1,
2]. CubeSats offer a low cost alternative to traditional satellites due to their low Size, Weight and Power (SWaP). Traditionally, CubeSats have had few options for on-board propulsion, but recent advances in fields such as additive manufacturing, microfluidics, MicroElectroMechanical Systems (MEMS), low power microelectronics, high efficiency solar cells, and advanced materials, as well as an increased interest from the commercial sector, are leading to new propulsion developments. Future CubeSats, incorporating these advances, could enable scientific missions that would be impossible or financially prohibitive using traditional satellites.
There are many potential advantages of incorporating propulsion systems on future CubeSat missions. Some of these advantages include extended mission lifetime, maneuvering ability, formation flying, proximity operations, fine attitude control, drag make-up, and de-orbiting [
3]. Today, NASA’s CubeSat Launch Initiative (CSLI) plans on providing low cost access to space for research purposes for over 100 CubeSats developed by both the United States government as well as non-profit organizations. Without on-board propulsion systems, these missions are expected to have a lifespan of approximately 90 days before they fall to Earth and burn up in the atmosphere [
4]. Through the integration of propulsion systems, lifetimes on missions similar to those of the CSLI missions could be greatly extended providing the government and independent researchers the ability to collect significantly more data.
There are numerous types of CubeSat propulsion systems in active development, including those based on solar sails, electrodynamic tethers, ion electrospray, miniaturized Hall thrusters, solid rocket motors, liquid chemical thrusters, cold gas thrusters, and pulsed plasma thrusters. Recent reviews of propulsion technologies and trends for small satellites and cubesats can be found in Refs. [
5,
6,
7,
8,
9]. While many of these technologies offer great promise, the high cost of manufacturing these systems along with the need for advancements in electric power generation and storage, thermal management, and power processing unit (PPU) technologies currently hinder the implementation of these propulsion systems on CubeSats. A reliable, low-cost, easily manufactured propulsion system is therefore necessary for CubeSat propulsion to be widely implemented.
Among the most appealing of these technologies are chemical propulsion systems, owing to their combination of simplicity, reliability, and low power requirements. Liquid propulsion systems are of particular interest both as primary propulsion and attitude control thrusters, as demonstrated by recent efforts such as the University of Vermont Discrete Monopropellant Microthruster [
10] and the ESA-sponsored PRECISE project [
11], which both use the catalyzed decomposition of a monopropellant to generate thrust. Each of these thruster initiatives, however, has reported challenges associated with the performance of the catalytic chamber due to scaling effects.
In a previous study by the authors [
12], a method for decomposing a monopropellant without a catalytic chamber was proposed that could address these challenges. Using this method, the monopropellant and an aqueous catalyst are fed into a mixing chamber to initiate decomposition, with the products ejected through a converging–diverging nozzle. A significant benefit of the homogeneous catalysis process—and the key contribution of this work—is the ability to operate in two distinct modes, a “pseudo-monopropellant” mode and a “bipropellant” mode. These different modes of operation are achieved by varying the the flow rates of the catalyst solution and hydrogen peroxide. By changing the ratio of catalyst solution to hydrogen peroxide in the mixing and reaction chamber, the resulting thrust and impulse bit can be altered and optimized for specific situations. The dual mode propulsion system offers simultaneous capabilities for both attitude control and primary propulsion for CubeSat-class satellites. Attitude control operations could include station keeping, detumble, and reaction wheel desaturation, whereas primary propulsion allows for CubeSat orbital maneuvering. Aside from the operational flexibility, a second major advantage of the dual mode system is the ability to use the same propellants, piping, and valving for both high and low thrust applications. It is noteworthy that the dual-mode propulsion concept has already been realized on the larger (or “macro”) scales with hypergolic propulsion systems. In contrast, however, implementing this design on the miniaturized scale associated with small satellites is inherently problematic due to the lack of flow mixing at the low Reynolds numbers and the emergence of capillary and surface wetting effects. In this sense, our implementation represents a new development in spacecraft propulsion.
One potential application for a dual-mode miniaturized propulsion system is that of cubesat-based exploration of near-Earth objects, asteroids and comets [
13,
14,
15,
16]. As these small celestial bodies typically have weak and irregular gravitational fields, they present an interesting propulsion challenge. Attitude control and station keeping for a cubesat orbiting an asteroid, say, will require low thrust levels and short duration firings that are better suited for a monopropellant thruster. To transit from Earth to the asteroid, however, a high-thrust, high-specific-impulse bipropellant thruster would be desirable, to reduce the wet mass of the cubesat at launch. A dual-mode propulsion system allows the satellite to operate in the most efficient mode for the mission profile without the need for multiple independent propulsion systems.
In this work, the design overview of a dual-mode propulsion system is presented along with supporting results from preliminary experiments demonstrating the concept. This article describes the selection of the fuel/catalyst combination (in both monopropellant and bipropellant operation), development of the monopropellant attitude control thrusters, and development of the dual-mode primary divert thrusters.
2. Propulsion Mechanism Overview
For a propulsion system based on a hydrogen peroxide monopropellant, the operation is based on the idealized chemical decomposition reaction that proceeds according to the reaction described in Equation (
1).
Here,
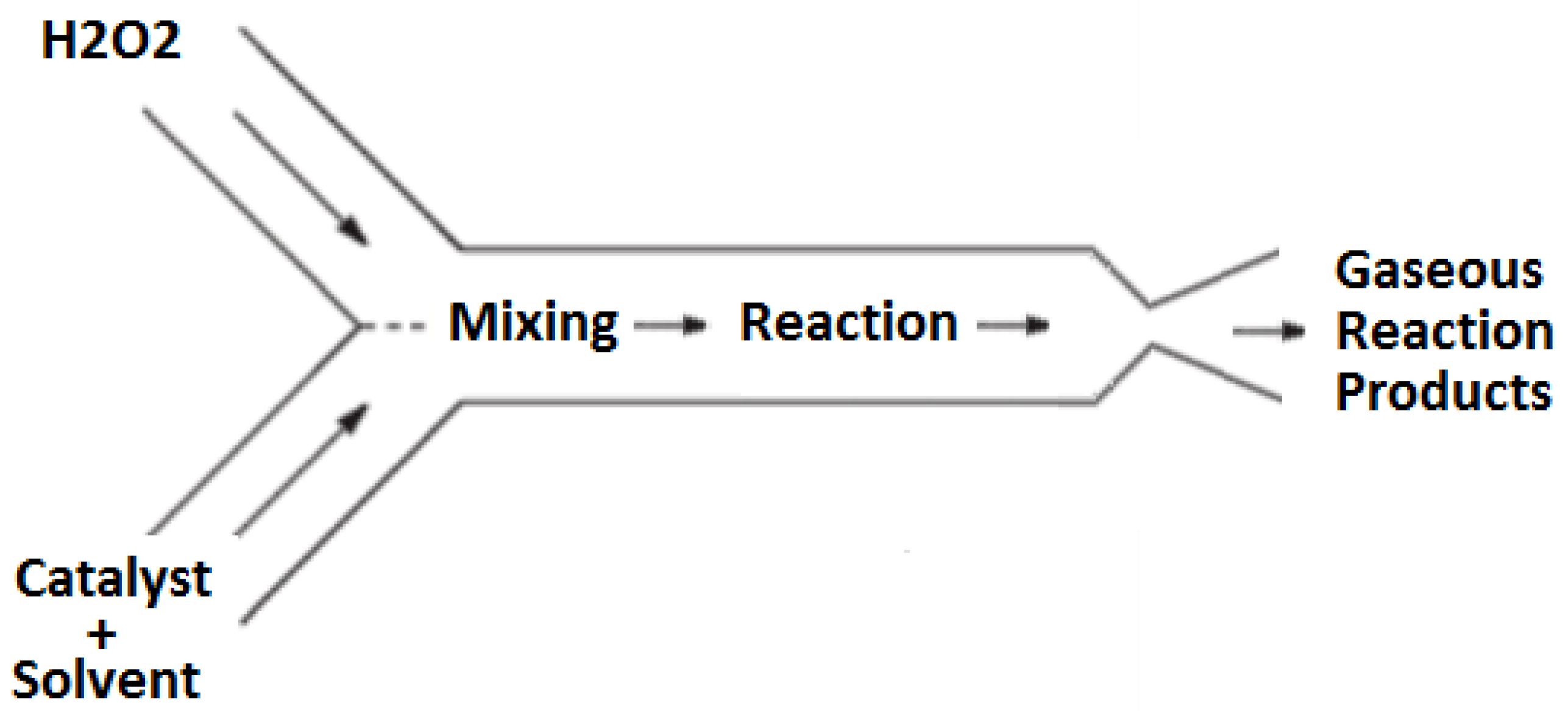
n represents a mole fraction of water present in the hydrogen peroxide fuel; typically, the hydrogen peroxide concentrations ranges 85–90% by mass. In this propulsion design, the decomposition is achieved using a homogeneous catalysis approach wherein the catalyst is dissolved in a liquid solvent and reacts with the monopropellant in the liquid phase. The resulting exothermic reaction produces high temperature gaseous products that are subsequently passed through a converging–diverging supersonic nozzle to produce thrust. A high-level representation of this process is shown schematically in
Figure 1.
The detailed operation of this propulsion concept can be broken down into three stages: storage, mixing and reaction, and expulsion. First, in the storage stage, the catalyst solution and hydrogen peroxide are both stored separately in chemically compatible vessels. In this system, the storage vessels are pressurized by the slow, controlled auto-decomposition of hydrogen peroxide, resulting in a pressure increase in the storage vessels without the need for an external pressure source. Not only does this approach mitigate the need for a separate pressurization system aboard the cubesat, but it also satisfies current NASA regulations for pressurized elements on cubesats during launch.
The second stage, mixing and reaction, occurs when the hydrogen peroxide and catalyst solutions are allowed to flow into the mixing and reaction chamber of the thruster. Here, the catalyst solution is mixed with the hydrogen peroxide resulting in a fast decomposition reaction. This reaction produces steam, oxygen, and heat. The heat released by the decomposition reaction vaporizes the catalyst solvent. Due to the high temperature created by the decomposition reaction, the catalyst solvent vapors react with the oxygen resulting in a secondary combustion reaction. The secondary combustion reaction releases additional thermal energy into the flow thereby increasing the of the propulsion system; this aspect represents a significant and novel aspect of this design.
After the reactions have occurred in the mixing and reaction stage, the hot product gases enter the third stage of the propulsion system, the converging–diverging nozzle. The decrease in mass density of the gaseous reaction products compared to the density of the liquid reactants results in substantial pressure increase in the mixing and reaction chamber. This pressure drives the reaction products through the converging–diverging nozzle to achieve supersonic conditions and thereby generate the thrust for the device.
At the lowest operational ratio of catalyst solution to hydrogen peroxide, the thrust produced will approach the results of a monopropellant thruster; this is the “pseudo-monopropellant” mode of operation. When operating in pseudo-monopropellant mode, the energy released approaches that of a monopropellant hydrogen peroxide system. In this mode, approximately 586 calories of heat per gram of 85% hydrogen peroxide is released and the adiabatic flame temperature during operation is 886 K [
17].
In contrast, by increasing the ratio of catalyst solution to hydrogen peroxide to a stoichiometric ratio allows all of the oxygen produced by the hydrogen peroxide decomposition to participate in a subsequent combustion reaction with the catalyst solvent. Coupling the combustion of the catalyst solvent with the decomposition of hydrogen peroxide results in a dramatic increase in thrust. This mode of operation is similar to a traditional bipropellant system, and is thus referred to as the “bipropellant mode” of operation. When the decomposition of hydrogen peroxide is coupled with the combustion of a catalyst solvent, the overall reaction products are carbon dioxide and steam and the total number of gas particles created by the reaction is increased. The addition of a stoichiometric ratio of energy-dense solvent significantly increases the energy evolved resulting in an increased adiabatic flame temperature. In comparison to the pseudo-monopropellant mode, the use of a stoichiometric mixture of hexanol and pure hydrogen peroxide results in an energy density of approximately 1050 calories per gram of mixture with an adiabatic flame temperature of 2670 K.
The theoretical differences between the pseudo-monopropellant mode and bipropellant mode are summarized in
Table 1.
3. Propellant and Catalyst/Solvent Selection
The CubeSat Design Specification, which creates a standard that ensures that CubeSats and their launch vehicle are compatible, sets strict limiting factors for the characteristics of CubeSat propulsion systems. Along with overall mass limitations for a 1U and 3U CubeSat at 1.33 kg and 4 kg, respectively, the maximum pressure contained in any component is limited to 1.2 atmospheres. Due to the high energy density, low vapor pressure, and nontoxic characteristics of hydrogen peroxide, it is a promising choice for use in a CubeSat propulsion system. In comparison to many other compounds used in propulsion today, such as hydrazine and oxides of nitrogen, high test hydrogen peroxide (HTP) has low toxicity and is considered a “green” monopropellant. The volume specific impulse of 90% HTP is greater than most comparable green propellants currently available. Another advantage of using HTP as a propellant is the benign nature of the products of hydrogen peroxide decomposition, steam and diatomic oxygen; unlike other promising high-energy green monopropellants such as ADN, HAN, and HNF, the decomposition of HTP results in products with relatively low molecular weight. This is an advantage because the operational temperature of the exhaust gases in HTP thrusters can be maintained much lower than that of other “green” propellants resulting in decreased materials and manufacturing costs [
18]. Due to these characteristics, HTP is a relevant propellant with a significantly reduced cost of manufacturing, storage, and handling when compared to many other fuels on the market today.
To decompose the HTP, a catalyst is used to accelerate the rate of the decomposition reaction. Previous work using heterogeneous ruthenium-oxide (RuO
) nanostructures as an in situ microchannel catalyst showed signs of incomplete decomposition, most likely caused by the inability of the HTP to wet the surface of the catalyst [
10,
19]. For the present work, homogeneous catalysis is used. This approach has many advantages over heterogeneous catalysis. Perhaps the most compelling advantage is the ability to use additive manufacturing for the device fabrication, decreasing the manufacturing cost and lead time. Two aqueous catalysts were chosen based on the available literature available: ferric chloride
and ferrous chloride
[
20]. Due to the ionic nature found in the bonds of both the catalysts, they are readily disassociated into their constituent ions; this results in a high concentration of iron ions in the solution that catalyzes the decomposition of hydrogen peroxide [
21].
An advantage of using an ionic compound as the catalyst for the reaction is the ability to use a polar solvent in the catalyst solution. Many polar solvents have high energy densities, low vapor pressures, and include many low toxicity compounds. Among such solvent options are water and alcohols. Several different catalyst solvents were tested in this study, each with a varying degree of polarity. The catalyst solvents were chosen based on several criteria: the ability to dissolve the catalyst, the miscibility with hydrogen peroxide, energy density, auto ignition temperature, and vapor pressure. Based on published data or estimates of these characteristics, six solvents of interest were chosen: hexanol, 2-propanol, ethanol, methanol, hexane, and water. Water was chosen as a baseline, non-reactive solvent. The polarity of the catalyst solvent allows the catalyst solution to be miscible with hydrogen peroxide. Although hexane’s dipole moment is significantly lower than the other solvents of interest, it was chosen to determine the degree of polarity necessary to dissolve the catalyst solutes. Nonpolar catalyst solvents tend to be immiscible with hydrogen peroxide and would greatly hinder mixing and hence unacceptably slow reactions in the thruster mixing chamber.
The fast rate of decomposition of the hydrogen peroxide allows for exhaust gases to be generated on demand while maintaining high flow rates during steady state operation. Therefore, it is important that the catalyst used for the reaction results in sufficiently fast reaction rates. The use of homogeneous catalysis eliminates catalyst beds completely, resulting in the elimination of problems caused by a contaminated catalyst surfaces (i.e., fouling). The constant mixing of fresh catalyst in the homogeneous catalysis design means that catalyst degradation over time is not an issue of concern in this design.
A key advantage of using the fuel as the catalyst solvent is the ability to vary the reaction kinetics by varying the ratio of hydrogen peroxide to fuel. If the system is run at the full stoichiometric ratio (1:9 fuel-to-oxidizer ratio for 2-propanol), the system operates as a bipropellant thruster with complete combustion of the fuel. By reducing the fuel injected into the reaction chamber, the system will transition to pseduo-monopropellant mode, where the majority of the reaction energy is coming from the exothermic decomposition of the hydrogen peroxide. The result of this control is the ability to use the same thruster, and related hardware, with a number of different operating profiles.
When the decomposition of hydrogen peroxide is coupled with the combustion of a catalyst solvent, the overall reaction products are carbon dioxide and steam. The total number of gas particles created through the combustion reaction is increased. This, along with an increase in the enthalpy of reaction results in larger stagnation pressures and temperatures upstream of the nozzle. The bipropellant mode of the microthruster design takes advantage of these properties resulting in an increased thrust and impulse bit.
When the desired values of thrust and impulse bit are below the minimum threshold for bipropellant operation, the ratio of catalyst solution to hydrogen peroxide is decreased significantly. This causes the enthalpy of reaction and product stream compositions to shift towards that of a hydrogen peroxide monopropellant thruster. The overall performance shift can be used when small adjustments are necessary, such as attitude control and small delta-V maneuvers.
For operation in a deep space environment, it is worth noting the freezing point of 90% propellant grade hydrogen peroxide is . Due to the freezing point depression caused by the colligative properties in the catalyst solution, the freezing points are depressed far below the freezing point of hydrogen peroxide for each combination of catalyst and solvent of interest. As a result, no additional heat maintenance is required for the described bipropellant system than a hydrogen peroxide monopropellant thruster.
The decomposition of hydrogen peroxide is extremely exothermic; when used in monopropellant mode, the energy released is approximated by the energy released through only the decomposition of hydrogen peroxide, 586 calories of heat per gram of 85% HTP decomposed with a resulting adiabatic flame temperature of
[
17]. The addition of an energy-dense solvent for the catalyst solution allows a co-combustion reaction to occur, increasing the energy released by the reaction, resulting in an increased adiabatic flame temperature. In comparison to the monopropellant mode, the use of a stoichiometric mixture of hexanol and pure hydrogen peroxide results in an energy density of approximately 1050 calories per gram of mixture with an adiabatic flame temperature of
. During bipropellant mode of operation, much more chemical energy will be liberated resulting in increased thrust compared to monopropellant mode.
The addition of water to the hydrogen peroxide through the use of aqueous ferric chloride solution decreases the adiabatic flame temperature, as shown in
Figure 2, but increases the total mass flow rate through the nozzle. The decrease in temperature can be negated by increasing the purity of hydrogen peroxide. By decreasing the water in the fuel, the same adiabatic flame temperature can be achieved using the aqueous catalyst solution. For example, the use of 98% hydrogen peroxide as fuel would allow a mixing ratio of approximately 15% catalyst solution before the adiabatic flame temperature dipped below
.
By replacing water solvent with a fuel at a stoichiometric ratio of fuel to hydrogen peroxide, the adiabatic flame temperature of the mixture is increased significantly. If ethanol, methanol, 2-propanol, hexane, or hexanol are used as the catalytic solvent, the resulting adiabatic flame temperatures are significantly higher as shown in
Figure 3. The adiabatic flame temperature increase when compared to using water as a solvent is a direct consequence of the substantial increase of chemical energy released through the combustion of the fuel in combination with the decomposition of hydrogen peroxide.
3.1. Monopropellant/Catalyst Solution Suitability Results
Results from monopropellant and bipropellant data were divided into three categories based on the observed reaction delay time. The “long delay” category contained solutions with an observed delay of greater than one second, the “short delay” category contained solutions with a delay less than or equal to one second, and the “no delay” category contained solutions with no observed delay before the reaction. The reactions were classified into “fast” or “slow” reactions if the reaction after the delay period was shorter or longer than 0.5 s. Using data from the reaction observations, each solvent was determined to be either fit or unfit for use in this application. If the solution had no delay and a fast reaction, it was put into the fit category; if the solution had a delay longer than 1 s or a reaction longer than 0.5 s after the delay, the solution was unfit. These data are shown in combination with collected solubility data in
Table 2.
3.2. Catalyst Solution Selection
The viable catalyst solutions were chosen by combining the solubility data and the chemical reactivity data. Viable catalyst solutions, which possess characteristics necessary for use in a functioning thruster, were chosen as solutions that were homogeneous and noncolloidal at −10C, and have no observed delay followed by a fast decomposition reaction during reactivity testing. Through this process, the original 36 possible catalyst solutions were further reduced to seven viable catalyst solutions.
Other characteristics that impact the catalyst solution effectiveness are the energy density of the chemical storage space, the solute concentration, and the relative theoretical maximum specific impulse. Each of these characteristics contribute to the thruster’s ability to provide consistent and reliable performance. The energy density of interest is the total energy per unit volume of the stoichiometric ratio of catalyst solution to hydrogen peroxide. When operating in bipropellant mode, the volumetric ratio of hydrogen peroxide and solvent differs between solvents due to the combustion chemistry of the reaction. When the volumetric ratios of hydrogen peroxide and solvent are taken into consideration, the solvent with the highest combined energy density will provide the thrust system with the highest delta-V per unit of combined hydrogen peroxide and catalyst storage space. This is important because of the tight volume constraints imposed by the CubeSat platform.
Figure 4 shows the energy density of each solvent.
There is a possibility that the catalyst will recrystallize and form a precipitate layer of catalyst on the walls of the nozzle during thruster operation. Due to this, the concentration of the catalyst in the solvent has the possibility to affect the performance of the thruster over its operational lifetime. This problem is mitigated by using the minimum catalyst concentration in the catalyst solution while maintaining high reaction kinetics in the device. Minimizing the mass of catalyst also decreases the total mass of the system.
To characterize the expected performance of each of the catalyst solutions, the theoretical relative specific impulse of each was calculated. Theoretical relative specific impulse is the theoretical maximum achievable specific impulse for each solvent normalized by the maximum achievable specific impulse of 85% hydrogen peroxide and water. By determining which solvents have the highest relative specific impulse, the performance of the solvents can be directly compared.
Figure 5, which is a plot of expected bipropellant performance, shows the rankings of relative expected performance of each catalyst solution based on the relative specific impulse (
), relative chemical storage energy density (
), and relative solute concentration (
). The expected performance was characterized using an empirical, weighted formula given by :
The relative expected performance was then determined by normalizing the expected performance with the highest performing solvent, in this case, 2-propanol.
Based on this metric, the catalyst solution predicted to provide the thruster system with the highest performance is 15% ferric chloride in 2-propanol. This solution was chosen by taking relative specific impulse, average storage energy density, and catalyst concentration into consideration. This solution showed promising results in preliminary chemical reaction and solubility testing. It was determined that this solution will take advantage of high performance of the combustion of 2-propanol with a relatively low concentration of catalyst, increasing thrust and reliability.
5. Experimental Device Testing and Characterization
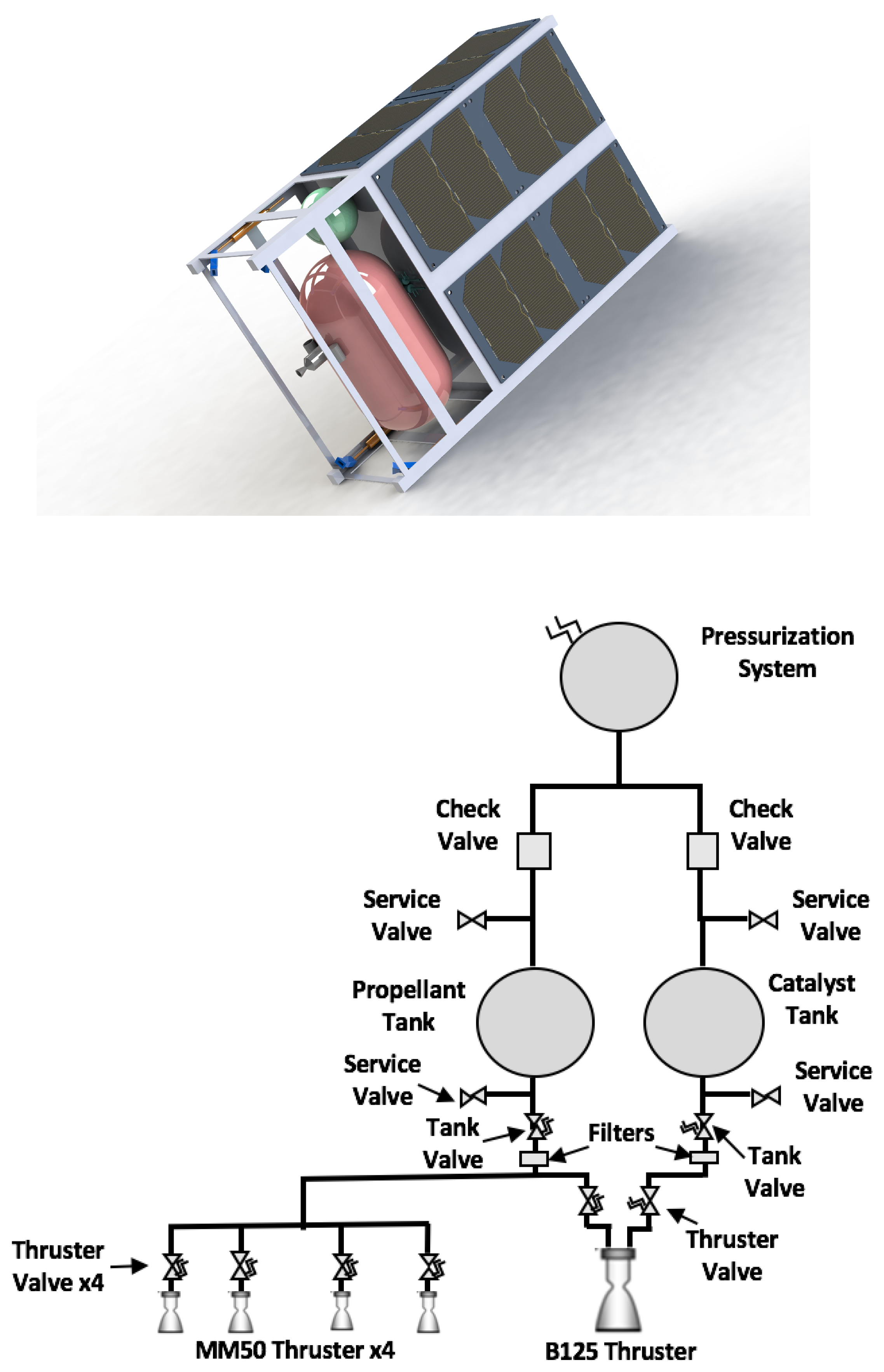
Due to the significant difference in thrust levels between the attitude control thrusters and the primary driver thruster, different measurement techniques were required for performance characterization. Three indirect measurement techniques were used to demonstrate the flow characteristics of the 50 mN attitude control thrusters: Schlieren imaging, thermal imaging, and exit plane temperature analysis. The thrust of the 1.25 N primary driver thruster was measured directly on a thrust stand. Specific descriptions of the methods used follow.
5.1. Schlieren Imaging of Micronozzle Operation
To assess the micronozzle performance, Schlieren photography was performed using ambient temperature compressed air. The house air stagnation pressure was measured to be approximately 55 psig. According to the compressible flow relation the ratio of the stagnation pressure to outlet pressure necessary to achieve sonic flow at the throat was 1.57 (using for air), resulting in a necessary minimum stagnation pressure of approximately 38 psig when vented directly to the laboratory. Due to this, the pressure drop over the nozzle resulting from the supplied house air was determined to be theoretically large enough for supersonic flow in the divergent section of the nozzle.
The Schlieren photography apparatus is shown in
Figure 9. The Schlieren apparatus used for this experiment was a Z-type system consisting of a bright light source that passes through a 0.25 mm hole, directed at a mirror that is placed one focal length away from the light source. The first mirror collimates the light and redirects the collimated light over the test region, where the nozzle was placed. After passing through the test region, another mirror refocuses the light towards the knife’s edge, in this case the edge of a razor blade, which is located one focal length from the second mirror. A camera located behind the knife edge was used to capture the Schlieren photographs.
For testing, a simplified flow network design was created that eliminated one of the inlets from the nozzle design, leaving one inlet for the house air line. A photograph of the nozzle that was used in the Schlieren imaging is shown in
Figure 10. The nozzle was additively manufactured using Formlabs Clear Photopolymer Resin, formulation FLGPCL02.
5.2. Thermal Imaging
Thermal imaging was used to visualize the temperature distribution within the device along with the plume at the nozzle exit during operation. A photograph of the experimental set-up is shown in
Figure 11. Images were captured during steady-state operation for the plume and the thruster and subsequently examined. During operation in the supersonic regime, the temperature of the working fluid after exiting the nozzle is significantly lower than the temperature upstream of the nozzle and within the reaction chamber. In particular, the thermal image of the plume was used to determine the shape, direction, and temperature characteristics of the flow after exiting the nozzle. From this information, the ratio of catalyst solvent to hydrogen peroxide as well as the total flow rate of gases through the nozzle were examined to determine how these parameters affect the operation of the propulsion system.
Thermal imaging of the system was performed using a thermoIMAGER TIM T900 manufactured by Micro-Epsilon (Raleigh, NC, USA). Thermal images were collected and analyzed using Micro-Epsilon’s thermal imaging software, TIM Connect. Along with thermal images, standard video was recorded using a PixeLink PL-B774U Color Camera. The thermal imaging camera was supported above the thruster, while the PixeLink camera was mounted to capture the side view of the thruster. Although the thermal camera was not calibrated to measure precisely the absolute temperatures of the materials, the thermal images nonetheless provide invaluable insight in the flow dynamics and operation of the system.
5.3. Exit Plane Temperature Measurement
The measurement of the temperature of the working fluid at the exit plane of the nozzle can be used to verify that the flow within the nozzle is in the supersonic regime. Supersonic flow through the nozzle is achieved if the pressure drop over the nozzle is high enough to choke the flow at the nozzle throat. As the nozzle diverges, a supersonic flow will accelerate as the flow area increases. The flow through a converging–diverging nozzle can be described using the well-known quasi-one-dimensional isentropic flow relation.
Equation (
3) relates the Mach number,
M, the ratio of the flow area to the area of the throat,
, and the specific heat ratio of the gases flowing through the nozzle,
. From this relationship, the geometry of the nozzle can be used to determine a theoretical Mach number at the nozzle exit plane
. Using the temperature relation for isentropic supersonic flow,
at this exit Mach number, the ratio of the stagnation temperature
to the exit temperature
can be calculated.
The adiabatic flame temperature of the reactants for each trial is the approximate stagnation temperature in the system. From this, it is possible to determine a theoretical working fluid temperature at the exit plane of the nozzle. These calculations result in the lowest achievable fluid temperature at the exit plane during experimentation due to the isentropic assumptions made in the calculation of the fluid exit temperature. Further, these temperature calculations allow the experimental exit plane temperature to be compared to the theoretical (isentropic) temperature. For these experiments, the catalyst solution used was 15 weight % aqueous ferric chloride. The ferric chloride itself was a laboratory grade anhydrous powder. The catalyst solution was prepared less than an hour prior to testing to eliminate any possible catalyst degradation due to oxygen exposure. Hydrogen peroxide used for the experiment was HTP manufactured by the FMC Corporation (Philadelphia, PA, USA); the concentration was measured as 87.6 % using a Brix refractometer. With this combination of aqueous catalyst and HTP, the maximum adiabatic flame temperature expected during steady-state operation of the thruster was 685 K.
The exit plane temperature measurement was performed using an Omega digital thermometer, model HH501AK, and a type K thermocouple. The thermocouple was placed directly at exit plane of the nozzle so that the end of the thermocouple was in the center of nozzle exit. The thermal imaging system was also used to capture thermal images of the plume during the tests. Along with thermal images, standard video was recorded using a PixeLink PL-B774U color camera. The experimental set-up for this experiment was similar to the arrangement used for the thermal imaging of the plume experiment and is shown in
Figure 12.
5.4. Thrust Stand Measurement
Measurements of thruster performance were taken on a custom-built torsional thrust stand, with a sensitivity of 1.0 mN and a sampling rate of 20,000 samples/s. The position of the torsion arm is measured by a Digital Voltage Reluctance Transmitter (DVRT, Lord MicroStrain, Williston, VT, USA), and captured using a National Instruments Model USB-6001 Digital Acquisition System (National Instruments Corporation, Austin, TX, USA). The positional data were converted real-time into raw thrust data and recorded for post-processing. The flow rate of the hydrogen peroxide and catalyst solutions entering the prototype thrusters was controlled via syringe pump. The prototype thruster was attached to the thrust stand and data collection began once the hydrogen peroxide and catalyst solution entered the mixing chamber.