The Potential Changes and Stereocilia Movements during the Cochlear Sound Perception Process
Abstract
:1. Introduction
2. Model
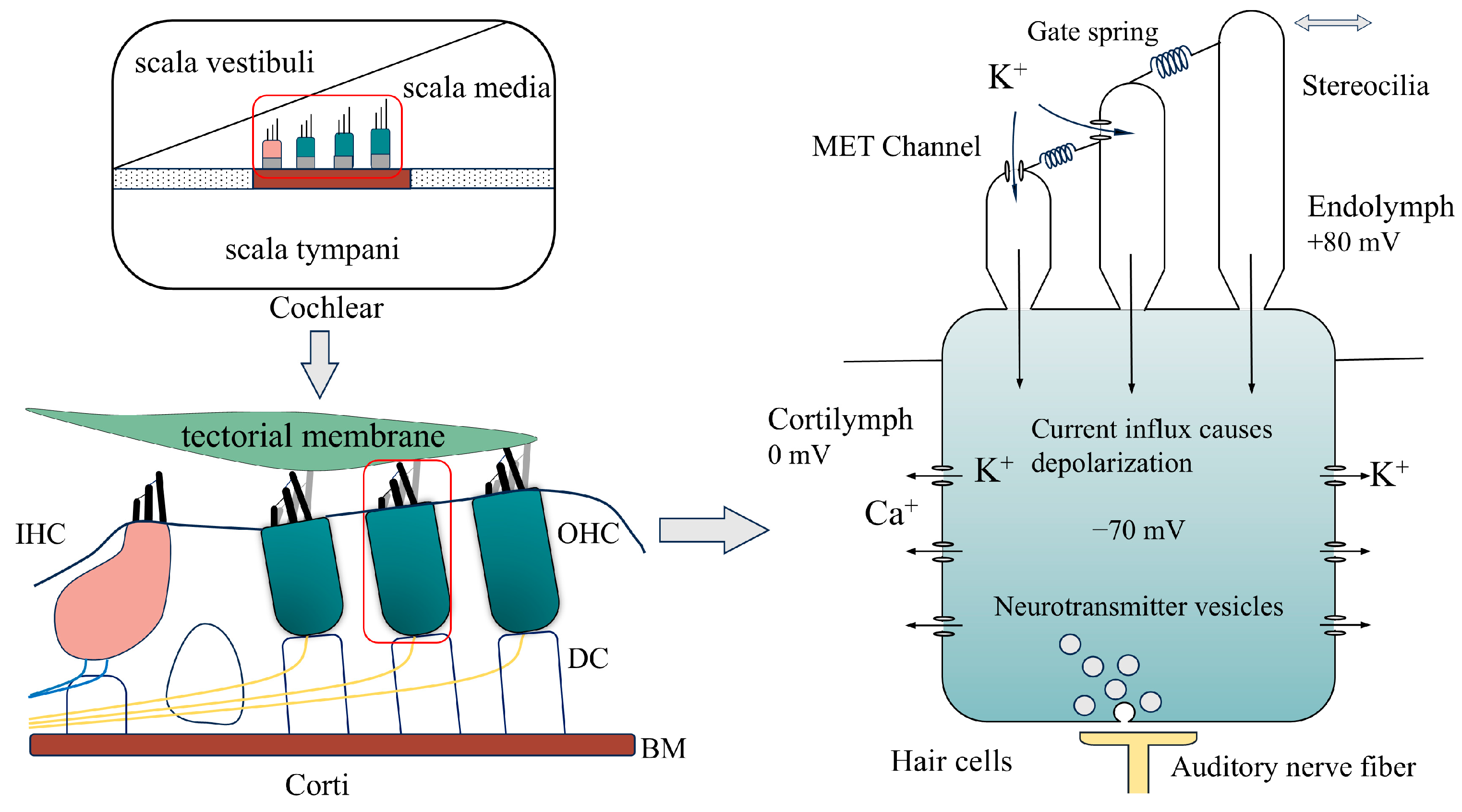
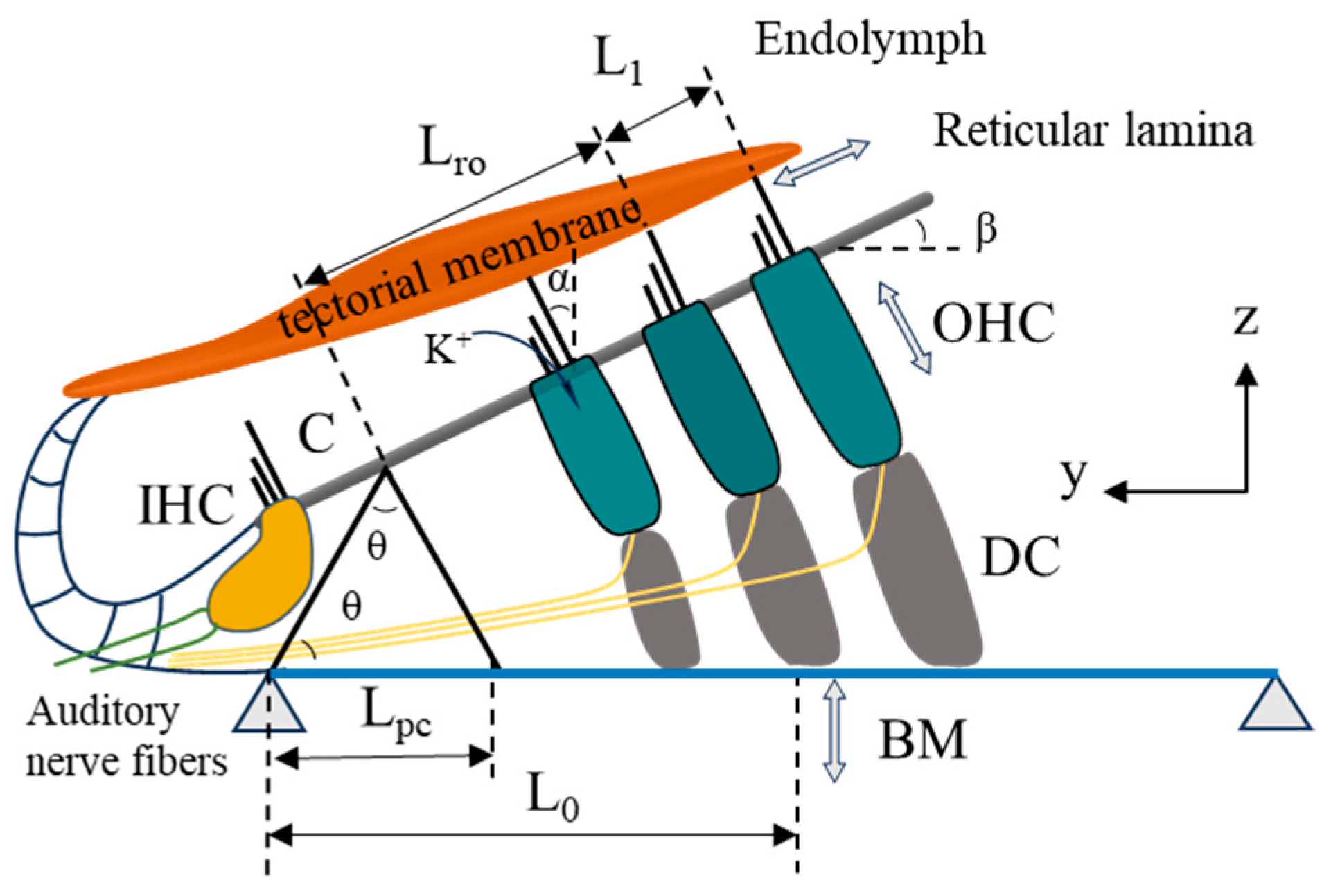
2.1. The Movement of the Organ of Corti
2.2. The Opening and Closing Characteristics of MET Channels and Electromechanical Characteristics of OHCs
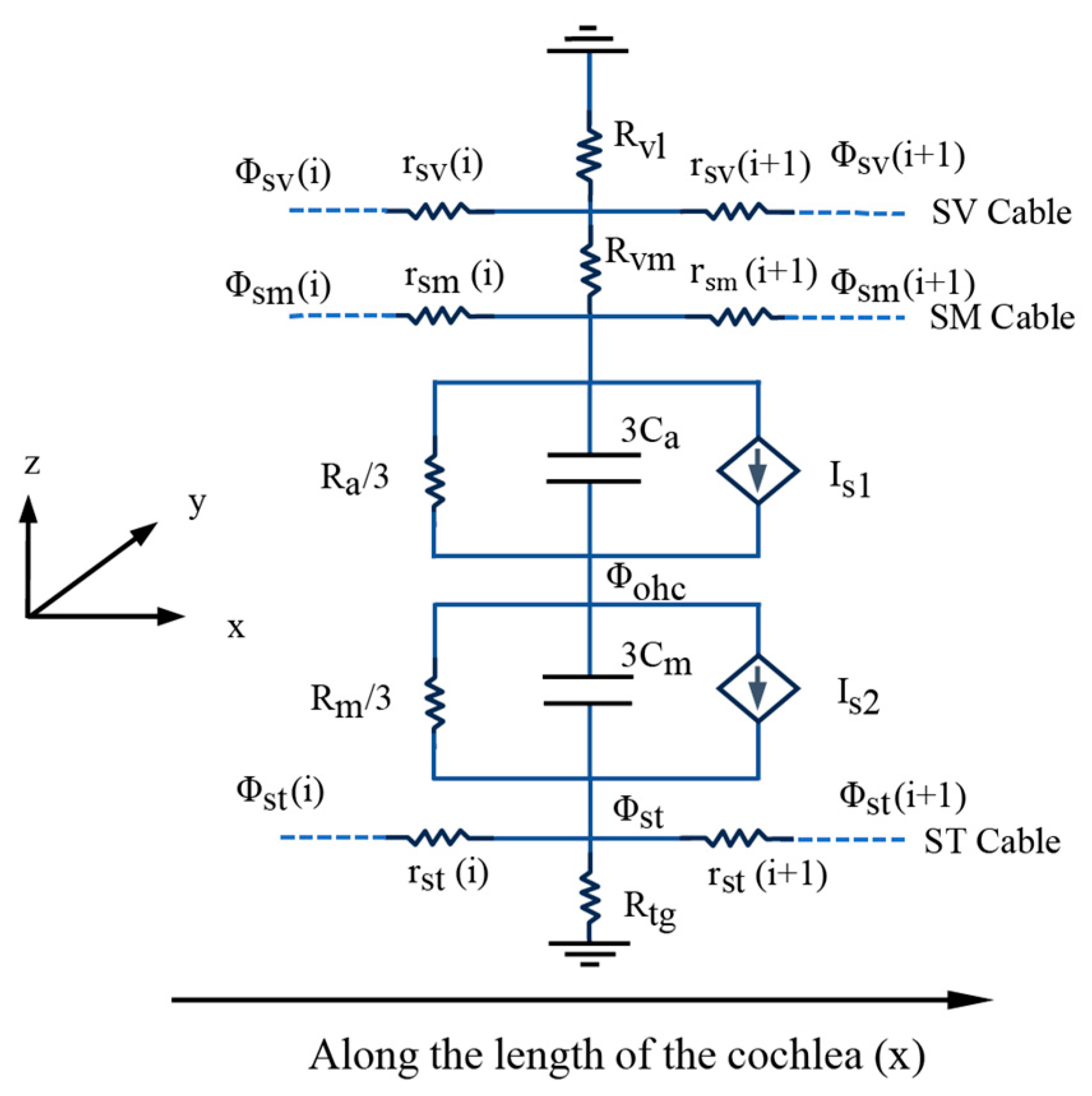
2.3. Electrical Environment of the Cochlear Cavity
2.4. Active Force Feedback
2.5. Material Properties
2.6. Control Equations and Boundary Conditions for Numerical Models
3. Results
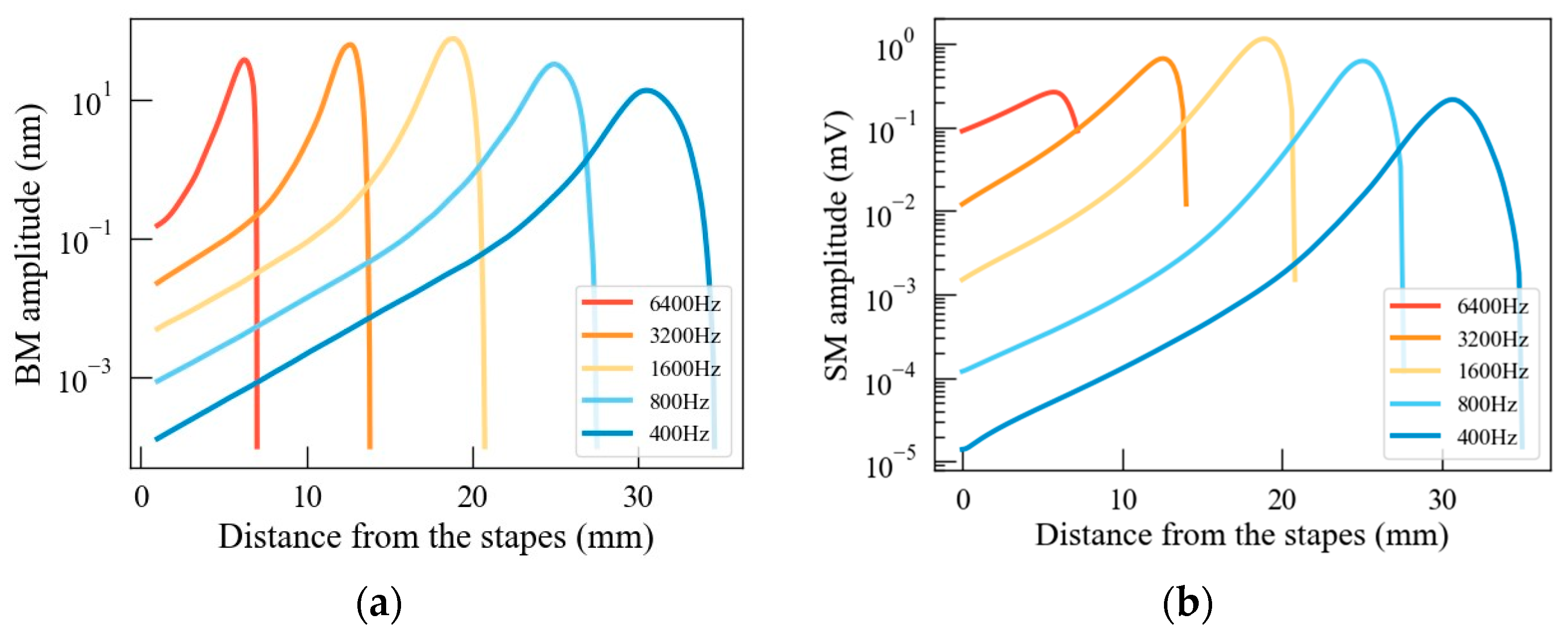
3.1. Model Validation
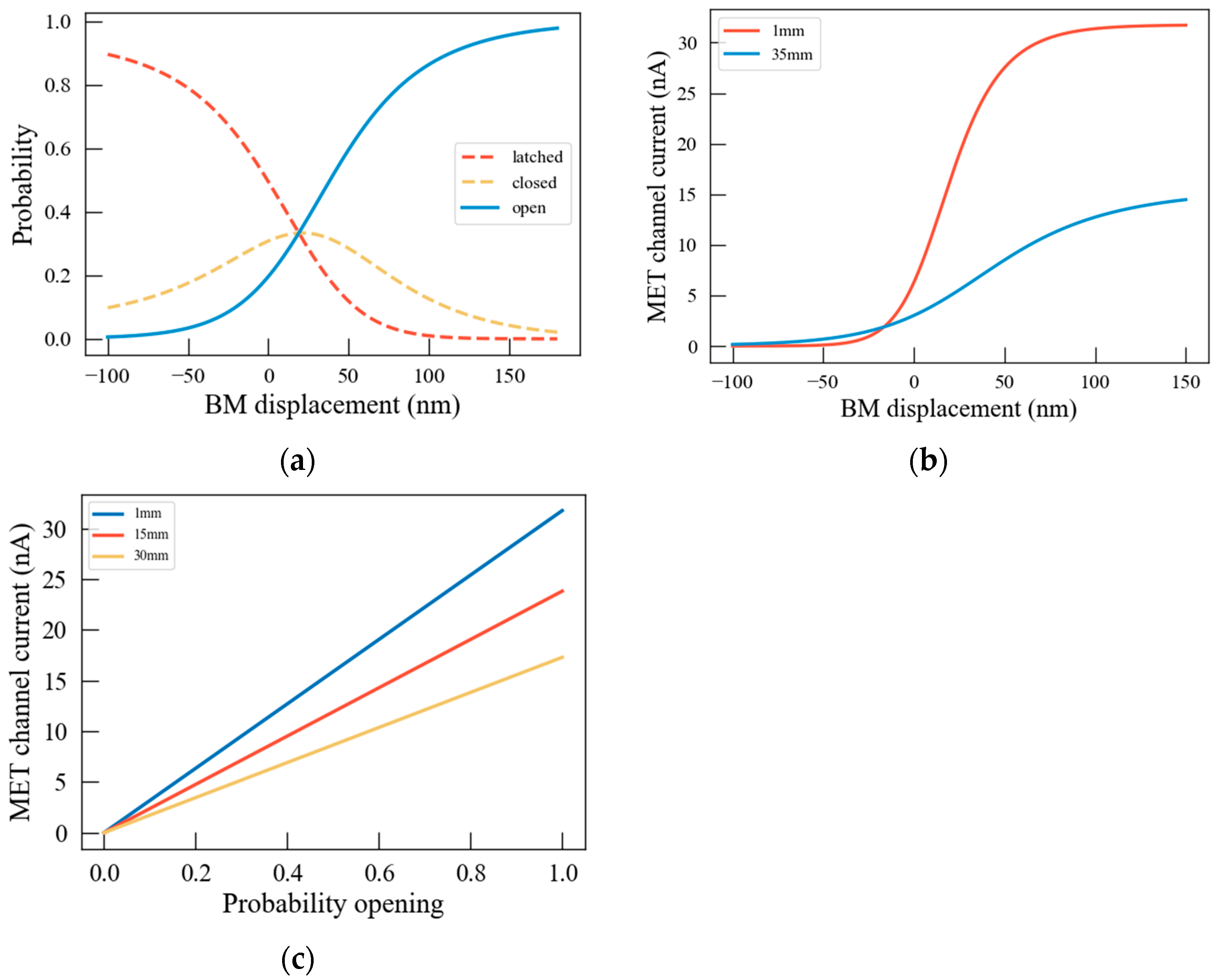
3.2. The Transduction Current of Hair Bundles
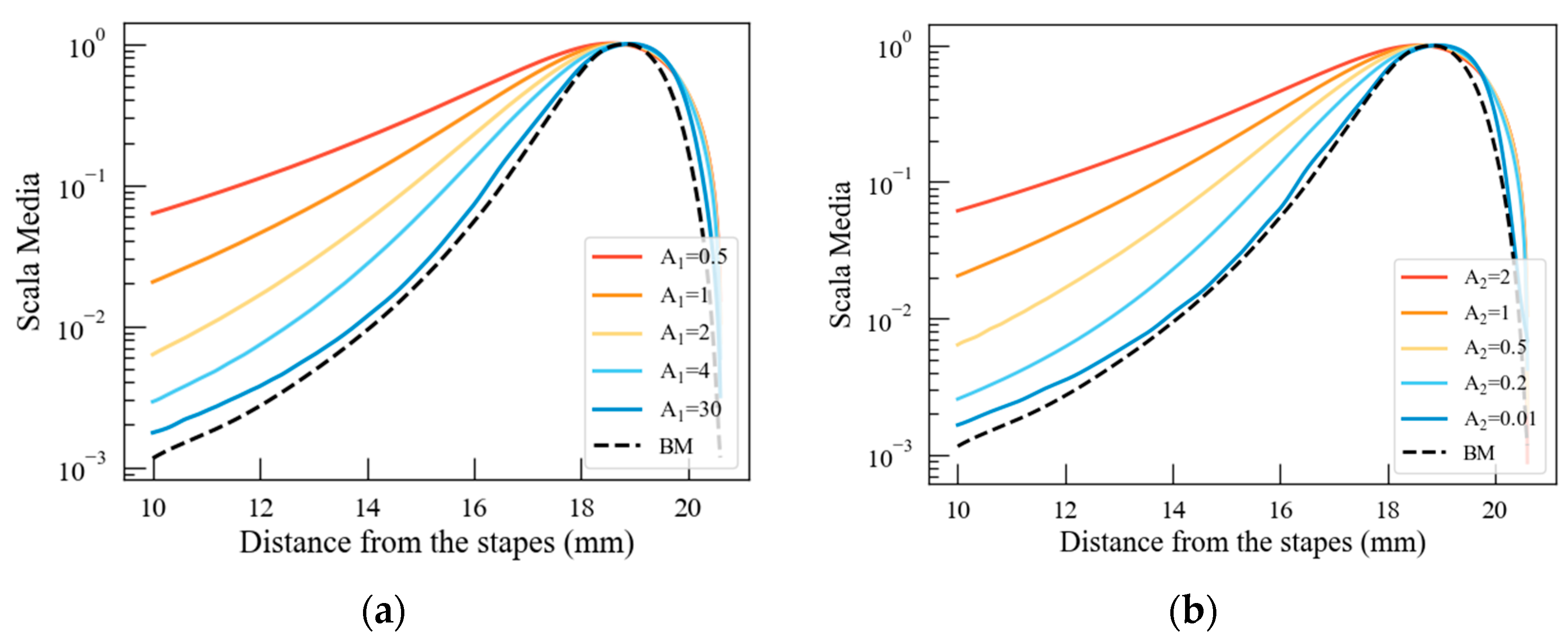
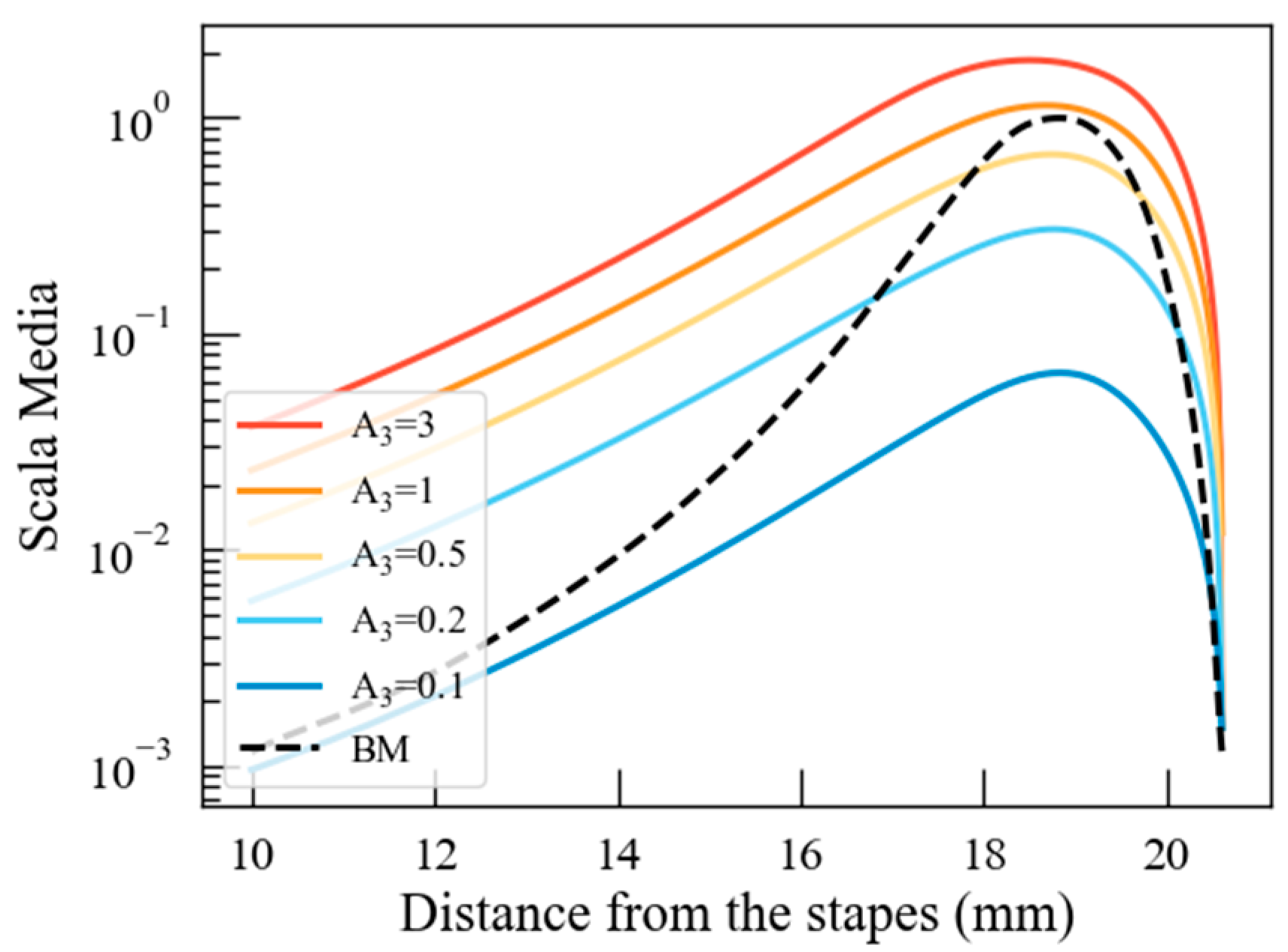
3.3. The Effect of Stereocilia Structure on Longitudinal Conduction Current
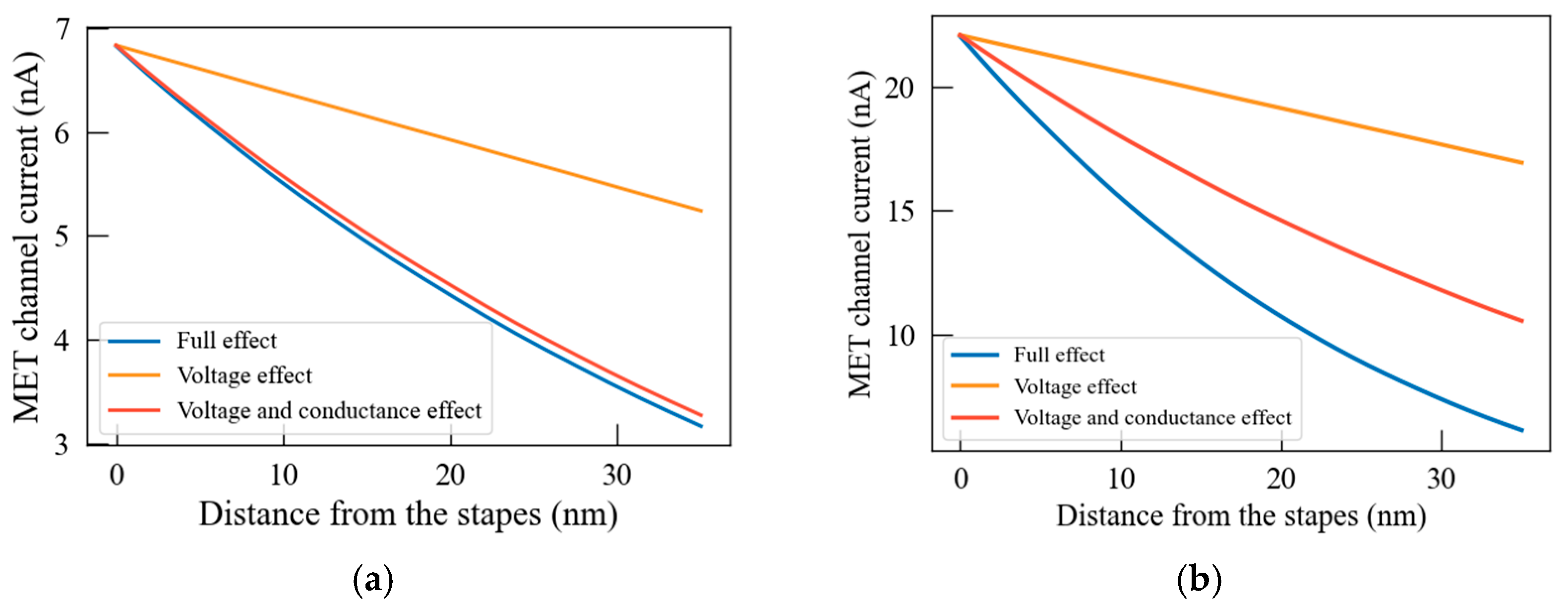
3.4. The Effect of Resistance on CM Potential
4. Discussion
4.1. Potential Distribution Pattern
4.2. The Effect of Stereocilia Stiffness on Cochlear Potential
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dallos, P.; Cheatham, M.A. Production of cochlear potentials by inner and outer hair cells. J. Acoust. Soc. Am. 1976, 60, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.N.; Hackney, C.M.; Evans, M.G. Localisation of the mechanotransducer channels in mammalian cochlear hair cells provides clues to their gating. J. Physiol. 2010, 588, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Robles, L.; Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. Rev. 2001, 81, 1305–1352. [Google Scholar] [CrossRef] [PubMed]
- Dallos, P. Cochlear amplification, outer hair cells and prestin. Curr. Opin. Neurobiol. 2008, 18, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Gummer, A.W.; Hemmert, W.; Zenner, H.P. Resonant tectorial membrane motion in the inner ear: Its crucial role in frequency tuning. Proc. Natl. Acad. Sci. USA 1996, 93, 8727–8732. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.A.; Naik, K.; Dallos, P. Using the Cochlear Microphonic as a Tool to Evaluate Cochlear Function in Mouse Models of Hearing. J. Assoc. Res. Otolaryngol. 2011, 12, 113–125. [Google Scholar] [CrossRef]
- Mistrik, P.; Mullaley, C.; Mammano, F.; Ashmore, J. Three-dimensional current flow in a large-scale model of the cochlea and the mechanism of amplification of sound. J. R. Soc. Interface 2009, 6, 279–291. [Google Scholar] [CrossRef]
- Howard, J.; Roberts, W.M.; Hudspeth, A.J. Mechanoelectrical transduction by hair cells. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 99–124. [Google Scholar] [CrossRef]
- Markin, V.S.; Hudspeth, A.J. Gating-Spring models of mechanoelectrical transduction by hair cell of the internal ear. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 59–83. [Google Scholar] [CrossRef]
- Nadrowski, B.; Martin, P.; Julicher, F. Active hair-bundle motility harnesses noise to operate near an optimum of mechanosensitivity. Proc. Natl. Acad. Sci. USA 2014, 101, 12195–12200. [Google Scholar] [CrossRef]
- Furness, D.N.; Zetes, D.E.; Hackney, C.M.; Steele, C.R. Kinematic analysis of shear displacement as a means for operating mechanotransduction channels in the contact region between adjacent stereocilia of mammalian cochlear hair cells. Proc. R. Soc. B-Biol. Sci. 1997, 264, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zetes, D.E.; Steele, C.R. Fluid-structure interaction of the stereocilia bundle in relation to mechanotransduction. J. Acoust. Soc. Am. 1997, 101, 3593–3601. [Google Scholar] [CrossRef]
- Matsui, T.; Nakajima, C.; Yamamoto, Y.; Andoh, M.; Iida, K.; Murakoshi, M.; Kumano, S.; Wada, H. Analysis of the dynamic behavior of the inner hair cell stereocilia by the finite element method. JSME Int. J. Ser. C-Mech. Syst. Mach. Elem. Manuf. 2006, 49, 828–836. [Google Scholar] [CrossRef]
- Davis, H. A model for transducer action in the cochlea. Cold Spring Harb. Symp. Quant. Biol. 1965, 30, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Strelioff, D. A computer simulation of the generation and distribution of cochlear potentials. J. Acoust. Soc. Am. 1973, 54, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Deo, N.V.; Grosh, K. A mechano-electro-acoustical model for the cochlea: Response to acoustic stimuli. J. Acoust. Soc. Am. 2007, 121, 2758–2773. [Google Scholar] [CrossRef]
- Mayon Ku, E. Modelling the human cochlea. J. Acoust. Soc. Am. 2009, 126, 3373. [Google Scholar] [CrossRef]
- Ayat, M.; Teal, P.D.; McGuinness, M. An integrated electromechanical model for the cochlear microphonic. Biocybern. Biomed. Eng. 2014, 34, 206–219. [Google Scholar] [CrossRef]
- Teal, P.D.; Ni, G. Finite element modelling of cochlear electrical coupling. J. Acoust. Soc. Am. 2016, 140, 2769–2779. [Google Scholar] [CrossRef]
- Lamb, J.S.; Chadwick, R.S. Phase of Shear Vibrations within Cochlear Partition Leads to Activation of the Cochlear Amplifier. PloS ONE 2014, 9, 85969. [Google Scholar] [CrossRef]
- Dong, W.; Olson, E.S. Detection of Cochlear Amplification and Its Activation. Biophys. J. 2013, 105, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Oghalai, J.S. The cochlear amplifier: Augmentation of the traveling wave within the inner ear. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, H.J.; Crawford, A.C.; Fettiplace, R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 2005, 433, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-H.; Fettiplace, R. A Cochlear Partition Model Incorporating Realistic Electrical and Mechanical Parameters for Outer Hair Cells. In Proceedings of the AIP Conference Proceedings, Williamstown, MA, USA, 16–22 July 2011. [Google Scholar]
- Watts, L. Cochlear Mechanics: Analysis and Analog VLSI. Ph.D thesis, California Institute of Technology, Pasadena, CA, USA, 1993.
- He, D.Z.Z.; Jia, S.; Dallos, P. Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 2004, 429, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yao, W.; Hu, B. Simulation of the Multiphysical Coupling Behavior of Active Hearing Mechanism within Spiral Cochlea. J. Biomech. Eng. 2020, 142, 091005. [Google Scholar] [CrossRef]
- Dallos, P. Some electrical circuit properties of the organ of Corti. II. Analysis including reactive elements. Hear. Res. 1984, 14, 281–291. [Google Scholar] [CrossRef]
- Fernández, C.; Butler, R.; Konishi, T.; Honrubia, V.; Tasaki, I. Cochlear Potentials in the Rhesus and Squirrel Monkey. J. Acoust. Soc. Am. 1962, 34, 1411–1417. [Google Scholar] [CrossRef]
- Fridberger, A.; de Monvel, J.B.; Zheng, J.; Hu, N.; Zou, Y.; Ren, T.; Nuttall, A. Organ of Corti Potentials and the Motion of the Basilar Membrane. J. Neurosci. 2004, 24, 10057–10063. [Google Scholar] [CrossRef]
- Honrubia, V.; Ward, P.H. Longitudinal Distribution of the Cochlear Microphonics inside the Cochlear Duct (Guinea Pig). J. Acoust. Soc. Am. 1968, 44, 951–958. [Google Scholar] [CrossRef]
- Tinevez, J.Y.; Jülicher, F.; Martin, P. Unifying the Various Incarnations of Active Hair-Bundle Motility by the Vertebrate Hair Cell. Biophys. J. 2007, 93, 4053–4067. [Google Scholar] [CrossRef]
- Saunders, T.J.C. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J. Cell Biol. 1983, 96, 807–821. [Google Scholar]
- Patuzzi, B.R. A model of the generation of the cochlear microphonic with nonlinear hair cell transduction and nonlinear basilar membrane mechanics. Hear. Res. 1987, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ayat, M.; Teal, P.D.; Searchfield, G.D.; Razali, N. Cochlear microphonic broad tuning curves. In Proceedings of the AIP Conference Proceedings, Cape Sounio, Greece, 23–29 June 2014. [Google Scholar]

| Parameters | Parametric Description | Value |
|---|---|---|
| b | BM width | 100 nm~400 nm |
| The distance from the second OHC to the cochlear axis | BM width/2 | |
| Width of the arcuate structure | BM width/3 | |
| θ | Included angle of the arcuate structure | 60 deg |
| β | The angle between OHC and the z axis | 60 deg |
| α | The angle between the stereocilia and the z axis | 25 deg~45 deg |
| Stereocilium length | 1 µm | |
| Single-channel horizontal gating force | 3 pN | |
| γ | Geometric magnification factor | |
| T | Absolute ambient temperature | 300 K |
| Boltzmann constant | J/K |
| Parameters | Parametric Description | Value |
|---|---|---|
| Resistance of scala vestibuli cable | 0.1 MΩ/m | |
| Resistance of scala media cable | 0.2 MΩ/m | |
| Resistance of scala tympani cable | 100 Ω/m | |
| Resistivity between scala vestibuli cable and scala media cable | 5 Ωm | |
| Resistivity between scala vestibuli cable to ground | 10 Ωm | |
| Resistivity between scala tympani cable to ground | 10 Ωm | |
| Resistance at the top of the OHC | 100 µS/m | |
| Capacitance at the top of the OHC | 50 nF/m | |
| Resistance at the bottom of the OHC | 5100 µS/m~360 µS/m | |
| Capacitance at the bottom of the OHC | 1800 nF/m~4200 nF/m | |
| Mechanical electrical coupling coefficient | −1.04·(10−5 + x·10−6) N/m/mV | |
| Stereocilia saturated conductance | 72exp(−13.5x) nS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liang, J.; Yao, W.; Xu, C. The Potential Changes and Stereocilia Movements during the Cochlear Sound Perception Process. Mathematics 2024, 12, 2470. https://doi.org/10.3390/math12162470
Liu B, Liang J, Yao W, Xu C. The Potential Changes and Stereocilia Movements during the Cochlear Sound Perception Process. Mathematics. 2024; 12(16):2470. https://doi.org/10.3390/math12162470
Chicago/Turabian StyleLiu, Bin, Junyi Liang, Wenjuan Yao, and Chun Xu. 2024. "The Potential Changes and Stereocilia Movements during the Cochlear Sound Perception Process" Mathematics 12, no. 16: 2470. https://doi.org/10.3390/math12162470





