Abstract
Osteoporosis is frequent in elderly people, causing bone fractures and lowering their quality of life. The costs incurred by these fractures constitute a problem for public health. Markov chains were used to carry out an incremental cost-utility analysis of the four main drugs used in Spain to treat osteoporosis (alendronate, risedronate, denosumab and teriparatide). We considered 14 clinical transition states, from starting osteoporotic treatment at the age of 50 until death or the age of 100. Cost-effectiveness was measured by quality adjusted life years (QALYs). The values used in the Markov model were obtained from the literature. Teriparatide is the cost-effective alternative in the treatment of osteoporosis in patients with fractures from the age of 50, establishing a payment threshold of 20,000 EUR/QALY. However, it is the most expensive therapy, not appearing cost-effective in cases that do not present fracture and in ages over 80 years with fracture. Alendronate and denosumab therapies are presented as cost-effective osteoporosis treatment alternatives depending on the age of onset and duration of treatment. From the perspective of cost-effectiveness, establishing a payment threshold of 20,000 EUR/QALY, teriparatide is the cost-effective alternative in patients with fracture from the age of 50 to 70 years old in Spain.
1. Introduction
Osteoporosis is a chronic disease, characterized by the loss of bone quality and mass. Generally, there is no symptomatology until a fracture occurs. In the age group of 50–84, approximately 6% of men and 21% of women suffer from osteoporosis [1]. The principle risk factors for the disease are age and being female, and it is calculated that 2% of 50-year-old women suffer from it, rising to 25% at 80 [2]. Additionally, sustaining a fragility fracture is considered to be one of the most important factors in experiencing subsequent fractures [3,4]. At present, it is estimated that there are 323 million people over 65 with the disease worldwide, and, given the increase in life-expectancy, this is expected to increase to up to 1.555 billion by 2050 [5], thus increasing the prevalence of fragility fractures. In Europe in 1990 [6], there were 2.7 million osteoporotic fractures, giving rise to a direct cost of 36 billion euros, of which 24.3 billion were for hip fractures. Moreover, it was estimated that this cost would rise to 76.8 billion euros in 2050 [7]. Similar figures were reached by another European study [8], which foresaw a 25% increase in osteoporosis costs, from 37.4 billion euros in 2010 to 46.8 billion in 2025. To evaluate the efficacy of a therapy from an economic viewpoint, cost-utility analysis is used, which has the aim of quantifying how much a health system is willing to pay for quality-adjusted life years (QALYs) [9]. In Spain, the total cost in QALYs lost due to fractures is estimated to increase from 70,800 in 2010 to 89,000 in 2025, which corresponds to a 26% increase, and in the overall cost of the disease, the QALYs lost to osteoporosis will be 7.8 billion euros in 2025 [10]. From the above, health care for osteoporotic fractures and the reduction in quality of life this causes for patients gives rise to a significant clinical impact and healthcare cost, which can be reduced principally with the pharmaceutical treatment of osteoporosis.
There are different drugs for the treatment of osteoporosis, mainly classified into antiresorptive drugs, which impede the resorption and loss of bone, and anabolic drugs, which promote bone formation. In Spain, the majority of drugs used are antiresorptive, among which are bisphosphonates (alendronate and risedronate) and denosumab; the only bone-forming agent used and sold in Spain is teriparatide. All of these have demonstrated their efficiency in reducing fractures [11,12,13,14,15] and are included in the national clinical recommendations [16,17].
To analyze the cost-utility of healthcare interventions in the context of a chronic disease, Markov chains are used. Several studies have used this model for the economic analysis of osteoporosis treatment in Spain. One compares risedronate with no treatment in Spain, together with three other European countries [18], while another analyzes alendronate against a placebo, also in Spain together with eight more European countries [19]. Regarding studies carried out exclusively in Spain [20,21,22], one compares a daily administration of risedronate with alendronate given weekly [22]; to these two drugs, another [20] adds strontium ranelate, ibandronate and raloxifene compared to a placebo or calcium and vitamin D, and the most recent work [21] adds denosumab to compare with the above-mentioned drugs. The transition cycles of the cited studies was one year, except for that of Darbà et al. [21], which was only six months. Additionally, the models considered between seven [18,19,20] and eight states [21,22].
Given that healthcare and economic resources are limited, the goal of the study was to evaluate the economic impact on quality of life of the main treatments and drugs used in Spain for osteoporosis and in preventing fragility fracture, to help facilitate decision making by healthcare professionals and managers. Additionally, we analyzed the cost-utility impact of the age at which treatment was started. A total of 14 possible health states were considered, which can include a patient without fracture or after experiencing an osteoporotic fracture, that best reflect reality and with a transition period from one state to another of 12 months. We analyzed five strategies: no intervention and treatment with four drugs—alendronate, risedronate, denosumab and teriparatide. Population quality of life values were expressed in years of life with full health (QALYs).
2. Materials and Methods
2.1. Design of the Markov Models
The methodology of discrete Markov chains has been used in medicine to model events over uniformly spaced times or cycles [23,24]. It is a special type of discrete stochastic process in which the probability of an event occurring depends only on the immediately preceding event. This process is defined by a sequence of random variables, X1, X2, X3, …, called states.
We defined 14 possible health states in which a person may be placed, from starting osteoporotic treatment at age 50, through its evolution, to death or reaching 100 years of age:
X1 Event free: no fracture.
X2 Hip fracture: the patient experiences their first fracture, in this case, of the hip.
X3 Vertebral fracture: the patient experiences their first fracture, in this case, of the vertebral column.
X4 Wrist fracture: the patient experiences their first fracture, in this case, of the wrist.
X5 Other fractures: the patient experiences their first fracture, in this case, not hip, wrist or vertebral.
X6 Subsequent hip fracture: the patient experiences a subsequent fracture, in this case, of the hip.
X7 Subsequent vertebral fracture: the patient experiences a subsequent fracture, in this case, of the vertebral column.
X8 Subsequent wrist fracture: the patient experiences a subsequent fracture, in this case, of the wrist.
X9 Subsequent other fracture: the patient experiences a subsequent fracture, in this case, not hip, wrist or vertebral.
X10 Hip post-fracture: the health state of the patient after experiencing a hip fracture.
X11 Vertebral post-fracture: the health state of the patient after experiencing a vertebral fracture.
X12 Wrist post-fracture: the health state of the patient after experiencing a wrist fracture.
X13 Other post-fractures: the health state of the patient after experiencing a fracture, in this case, not hip, wrist or vertebral.
X14 Dead (absorbing state to which there is a probability of transit from any other state).
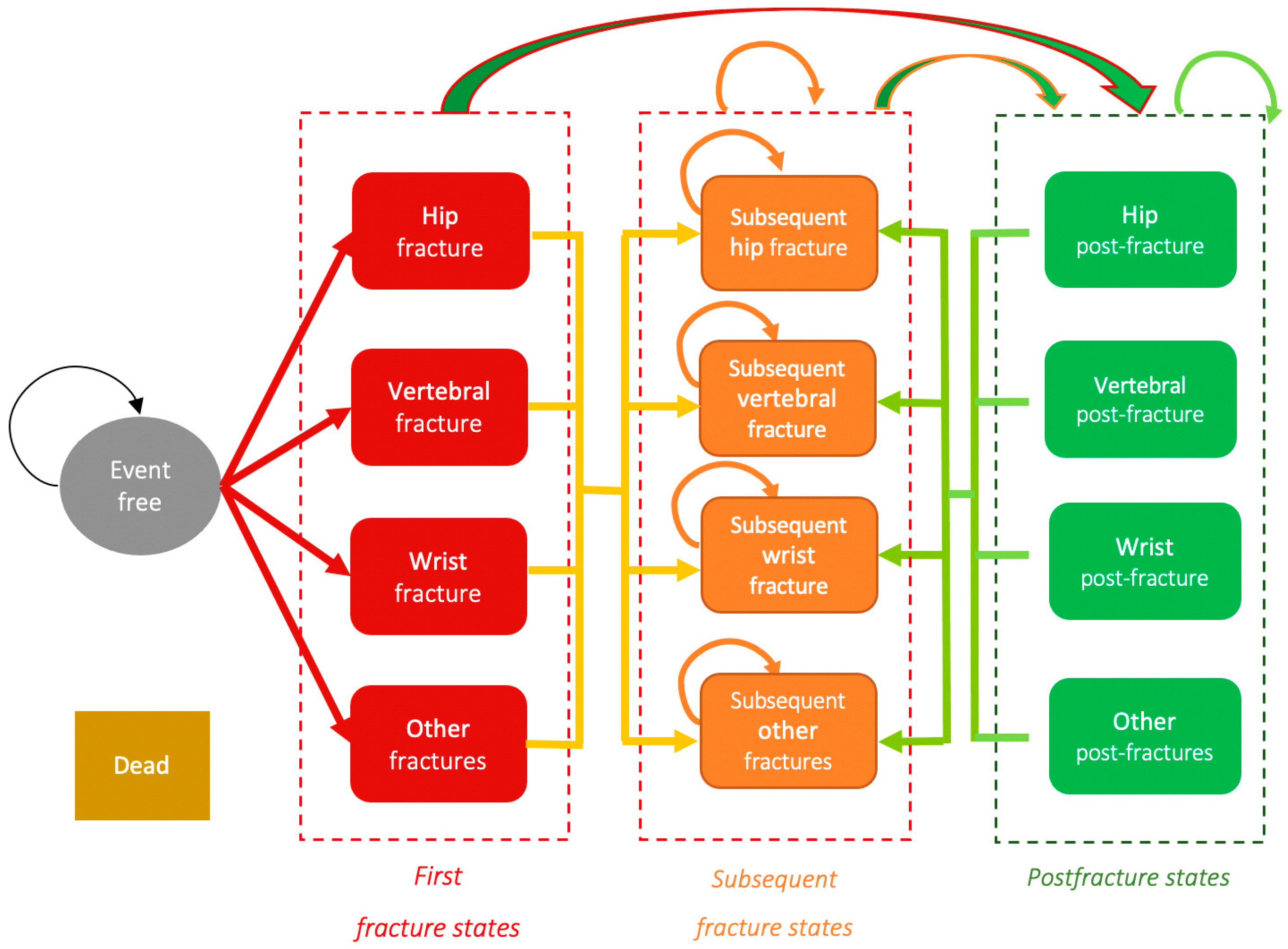
The transitions between the states in the Markov model is shown in Figure 1.
Figure 1.
Diagram of the Markov model. States and transitions. Source: prepared by the authors.
The Markov model is formulated as follows:
where X1,n, X2,n, …, X14,n are the proportion of population in each of the 14 states in the n cycle. It is assumed that X1,n + X2,n+ … + X14,n = 1 for each n cycle. As initially, it is assumed all the people are event-free, the initial cohort corresponds to the deterministic vector (x1,0, 0, …, 0)⊤, x1,0 = 1. If pi,j is the probability of transition from state i to state j in each of the n cycles, it is assumed that the transition matrix is given by:
The probabilities pi,j that an event occurs (changing from one state to another, or remaining in the same state) between two consecutive cycles n and n + 1 constitute the entries of the transition matrix. These entries are constant, deterministic, are not random variables and take a value between 0 and 1. A transition matrix was compiled for each of the five possible treatments, as the probabilities of experiencing a fracture vary for each treatment.
It is guaranteed that the condition is met that on computing the probability of remaining in the present state, or of passing to the corresponding post-fracture state in the case of states X2 to X9, as the complementary probability to the rest of events, that is:
where k takes the value of i if the patient can remain in the same state the next cycle, or the value corresponding to the appropriate post-fracture state in the case of states X2 to X9. It has been verified that in no case is the summation of probabilities greater than 1, to guarantee that it is a closed cycle in which the number of patients does not vary.
The probabilities vary according to the value of n, associating the number of cycles with the age of the patient according to the starting age considered. To modify these, lookup tables registering the probability values associated with the age ranges were used.
Each transition cycle n between the 14 possible states has a duration of 12 months, taking this period to be the duration of the clinical impact of recovery and costs for these fractures. All begin in the “event-free” state, from which they can experience any fracture in the following cycle, remain event-free or die. The patients who enter the “dead” state will remain there for the whole simulation. Those who experience a fracture can develop a subsequent fracture in the following cycle or pass to the post-fracture state of the fracture experienced. In the case of a patient presenting various fractures, they will go to the post-fracture state with greatest clinical impact, taken in this order: hip, vertebral and, at the same level, wrist or others. Once having experienced a fracture, a patient cannot pass to the “event-free” state, but must remain in the “post-fracture” state. A patient can pass to the “dead” state from any other state.
For each of the five strategies, the annual cost caused by osteoporosis in each possible state was obtained. The total costs for the treatment of the possible fractures, the costs derived from possible post-fractures and the pharmaceutical treatments according to the particular characteristics of each state were taken into consideration.
The utility in each health state was also measured by the QALYs gained in each strategy. There are different questionnaires that serve to measure and quantify the quality of life of a person: EuroQol-5D, SF-36 and SF-12, among others [25,26]. In all of them, utility takes the value 0 for dead and the value 1 for a perfect state of health. The gain in quality of life from a therapeutic alternative is expressed in QALYs and allows comparison of improvement in health for different alternatives.
To obtain the results for costs and utility, the number of patients remaining in each of the states at the end of the cycle is multiplied by the cost or utility associated with this state and cycle. Given that the Markov model takes into consideration costs incurred over different years in a quite wide time frame, it is necessary to homogenize these amounts to the present time. To achieve this, costs and utility were adjusted by applying a 3% discount rate according to the recommended practice in Spain [27], in order to update the values along the chain and compare them in terms of present value, which is why the values for costs and utility vary with value of n.
The values for each cycle can be calculated by matrix using the vector of resulting states (X) and the corresponding vector of costs or utilities (Y) as follows:
where V indicates the total of costs or utilities for the cycle n, depending on the variable chosen for vector Y. The total costs or utilities after n cycles can be calculated by the summation of the individual n values obtained (.
Given the aggregated utility and cost values obtained after n cycles, the comparison between two options is carried out through the ratio of the increment in cost over the increment in effectiveness (ICER), according to the following formula:
The results are expressed in euros (EUR)/quality adjusted life years (QALYs).
We carried out a cost-utility analysis for the base scenario and subsequently, a sensitivity analysis, modifying possible treatment alternatives.
In the base scenario, the patients commence treatment for five years with alendronate, risedronate and denosumab at the beginning of the model. However, teriparatide is administered for two years from the time of experiencing fracture. The duration of each treatment was established according to the clinical guidelines [17,28,29]. Once the treatment is finished, an efficiency of two years for all the drugs was considered (known as offset time), with a linear reduction in the effect until reaching 0 during this period.
A Markov model was developed for each strategy, that is, non-intervention and treatment with each of the four drugs (alendronate, risedronate, denosumab and teriparatide). Furthermore, the base scenario was analyzed according to different ages of starting treatment: 50, 60, 70 and 80 years old.
A value for cost and for utility is obtained as a result of each of the Markov models, with the goal of maximizing health gains of the patients. Following this, the ICER is calculated for the treatments with the four drugs compared with non-intervention (the control group), as well as between the four drugs themselves. In the comparison with no intervention, a willingness-to-pay (WTP) threshold of 20,000 EUR/QALY was established, in accordance with initial work carried out in Spain that proposed an WTP threshold of 20,000–30,000 EUR/QALY [30,31].
Additionally, two sensitivity analyses were carried out:
- The duration of treatment with denosumab is extended to 10 years, once its efficacy and safety are demonstrated [32].
- Treatment with teriparatide is started at the beginning of the model in patients without fractures.
The program TreeAge Pro Healthcare 2020 was used in the design and analysis of Markov modeling.
2.2. Materials
The following factors were used to calculate probabilities in the design and development of the five transition matrices: risk of experiencing a fracture without intervention, efficacy and safety of the pharmaceutical treatment, and mortality. To calculate the state variables, we estimated the costs, quality of life and treatment adherence and persistence. The values for these factors considered in the model have been extracted from other previously published works, detailed below.
2.2.1. Incidence of Fractures without Intervention
Given the lack of a national registry of osteoporotic fractures in Spain, the data for the incidence of fractures without intervention were compiled from Svedbom et al. [10], who published the epidemiological data of osteoporosis for 27 countries of the European Union. These data were adjusted for the Spanish population in 2010 according to the National Institute of Statistics (Instituto Nacional de Estadística—INE) and were merged by gender for each age range in the Markov model (Table 1).

Table 1.
Fracture incidence × 100,000 inhabitants/year in the Spanish population in 2010, adjusted according to INE.
Furthermore, patients who have experienced an osteoporotic fracture have a greater risk of experiencing further fractures compared to those who have not yet developed any. For this reason, a distinction has been made in the model, using states, between the first osteoporotic fracture and the second and subsequent fractures. The data for the incidence for second and subsequent fractures were obtained from Klotzbuecher et al. [3] (Table 2). This work estimates the risk of experiencing a second fracture for post-menopausal women according to the type of fracture previously experienced. Given the lack of works in the literature that provide data for men, we considered the risk of subsequent fracture for both sexes to be the same.

Table 2.
Relative risk of experiencing a second osteoporotic fracture according to previous type of fracture in peri/post-menopausal women.
2.2.2. Efficacy and Safety in the Pharmaceutical Treatments
The efficacy of the four drugs analyzed in the model was taken from the meta-analysis [33] published in 2020 by the National Institute for Health Research in the UK. These data are shown in Table 3.

Table 3.
Model parameters in this study.
2.2.3. Costs of Fractures and Treatments
The cost of each osteoporotic fracture in Spain is published in the work by Imaz et al. [20] for the direct and indirect healthcare costs of a hip fracture in the first year, second and subsequent years, as well as the costs of vertebral fracture and wrist fracture in the first year, in 2010. These costs have been updated for 2018 according to the consumer price index (Table 3). The same cost for the first and for subsequent vertebral fractures has been assumed. Additionally, the cost of other fractures has been accepted to be the same as that of a wrist fracture.
The cost of each drug used in the model was obtained from the price published in the official list of medicines published by the Ministry of Health of Spain in 2018 (Table 3).
2.2.4. Mortality
The mortality rate used for healthy individuals in the model is that published by the INE in 2016 [34] by age group.
Regarding the mortality rate caused by fractures, the most complete publication found in the literature is Svedbom et al. from 2013 [10]. This work presents the information segregated by gender and for its use in our model, the average has been calculated (Table 4). Nevertheless, it was observed that the published rates were lower than those for the healthy population. Therefore, it was decided to add the mortality rate in the first year after fracture from Svedbom’s work to that published by the INE (Table 5), to emphasize in the model the increase in mortality that occurs in the first year of the fracture and that in subsequent years is the same as in the rest of the population. The assigned mortality rate at the beginning of the model was zero, thus allowing all people to participate.

Table 4.
Mortality rate per 100,000 inhabitants in the first year after fracture adjusted by comorbidities in Spain in 2010.

Table 5.
Gross mortality rate for both sexes in Spain in 2016 according to INE.
2.2.5. Utility or Quality of Life
Fractures result in a reduction in the quality of life of a patient, which varies according to the type of fracture. The values used for the utility of each fracture were obtained from the Australian work by Abimanyi-Ochom et al. [35], published in 2015, and which used the EQ-5D questionnaire. This work breaks down the utilities according to the type of osteoporotic fracture and the point at which the patient finds themself (before experiencing fracture, experiencing fracture and post-fracture state at 4, 12 and 18 months). In the model, for each state of fracture, we used the utility produced at the moment of the fracture for the cycle corresponding to that fracture and according to the type of fracture, and for the “hip post-fracture” and “vertebral post-fracture” states, we used the values published for 12 months post-fracture (Table 6).

Table 6.
Utility at 12 months after each type of fracture.
2.2.6. Treatment Adherence and Persistence
The Professional Society for Health Economics and Outcomes Research (ISPOR) defines therapeutic compliance or adherence as “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen”. As well as the manner in which a treatment is taken, the results also depend on time. Therefore, the concept of persistence has been added, which is defined as “the duration of time from initiation to discontinuation of therapy” [36]. In order to simplify the model, the present work has assumed complete adherence and persistence by the patients.
3. Results
The results obtained in the cost-utility analysis are presented below for treatment starting at 50, 60, 70 and 80 years old with alendronate, risedronate and denosumab, and at the time of the fracture in the case of teriparatide. A comparison was carried out of the treatments with that of non-intervention (control group), and later between each of the interventions.
Comparing the four treatments in the scenario starting at 50 years old, all of them are effective alternatives with regard to non-intervention (c) and, therefore, could be cost-effective alternatives, depending on the WTP threshold established (Table 7). In this case, teriparatide is the most cost-effective alternative and is within the established WTP threshold of 20,000–30,000 EUR/QALY. Regarding the antiresorptive drugs, alendronate and risedronate are dominated by teriparatide, as they are less effective and more expensive. Denosumab shows greater utility than teriparatide, but its ICER is very high, surpassing the established WTP thresholds. As such, for this age range, it could be said that teriparatide, begun with the appearance of the fracture, is the cost-effective therapy. The rest of the treatments should be discouraged, as the cost is very high for the gain obtained.

Table 7.
The ratio of the increment in cost over the increment in effectiveness (ICER) for the 4 drugs compared with non-intervention and with each other.
In the scenario with the starting age of 60 (Table 7), as with that of 50, all the therapies are more useful than that of non-intervention. In this case, teriparatide continues to be the alternative with greatest cost-utility. An important change appears with the antiresorptives, as alendronate is the most cost-effective therapy within the WTP threshold of 20,000–30,000 EUR/QALY, with risedronate dominating. Denosumab would also be a cost-effective treatment with a much higher WTP threshold established (ICER of 72,905.15 EUR/QALY) than that of alendronate (26,838.09 EUR/QALY). Therefore, for the starting age of 60, teriparatide would be the cost-effective treatment when there is a fracture and alendronate would be a cost-effective starting treatment in patients with osteoporosis.
In the scenario with the starting age of 70 (Table 7), as with the previous ages, all the treatments are more cost-effective than non-intervention. As in the scenario with a starting age of 60, teriparatide is the most cost-effective alternative. It should be noted that treatment with denosumab, which at 60 was not within the established WTP threshold, now is. Therefore, at 70, alendronate and denosumab would be two cost-effective antiresorptive alternatives.
In the scenario with the starting age of 80 (Table 7), continuing with the trend of the previous results, all the treatments are more cost-effective than non-intervention. Nevertheless, there are important changes in cost-utility for this age with respect to the previously described scenarios. In this case, teriparatide and risedronate are dominated by alendronate, due to lower utility. For this age range, alendronate is the most cost-effective treatment, followed by denosumab, both included within the established WTP threshold of 20,000–30,000 EUR/QALY.
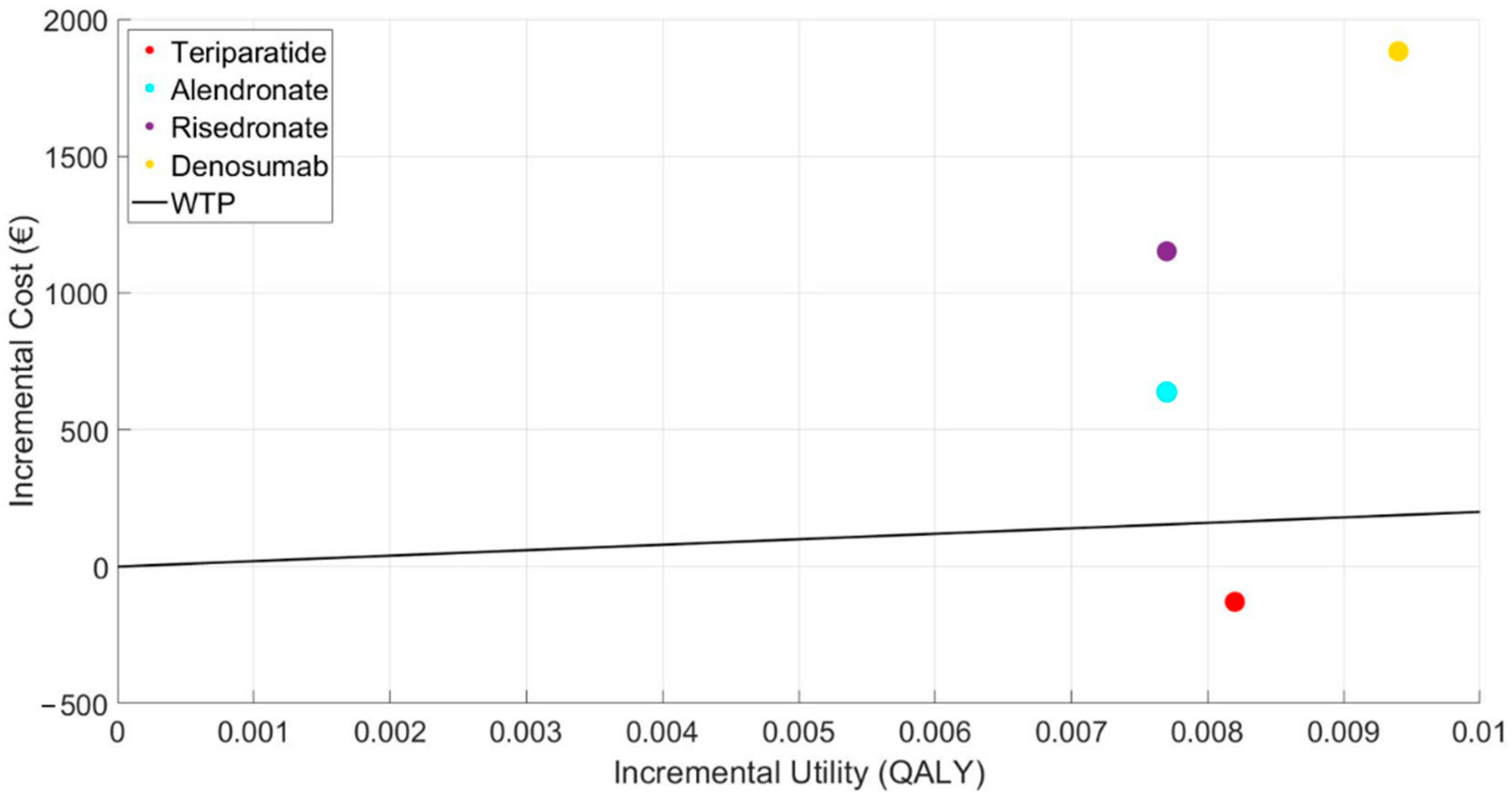
Below, the results of the cost-utility plane are shown (Figure 2), with the cost-utility increments compared to the control or reference point, and the typical WTP threshold established at 20,000 EUR/QALY in the case base scenario with a starting age of 50. This figure graphically expresses the results commented previously, showing that teriparatide is the only alternative below the WTP line.
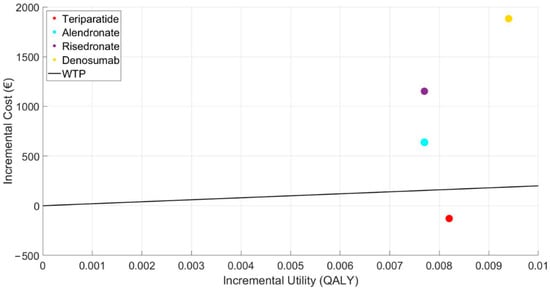
Figure 2.
Representation of the incremental cost-utility plane of the treatments.
3.1. Sensitivity Analysis
3.1.1. Denosumab for 10 Years
The results obtained in the cost-utility analysis are shown for a treatment starting age of 50 for alendronate and risedronate, both with a treatment duration of five years, and with treatment extended to 10 years for denosumab. Teriparatide is started with the appearance of the fracture as in the base scenario. A comparison of the treatments with non-intervention (control group) was carried out and later between each of the interventions (Table 8).

Table 8.
The ratio of the increment in cost over the increment in effectiveness (ICER). Extension of denosumab to 10-year treatment.
As with the base scenario starting treatment at 50, all the therapies are more useful than non-intervention. Teriparatide remains the most cost-effective. However, there is a substantial change in the treatment with denosumab, as, by extending the treatment to 10 years, the ICER values descend drastically (from 200,005.65 EUR/QALY to 13,799.04 EUR/QALY), and it becomes a cost-effective therapy within the previously established WTP threshold. Thus, when starting a therapy with an antiresorptive, denosumab would be the choice for the long term.
3.1.2. Teriparatide Given at the Start of the Model
The results are shown for the analysis giving all the drugs, including teriparatide, at the start of the model—that is, in patients with osteoporosis who have not experienced a fracture, from 50 years of age (Table 9). For this case, all the treatments continue to be more useful than non-intervention. However, the ICER of the treatments are very high, far above the 20,000–30,000 EUR/QALY WTP threshold, with teriparatide having the highest cost, dominated by denosumab. As such, teriparatide is not cost-effective if administrated without the appearance of a fracture.

Table 9.
The ratio of the increment in cost over the increment in effectiveness (ICER). Teriparatide administered at the start of the model.
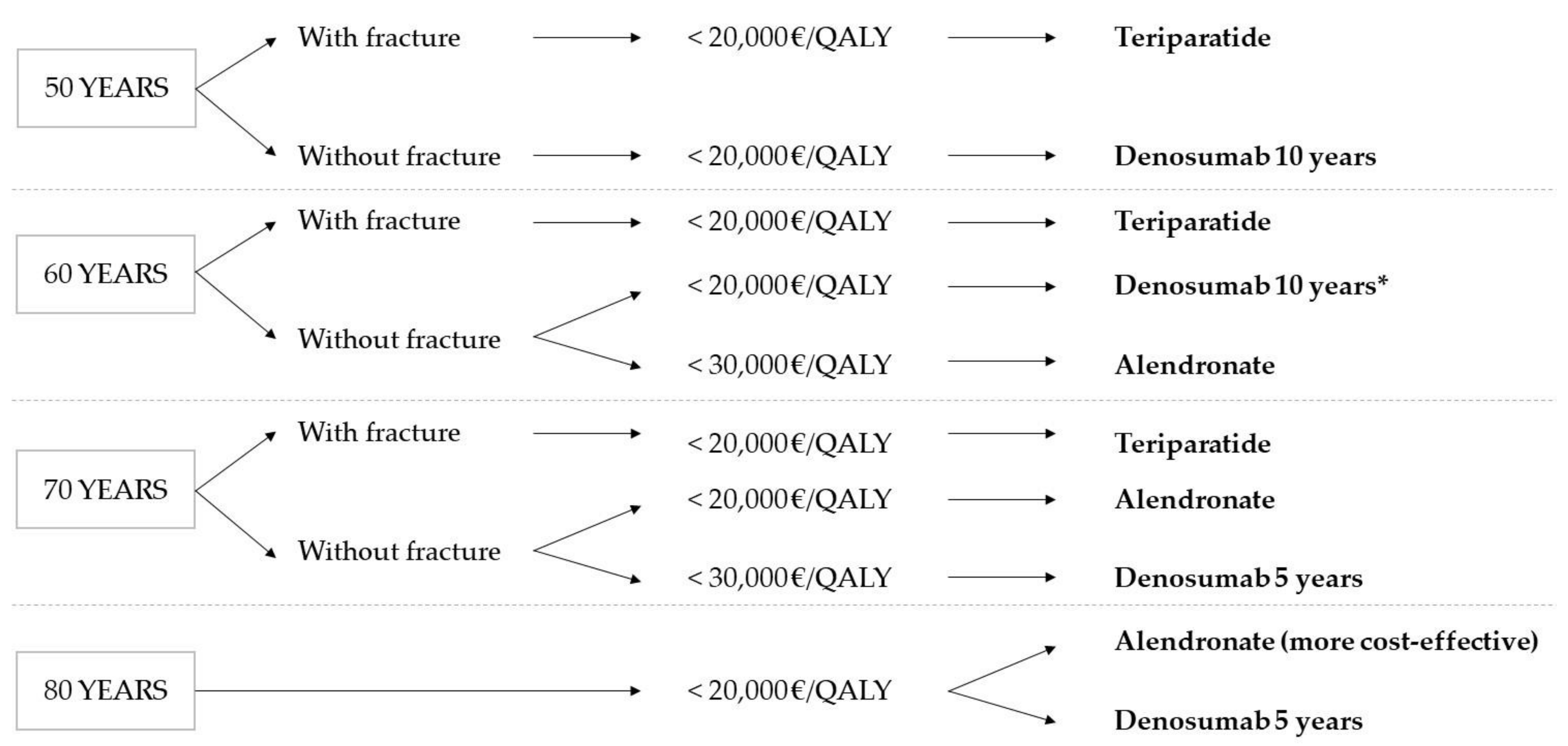
Figure 3 shows the treatment decision tree according to the cost-utility results obtained in this work.
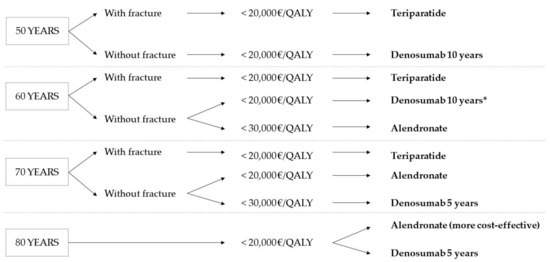
Figure 3.
Treatment decision tree according to cost-utility results. * The same result as at 50 is assumed.
The results obtained indicate teriparatide as the cost-effective alternative in the treatment of osteoporosis in patients with a fracture aged 50 and above, establishing a WTP threshold of 20,000 EUR/QALY. Nevertheless, it is the most expensive therapy, not being cost-effective in cases without fracture or in ages above 80 with fracture. The therapies with alendronate and denosumab are shown to be cost-effective treatment alternatives for osteoporosis, depending on the starting age and duration of the treatment. Thus, with a WTP threshold of 20,000 EUR/QALY, treatment with denosumab started at 50 is only cost-effective when given for 10 years. However, for older patients, the best choice from the point of view of cost-utility is alendronate for five years.
4. Discussion
Using Markov models, in this work we have analyzed the principal strategies used to treat osteoporosis in Spain from a point of view of cost and clinical utility. It is the first cost-utility work in Spain to compare a bone-forming agent, teriparatide, with other antiresorptive drugs, as other works [18,19,20,21] have only analyzed antiresorptive or dual action drugs, such as strontium ranelate.
Furthermore, the majority of works have used simpler models, including only hip or vertebral fractures [37], or later adding other locations and with certain limitations to transition between states [38,39]. In the model we developed, we consider all the possible states in the evolution of osteoporosis in the patient. We carried out a simulation the most similar to reality as possible, represented by 14 states in which the patient may be placed, allowing transition between all fracture states. All patients can experience a subsequent fracture, even if having previously presented a hip or vertebral fracture, emphasizing the risk factor of experiencing another osteoporotic fracture when having experienced one previously. This has not been carried out in the Spanish [20,21] and European works [18,19], in which patients who had experienced hip or vertebral fractures could not go on to have another in the wrist or other location.
As in other Spanish [20,22] and European works [18,19], as well as in different international studies [37,39,40,41,42], the proposed transition cycle between states is 1 year, given that it is unlikely that another fracture occurs in this period and in order to facilitate analysis and evaluation of the model’s results.
Faced with the lack of primary data in Spain for developing the models, data from the literature were used, as in other Spanish studies [20,21]. The data for fracture incidence were taken from epidemiological references of other countries in the European Union. Additionally, are there no specific data for the mortality rate by age, sex or fracture in Spain; therefore, the mortality rates published by the INE were used, adding the mortality rate published in the work by Svedbom et al. [10]. Other Spanish works have used regional mortality rates [43], have only adjusted the increase in mortality in the first year of the fracture [44] or only in hip and vertebral fractures in the case of other publications, and later extrapolated the data to the whole Spanish population [21].
There is no consensus on the correct value for the WTP threshold per QALY. Each country establishes an unofficial guideline value for agents to make decisions in medication and healthcare technology policies, and very different values can be found between them. In the USA, the values are between 100,000–297,000 USD/QALY [45]. In Great Britain, the NICE recommendations are for 20,000–30,000 GBP/QALY, giving higher values in pathologies with a life expectancy of less than two years [46,47]. The WHO suggests that a procedure is cost-effective if the value is between 1 and 3 times the per capita income of the country [48]. A Greek work carried out in 2015 [42] proposed a cost-utility threshold of 30,000 EUR/QALY, which would correspond to twice the per capita income for 2014. There is no officially recommended figure in Spain. Initial works situated it at 20,000–30,000 EUR/QALY [30,31]. Later, a Spanish revision carried out by De Cock et al. [9] in 2007 suggested that a reasonable value would be in the range of 30,000–45,000 EUR/QALY.
In the initial analysis of the present work, all the drugs (alendronate, risedronate, denosumab and teriparatide) were more useful than non-intervention. Therefore, depending on the WTP threshold established, all of them could be cost-effective alternatives to non-intervention. Our work established the threshold at 20,000 EUR/QALY, situated in the lower range of the values mentioned above. In the base scenario, the cost-utility results vary according to the treatment starting age. From 50 to 70 years old, teriparatide is the cost-effective alternative when administered to patients with a fracture. Nevertheless, after 80 years old, it is dominated by alendronate and is not a cost-effective alternative. Furthermore, in the sensitivity analysis, it is seen that it is not cost-effective if administered before fractures appear, in this sense corroborating its indication in the clinical guidelines as a second-line treatment for osteoporosis—that is, for high-risk patients who have presented fracture.
Reviewing similar works, a model developed using Swedish data by Murphy et al. [49] justifies the use of teriparatide with a threshold of 50,000 EUR/QALY as first-line treatment compared to bisphosphonates in patients of 69 years old with low bone mineral density or under treatment with corticosteroids, and who had a recent fracture. In that study, for 70 years old, the ICER of teriparatide compared to no treatment is 18,701 EUR/QALY, and 36,995 EUR/QALY compared to the bisphosphonates, similar results, if a little higher, to those found in our work, which are 13,850 EUR/QALY and 18,301 EUR/QALY, respectively. Similarly, the Iranian model developed by Taheri et al. [50] considers teriparatide as cost-effective, with a probability of 51% or 83% and an accepted WTP threshold 2 or 3 times the gross domestic product/capita, respectively, when used in patients with severe osteoporosis (low bone mineral density and/or prior fracture). On the other hand, different Chinese [40] and American works [51,52,53] present greatly different results than ours and the aforementioned works for two fundamental reasons: the high price of teriparatide in those countries and its use in some of them as a first-line treatment in patients with osteoporosis and a high risk of fracture, but without having yet experienced one. The cost of teriparatide in 2018 was 20,161 USD/year in the USA [53], compared to 4888 EUR/year in Spain and 5380 EUR/year in Sweden in 2011 [49].
In the present work, at the age of 50, the cost-utility threshold for antiresorptives (bisphosphonates and denosumab) is very high (>50,000 EUR/QALY) compared to no treatment. However, if the duration of the denosumab treatment is extended to 10 years, it is a cost-effective alternative within the established WTP threshold. It is after ages 60 and 70 that alendronate and denosumab become cost-effective alternatives within the accepted threshold of 20,000–30,000 EUR/QALY compared with non-intervention. The great majority of published works on cost-utility refer to these two drugs, with different results according to the population analyzed. For example, Parthan et al. [39] published a work in 2013 with reference to the USA which compared denosumab and the bisphosphonates, finding alendronate to be a cost-effective alternative in patients with high risk of fracture, but the incremental cost with respect to denosumab is only 7900 dollars. On the other hand, denosumab is the cost-effective alternative for patients over 75, taking as reference a threshold of 100,000 USD/QALY. Two Japanese articles [53,54] found that denosumab is cost-effective with respect to alendronate in patients with high risk of fracture over 65, 75 and 80 years old, accepting a threshold of 50,000 USD/QALY. However, an Australian work carried out in 2016 [55] based on a transition model rather than Markov concluded that the price of denosumab needed to be reduced by 50% in order for it to be cost-effective compared to alendronate. A systematic review, recently published in June 2020 [33] that compares various non-bisphosphonate drugs (denosumab, raloxifene, romosozumab, teriparatide) with each other, with the bisphosphonates and with non-intervention, concluded that the non-bisphosphonate drugs are effective in reducing fractures, but their ICER is above the 20,000–30,000 GBP/QALY threshold generally applied. In said work, denosumab could be below a threshold of 30,000 GBP/QALY in very high risk levels or patients at high risk (low bone mineral density and high risk of fracture) with specific characteristics.
Analyzing the Spanish references on cost-utility, a work published in 2002 [22] compares alendronate and risedronate with a placebo, finding that risedronate gives better cost-utility results in patients over 70. By contrast, the work by Imaz et al. [20] using costs from 2010, finds, as in our work with costs from 2018, alendronate to be cheaper than risedronate for this age, and the cost-utility results change. In this same work, which analyzes various bisphosphonates, non-intervention is the cost-effective alternative if treatment is started between 50–72 years old, but after 73, alendronate dominates all the alternatives considered, taking as reference the NICE proposal for the WTP threshold (20,000–30,000 GBP/QALY), the same as the results of the present work. A recent Spanish study carried out by Darbà et al. [21] compares denosumab with non-intervention, generic bisphosphonates and strontium ranelate. However, unlike our work, teriparatide is not included in the analysis. In this study, published in 2015, denosumab is the cost-effective alternative, with a WTP threshold of 30,000 EUR/QALY in patients older than 70 and with prior fracture or low bone mineral density. The cost-utility values improve even more when the duration of treatment is extended to 10 years, as is observed in the present work. Thus, denosumab does not give cost-effective results in patients younger than 70 without fracture, with alendronate being the dominant alternative in this case.
In the design of our model, it was assumed that adherence and persistence were complete with respect to all the drugs, in line with other works such as the Greek by Makras [42] or the Chinese by Na Li [40] in order to facilitate modeling. Unfortunately, this does not occur in realty. Therefore, this must be taken into consideration when prescribing treatment in scenarios where the accepted WTP threshold offers various cost-effective alternatives, since, as observed in the work by Freemantle et al. [56], it seems that denosumab could have the greatest adherence. A French work [57] observed that risedronate, administered in gastro-resistant tablets to avoid side effects and improve adherence, was cost-effective with a WTP threshold below 60,000 EUR/QALY compared with alendronate taken weekly or non-intervention, the results improving with the greater the patient’s risk of fracture, reaching a threshold below 20,000 EUR/QALY in patients with low bone mineral density and prior fractures.
In conclusion, the choice of drug from the cost-utility perspective varies principally according to the WTP threshold accepted. It must be borne in mind that below 20,000 EUR/QALY, teriparatide is the dominant option when there is a fracture and an age range of 50–70 years old. Given its high price, it is not cost-effective in cases without fracture. Regarding the antiresorptives, from 50 years of age on, denosumab is a cost-effective alternative if administered for 10 years, possibly adding better treatment adherence according to the patient’s characteristics. On the other hand, alendronate would be indicated from a cost-utility perspective in patients with osteoporosis from 60 years of age on, and is the lowest cost option for those over 80.
Regarding limitations, the ideal scenario for constructing the economic evaluation Markov model would have been to obtain data extracted from a national register of osteoporotic fractures in Spain, something that does not yet exist. It is also possible to underestimate the occurrence of some events, for example, a new fracture while in a transition state or a patient with two fractures at the same time, events which are difficult to incorporate in this type of model that tries to simplify the processes to provide easier analysis. In scenarios where there are various cost-effective therapies according to the WTP threshold accepted, adherence and persistence should be considered in the prescription of treatment according to the characteristics of the patient.
5. Conclusions
From the cost-utility model designed from osteoporosis treatment in Spain, it can be concluded that the pharmaceutical therapies with alendronate, risedronate, denosumab and teriparatide are more useful than non-intervention. However, from a cost-utility perspective, the choice of treatment varies principally according to the WTP threshold established.
Teriparatide is the cost-effective alternative in treating osteoporosis in patients 50 years and older with a fracture, with a WTP threshold of 20,000 EUR/QALY. Nevertheless, it is the most expensive therapy, not being cost-effective in cases without fracture and in ages over 80 with fracture. Therefore, from an economic point of view, its use should follow its clinical guideline recommendations as a second-line therapy in patients with prior fractures and high risk of subsequent fracture, where other treatments have not been effective and/or it is indicated for the patient for its bone-forming characteristics.
The antiresorptive therapies with alendronate and denosumab are shown to be cost-effective alternatives for treatment of osteoporosis, depending on the starting age and duration of treatment. Thus, with a WTP threshold of 20,000 EUR/QALY, treatment with denosumab starting at 50 years old is only cost-effective when administered for 10 years. However, for older patients, in terms of cost-utility, the cost-effective treatment is alendronate for 5 years.
Author Contributions
Conceptualization, D.V.-C. and M.O.-G.; methodology, J.D.-C.; software, S.G.-d.J.; validation, M.O.-G., D.V.-C. and J.D.-C.; formal analysis, S.G.-d.J.; investigation, M.O.-G.; resources, D.V.-C.; data curation, S.G.-d.J.; writing—original draft preparation, M.O.-G.; writing—review and editing, D.V.-C.; visualization, J.D.-C.; supervision, D.V.-C.; project administration, S.G.-d.J.; funding acquisition, D.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee in Experimental Research of the University of Valencia” on 23 May 2016, and it received authorization from the director of the Hospital Obispo Polanco in Teruel (Spain) on 6 April 2016.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank John Wright for help with English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kanis, J.A.; on behalf of the World Healthy Organization Collaborating Centre for Metabolic Bone Diseases. Assessment of Osteoporosis at the Primary Health Care Level; University of Sheffield: Sheffield, UK, 2007. [Google Scholar]
- Osteoporosis: Assessing the Risk of Fragility Fracture; (NICE Clinical Guidelines, No. 146). National Institute for Health and Care Excellence (UK): London, UK, 2012. Available online: https://www.nice.org.uk/guidance/cg146 (accessed on 17 May 2021).
- Klotzbuecher, C.M.; Ross, P.D.; Landsman, P.B.; Abbott, T.A.; Berger, M. Patients with Prior Fractures Have an Increased Risk of Future Fractures: A Summary of the Literature and Statistical Synthesis. J. Bone Miner. Res. 2010, 15, 721–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanis, J.; Johnell, O.; De Laet, C.; Johansson, H.; Oden, A.; Delmas, P.; Eisman, J.; Fujiwara, S.; Garnero, P.; Kroger, H.; et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004, 35, 375–382. [Google Scholar] [CrossRef]
- Christodoulou, C.; Cooper, C. What is osteoporosis? Postgrad. Med. J. 2002, 79, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O. Requirements for DXA for the management ofosteoporosis in Europe. Osteoporos. Int. 2005, 16, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Viktoria Stein, K.; Dorner, T.; Lawrence, K.; Kunze, M. Economic concepts for measuring the costs of illness of osteoporosis: An international comparison. Wien. Med. Wochenschr. 2009, 159, 253–261. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic Burden: A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cock, E.; Miravitlles, M.; González-Juanatey, J.R.; Azanza-Perea, J.R. Valor umbral del coste por año de vida ganado para recomendar la adopción de tecnologías sanitarias en España: Evidencias procedentes de una revisión de la literatura. PharmacoEcon. Span. Res. Artic. 2007, 4, 97–107. [Google Scholar] [CrossRef]
- Svedbom, A.; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A.; the EU review panel of the IOF. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 1–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, D.M.; Thompson, D.E.; Bauer, D.C.; Ensrud, K.; Musliner, T.; Hochberg, M.C.; Nevitt, M.C.; Suryawanshi, S.; Cummings, S.R. Fracture Risk Reduction with Alendronate in Women with Osteoporosis: The Fracture Intervention Trial. J. Clin. Endocrinol. Metab. 2000, 85, 4118–4124. [Google Scholar] [CrossRef]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; Mckeever, C.D.; Hangartner, T.; Keller, M.; Iii, C.H.C.; Brown, J.; Miller, P.D.; Page, P. Effects of Risedronate Treatment on Vertebral and Nonvertebral Fractures in women with postmenopausal osteoporosis. J. Am. Med. Assoc. 1999, 282, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Boonen, S.; Adachi, J.D.; Man, Z.; Cummings, S.R.; Lippuner, K.; Törring, O.; Gallagher, J.C.; Farrerons, J.; Wang, A.; Franchimont, N.; et al. Treatment with Denosumab Reduces the Incidence of New Vertebral and Hip Fractures in Postmenopausal Women at High Risk. J. Clin. Endocrinol. Metab. 2011, 96, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Pang, Y.; Shi, Y.; Xu, M.; Xu, X.; Zhang, J.; Ji, L.; Zhao, D. Indirect comparison of teriparatide, denosumab, and oral bisphosphonates for the prevention of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. Menopause 2015, 22, 1021–1025. [Google Scholar] [CrossRef]
- Fahrleitner-Pammer, A.; Langdahl, B.L.; Marin, F.; Jakob, F.; Karras, D.; Barrett, A.; Ljunggren, Ö.; Walsh, J.B.; Rajzbaum, G.; Barker, C.; et al. Fracture rate and back pain during and after discontinuation of teriparatide: 36-month data from the European Forsteo Observational Study (EFOS). Osteoporos. Int. 2011, 22, 2709–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comité de Expertos de la SEIOMM. La Guías de Práctica Clínica en la osteoporosis posmenopaáusica, glucocorticoidea y del Varón. Sociedad Española de investigación Ósea y del metabolismo mineral. Rev. Clín. Esp. 2014, 208, 1–153. [Google Scholar]
- Etxebarria-Foronda, I.; Caeiro-Rey, J.R.; Larrainzar-Garijo, R.; Vaquero-Cervino, E.; Roca-Ruiz, L.; Mesa-Ramos, M.; Merino Pérez, J.; Carpintero-Benítez, P.; Fernández Cebrián, A.; Gil-Garay, E. Guía SECOT-GEIOS en osteoporosis y fractura por fragilidad. Actualización/SECOT-GEIOS guidelines in osteoporosis and fragility fracture. An update. Rev. Esp. Cir. Ortop. Traumatol. 2015, 59, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Borgström, F.; Carlsson, A.; Sintonen, H.; Boonen, S.; Haentjens, P.; Burge, R.; Johnell, O.; Jönsson, B.; Kanis, J.A. The cost-effectiveness of risedronate in the treatment of osteoporosis: An international perspective. Osteoporos. Int. 2006, 17, 996–1007. [Google Scholar] [CrossRef]
- Ström, O.; Borgström, F.; Sen, S.S.; Boonen, S.; Haentjens, P.; Johnell, O.; Kanis, J.A. Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries—An economic evaluation based on the fracture intervention trial. Osteoporos. Int. 2007, 18, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Imaz-Iglesia, I.; Rubio-Gonzalez, B.; Lopez-Delgado, M.E.; Amate, J.M.; Gomez-Pajuelo, P.; Gonzalez-Enriquez, J.; Agencia de Evaluación de Tecnologías Sanitarias (AETS), Instituto de Salud Carlos III—Ministerio de Ciencia e Innovación. Análisis Coste-Utilidad de los Tratamientos Farmacológicos para la prevención de Fracturas en Mujeres con Osteoporosis en ESPAÑA; IPE 63/2010; AETS—Instituto de Salud Carlos III: Madrid, Spain, December 2010.
- Darba, J.; Kaskens, L.; Sorio, F.; Lothgren, M. Cost-utility of denosumab for the treatment of postmenopausal osteoporosis in Spain. Clin. Outcomes Res. 2015, 7, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Hart, W.M.; Rubio-Terrés, C.; Burrell, A.; Arístegui, I.; Escobar-Jiménez, F. Análisis farmacoeconómico del tratamiento de la osteoporosis postmenopáusica con risedronato o alendronato. Rev. Esp. Enferm. Metab. Oseas 2002, 11, 97–104. [Google Scholar]
- Cortés, J.-C.; Navarro-Quiles, A.; Romero, J.-V.; Roselló, M.-D. Randomizing the parameters of a Markov chain to model the stroke disease: A technical generalization of established computational methodologies towards improving real applications. J. Comput. Appl. Math. 2017, 324, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Barrachina-Martínez, I.; Navarro-Quiles, A.; Ramos, M.; Romero, J.-V.; Roselló, M.-D.; Vivas-Consuelo, D. Probabilistic Study of the Effect of Anti-Epileptic Drugs Under Uncertainty: Cost-Effectiveness Analysis. Mathematics 2020, 8, 1120. [Google Scholar] [CrossRef]
- Herdman, M.; Badia, X.; Berra, S. El EuroQol-5D: Una alternativa sencilla para la medición de la calidad de vida relacionada con la salud en atención primaria. Atención Primaria 2001, 28, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: Componentes físico y mental. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Bastida, J.L.; Oliva, J.; Antoñanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac. Sanit. 2010, 24, 154–170. [Google Scholar] [CrossRef] [Green Version]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.S.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef]
- Hernández, A.N.; del Campo Fontecha, P.D.; Acín, M.P.A.; Rodríguez, L.A.; Burgos, E.C.; Castañeda, S.; Aresté, J.F.; Gifre, L.; Vaquero, C.G.; Rodríguez, G.C.; et al. Recomendaciones de la Sociedad Española de Reumatología sobre osteoporosis. Reumatol. Clínica 2019, 15, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.L.; Rodríguez, R. Cuánto Vale la Pena Gastarse para Ganar un Año de Vida Ajustado por la Calidad? Un Estudio empírIco. En: El Valor Monetario de la Salud; Springer: Barcelona, Spain, 2001. [Google Scholar]
- Sacristán, J.; Oliva-Moreno, J.; Del Llano, J.; Prieto, L.; Pinto, J. ¿Qué es una tecnología sanitaria eficiente en España? Gac. Sanit. 2002, 16, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Davis, S.; Simpson, E.; Hamilton, J.; James, M.M.-S.; Rawdin, A.; Wong, R.; Goka, E.; Gittoes, N.; Selby, P. Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: A systematic review and economic evaluation. Health Technol. Assess. 2020, 24, 1–314. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Patrones de Mortalidad en España, 2016; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2019. [Google Scholar]
- Abimanyi-Ochom, J.; Watts, J.J.; Borgström, F.; Nicholson, G.; Shore-Lorenti, C.; Stuart, A.L.; Zhang, Y.; Iuliano, S.; Seeman, E.; Prince, R.; et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos. Int. 2015, 26, 1781–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication Compliance and Persistence: Terminology and Definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Crandall, C.J.; Ganz, D.A. Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos. Int. 2017, 28, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Parthan, A.; Kruse, M.; Agodoa, I.; Silverman, S.; Orwoll, E. Denosumab: A cost-effective alternative for older men with osteoporosis from a Swedish payer perspective. Bone 2014, 59, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Parthan, A.; Kruse, M.; Yurgin, N.; Huang, J.; Viswanathan, H.N.; Taylor, U. Cost Effectiveness of Denosumab versus Oral Bisphosphonates for Postmenopausal Osteoporosis in the US. Appl. Health Econ. Health Policy 2013, 11, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, B.; Liu, M.; Zhou, H.; Zhao, L.; Cai, H.; Huang, J. Cost-effectiveness of antiosteoporosis strategies for postmenopausal women with osteoporosis in China. Menopause 2019, 26, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosteson, A.N.A.; Melton, L.J.; Dawson-Hughes, B.; Baim, S.; Favus, M.J.; Khosla, S.; Lindsay, R.L. Cost-effective osteoporosis treatment thresholds: The United States perspective. Osteoporos. Int. 2008, 19, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makras, P.; Athanasakis, K.; Boubouchairopoulou, N.; Rizou, S.; Anastasilakis, A.D.; Kyriopoulos, J.; Lyritis, G.P. Cost-effective osteoporosis treatment thresholds in Greece. Osteoporos. Int. 2015, 26, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.J.; Larrosa, M.; Surís, X.; Garcia-Ruiz, A.J. Análisis de coste-utilidad e impacto presupuestario de la prevención primaria con alendronato de la fractura osteoporótica de cadera en Cataluña. Reumatol. Clín. 2012, 8, 128–134. [Google Scholar] [CrossRef]
- González, D.J.; Marco, G.M.; Henríquez, S.M. Coste anual de los fármacos utilizados en el tratamiento de la osteoporosis tras la revisión de los precios de referencia Bibliografía. Rev. Osteoporos. Metab. Miner. 2012, 41, 43–44. [Google Scholar]
- Braithwaite, R.S.; Meltzer, D.O.; King, J.T.; Leslie, D.; Roberts, M.S. What Does the Value of Modern Medicine Say About the $50,000 per Quality-Adjusted Life-Year Decision Rule? Med. Care 2008, 46, 349–356. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Culyer, A.J. Education and debate. National Institute for Clinical Excellence and ist value judgments. BMJ 2004, 329, 224–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devlin, N.; Parkin, D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004, 13, 437–452. [Google Scholar] [CrossRef]
- Murray, C.J.; Evans, D.B.; Acharya, A.; Baltussen, R.M. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000, 9, 235–251. [Google Scholar] [CrossRef]
- Murphy, D.R.; Smolen, L.J.; Klein, T.M.; Klein, R.W. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet. Disord. 2012, 13, 213. [Google Scholar] [CrossRef] [Green Version]
- Taheri, S.; Fashami, F.M.; Peiravian, F.; Yousefi, P. Teriparatide in the Treatment of Severe Postmenopausal Osteoporosis: A Cost-Utility Analysis. Iran. J. Pharm. Res 2019, 18, 1073–1085. [Google Scholar]
- Silverman, S.; Agodoa, I.; Kruse, M.; Parthan, A.; Orwoll, E. Denosumab for elderly men with osteoporosis: A cost-effectiveness analysis from the us payer perspective. J. Bone Miner. Res. 2013, 28, 627631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidner, M.; Institute for Clincial and Economic Review. Anabolic Therapies for Osteoporosis in Postmenopausal Women: Effectiveness and Value. 2017. Available online: https://icer-review.org/wp-con-tent/uploads/2016/11/CTAF_Osteoporosis_Final_Evidence (accessed on 17 May 2021).
- Mori, T.; Crandall, C.J.; Ganz, D.A. Cost-Effectiveness of Sequential Teriparatide/Alendronate Versus Alendronate-Alone Strategies in High-Risk Osteoporotic Women in the US: Analyzing the Impact of Generic/Biosimilar Teriparatide. JBMR Plus 2019, 3, e10233. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, T.; Nishino, T.; Okubo, I.; Yamazaki, M. Cost-effectiveness analysis of drugs for osteoporosis treatment in elderly Japanese women at high risk of fragility fractures: Comparison of denosumab and weekly alendronate. Arch. Osteoporos. 2018, 13, 94. [Google Scholar] [CrossRef]
- Karnon, J.; Shafie, A.S.; Orji, N.; Usman, S.K. What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia. Cost Eff. Resour. Alloc. 2016, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Freemantle, N.; Satram-Hoang, S.; Tang, E.-T.; Kaur, P.; Macarios, D.; Siddhanti, S.; Borenstein, J.; Kendler, D.L. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: A 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos. Int. 2012, 23, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiligsmann, M.; Reginster, J.-Y. Cost-effectiveness of gastro-resistant risedronate tablets for the treatment of postmenopausal women with osteoporosis in France. Osteoporos. Int. 2019, 30, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).