Smart Operating Room in Digestive Surgery: A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Surgical Control Tower and Black Box
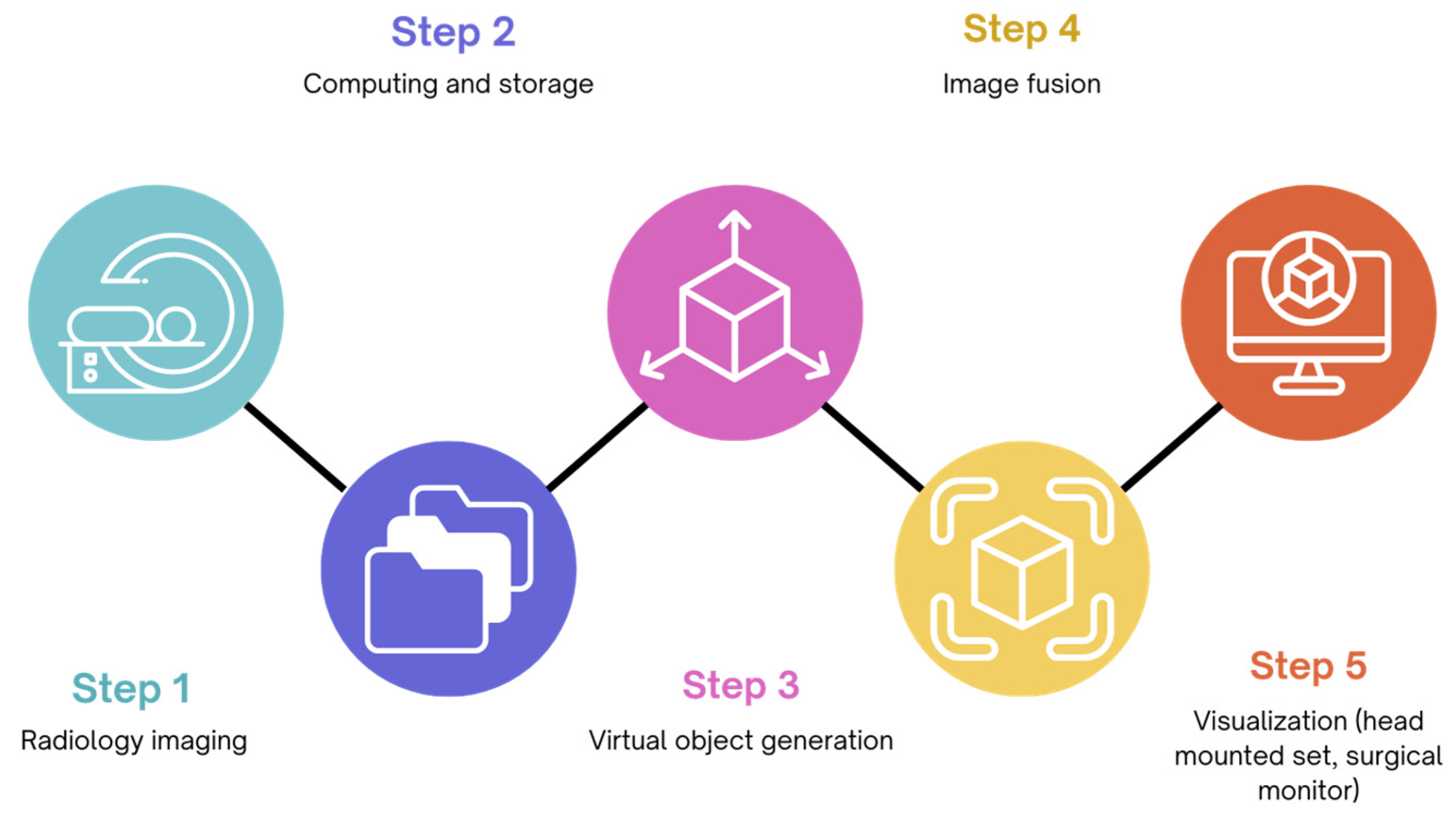
4. Augmented Reality in the Operating Room
5. Robotic Operating Room
6. Hybrid Operating Room
7. Telesurgery and Telementoring
8. Recommendations for Future Research: Is Artificial Intelligence (AI) the Answer?
9. Conclusions with Ethical and Legal Aspects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denton, B.; Viapiano, J.; Vogl, A. Optimization of Surgery Sequencing and Scheduling Decisions under Uncertainty. Health Care Manag. Sci. 2007, 10, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Weiser, T.G.; Haynes, A.B.; Molina, G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Fu, R.; Azad, T.; Chao, T.E.; Berry, W.R.; et al. Estimate of the Global Volume of Surgery in 2012: An Assessment Supporting Improved Health Outcomes. Lancet 2015, 385 (Suppl. S2), S11. [Google Scholar] [CrossRef] [PubMed]
- Zegers, M.; de Bruijne, M.C.; de Keizer, B.; Merten, H.; Groenewegen, P.P.; van der Wal, G.; Wagner, C. The Incidence, Root-Causes, and Outcomes of Adverse Events in Surgical Units: Implication for Potential Prevention Strategies. Patient Saf. Surg. 2011, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Daniel, M. Medical Error-the Third Leading Cause of Death in the Us. BMJ 2016, 353, i2139. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.; Macario, A. The Drive for Operating Room Efficiency Will Increase Quality of Patient Care. Curr. Opin. Anaesthesiol. 2006, 19, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gawande, A. Two Hundred Years of Surgery. N. Engl. J. Med. 2012, 366, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hein, L.; Vedula, S.S.; Speidel, S.; Navab, N.; Kikinis, R.; Park, A.; Eisenmann, M.; Feussner, H.; Forestier, G.; Giannarou, S.; et al. Surgical Data Science for Next-Generation Interventions. Nat. Biomed. Eng. 2017, 1, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Marescaux, J. Robotic Surgery. Br. J. Surg. 2015, 102, e15–e28. [Google Scholar] [CrossRef] [PubMed]
- Rus, D.; Meireles, O.R. The Ai Guardian for Surgery. In Healthcare and Artificial Intelligence; Nordlinger, B., Villani, C., Rus, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 105–114. [Google Scholar]
- Yeung, S.; Downing, N.L.; Fei-Fei, L.; Milstein, A. Bedside Computer Vision—Moving Artificial Intelligence from Driver Assistance to Patient Safety. N. Engl. J. Med. 2018, 378, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, K.R.; de Leval, M.R.; McEwan, A.; Pigott, N.; Elliott, M.J.; McQuillan, A.; MacDonald, C.; Goldman, A.J. Patient Handover from Surgery to Intensive Care: Using Formula 1 Pit-Stop and Aviation Models to Improve Safety and Quality. Paediatr. Anaesth. 2007, 17, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Helmreich, R.L. On Error Management: Lessons from Aviation. BMJ 2000, 320, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.G.; Jung, J.; Grantcharov, T.P. Using Data to Enhance Performance and Improve Quality and Safety in Surgery. JAMA Surg. 2017, 152, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Mascagni, P.; Padoy, N. Or Black Box and Surgical Control Tower: Recording and Streaming Data and Analytics to Improve Surgical Care. J. Visc. Surg. 2021, 158, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial Intelligence in Surgery: Promises and Perils. Ann. Surg. 2018, 268, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Spenkelink, I.M.; Heidkamp, J.; Futterer, J.J.; Rovers, M.M. Image-Guided Procedures in the Hybrid Operating Room: A Systematic Scoping Review. PLoS ONE 2022, 17, e0266341. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.C.; Ruiz, M.G. Telesurgery and Telementoring. Cir. Esp. (Engl. Ed.) 2024, 102, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, D.I.; Monfared, S.; Timsina, L.; Whiteside, J.; Banerjee, A.; Butler, A.; Stefanidis, D. Evaluation of Operating Room Inefficiencies and Their Impact on Operating Room Duration Using a Surgical App. Am. J. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Inaba, C.S.; Koh, C.Y.; Sujatha-Bhaskar, S.; Gallagher, S.; Chen, Y.; Nguyen, N.T. Operative Time as a Marker of Quality in Bariatric Surgery. Surg. Obes. Relat. Dis. 2019, 15, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Nensi, A.; Palter, V.; Reed, C.; Schulthess, P.; McLoone, M.; Grantcharov, T.; Shore, E.M. Utilizing the Operating Room Black Box to Characterize Intraoperative Delays, Distractions, and Threats in the Gynecology Operating Room: A Pilot Study. Cureus 2021, 13, e16218. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Juni, P.; Lebovic, G.; Grantcharov, T. First-Year Analysis of the Operating Room Black Box Study. Ann. Surg. 2020, 271, 122–127. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, A.S.H.M.; Strandbygaard, J.; van Herzeele, I.; Boet, S.; Grantcharov, T.P.; Schijven, M.P. Six Sigma in Surgery: How to Create a Safer Culture in the Operating Theatre Using Innovative Technology. Br. J. Anaesth. 2021, 127, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; McKechnie, T.; Kruse, C.C.; Aldrich, K.; Grantcharov, T.P.; Langerman, A. Surgical Data Recording in the Operating Room: A Systematic Review of Modalities and Metrics. Br. J. Surg. 2021, 108, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Raheem, S.; Ahmed, Y.E.; Hussein, A.A.; Johnson, A.; Cavuoto, L.; May, P.; Cole, A.; Wang, D.; Ahmad, B.; Hasasneh, A.; et al. Variability and Interpretation of Communication Taxonomy during Robot-Assisted Surgery: Do We All Speak the Same Language? BJU Int. 2018, 122, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dexter, F.; Hu, P.; Dutton, R.P. The Use of Distributed Displays of Operating Room Video When Real-Time Occupancy Status Was Available. Anesth. Analg. 2008, 106, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.F.; Xiao, Y.; Ho, D.; Mackenzie, C.F.; Hu, H.; Voigt, R.; Martz, D. Advanced Visualization Platform for Surgical Operating Room Coordination: Distributed Video Board System. Surg. Innov. 2006, 13, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Jue, J.; Shah, N.A.; Mackey, T.K. An Interdisciplinary Review of Surgical Data Recording Technology Features and Legal Considerations. Surg. Innov. 2020, 27, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, M. A Surgical Black Box to Prevent Mistakes. AORN J. 2015, 102, P15–P16. [Google Scholar] [PubMed]
- Al Abbas, A.I.; Sankaranarayanan, G.; Polanco, P.M.; Cadeddu, J.A.; Daniel, W.; Palter, V.; Grantcharov, T.; Bartolome, S.; Dandekar, P.; Evans, K.; et al. The Operating Room Black Box: Understanding Adherence to Surgical Checklists. Ann. Surg. 2022, 276, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.S.; Etheridge, J.; Palter, V.; Zeh, H., 3rd; Grantcharov, T.; Kaelberer, Z.; Sonnay, Y.; Smink, D.S.; Brindle, M.E.; Molina, G. Remote Assessment of Real-World Surgical Safety Checklist Performance Using the or Black Box: A Multi-Institutional Evaluation. J. Am. Coll. Surg. 2024, 238, 206–215. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, A.S.H.M.; Jansen, M.; van Haperen, M.; van Dieren, S.; Buskens, C.J.; van Dijkum, E.J.M.N.; Bemelman, W.A.; Grantcharov, T.P.; Schijven, M.P. Implementing Structured Team Debriefing Using a Black Box in the Operating Room: Surveying Team Satisfaction. Surg. Endosc. 2021, 35, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Doyen, B.; Gordon, L.; Soenens, G.; Bacher, K.; Vlerick, P.; Vermassen, F.; Grantcharov, T.; Van Herzeele, I. Introduction of a Surgical Black Box System in a Hybrid Angiosuite: Challenges and Opportunities. Phys. Med. 2020, 76, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Doyen, B.; Soenens, G.; Maurel, B.; Hertault, A.; Gordon, L.; Vlerick, P.; Vermassen, F.; Grantcharov, T.; van Herzeele, I. Assessing Endovascular Team Performances in a Hybrid Room Using the Black Box System: A Prospective Cohort Study. J. Cardiovasc. Surg. 2023, 64, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, Y.P.; Chiang, C.C.; Chang, M.C.; Lee, O.K. Real-Time Streaming of Surgery Performance and Intraoperative Imaging Data in the Hybrid Operating Room: Development and Usability Study. JMIR Med. Inform. 2020, 8, e18094. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, A.S.H.M.; Legemaate, J.; Schlack, W.S.; Legemate, D.A.; Schijven, M.P. Legal Perspectives on Black Box Recording Devices in the Operating Environment. Br. J. Surg. 2019, 106, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.; Mohan, H.; Ryan, J.R.; Schurch, C.M.; Nolan, G.P.; Frakes, D.H.; Coskun, A.F. Virtual and Augmented Reality for Biomedical Applications. Cell Rep. Med. 2021, 2, 100348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, V.; Khanduja, V. The Impact of Extended Reality on Surgery: A Scoping Review. Int. Orthop. 2023, 47, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Teber, D.; Guven, S.; Simpfendorfer, T.; Baumhauer, M.; Guven, E.O.; Yencilek, F.; Gozen, A.S.; Rassweiler, J. Augmented Reality: A New Tool to Improve Surgical Accuracy during Laparoscopic Partial Nephrectomy? Preliminary in Vitro and in Vivo Results. Eur. Urol. 2009, 56, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Onda, S.; Okamoto, T.; Kanehira, M.; Suzuki, F.; Ito, R.; Fujioka, S.; Suzuki, N.; Hattori, A.; Yanaga, K. Identification of Inferior Pancreaticoduodenal Artery during Pancreaticoduodenectomy Using Augmented Reality-Based Navigation System. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 281–287. [Google Scholar] [CrossRef]
- Kenngott, H.G.; Neuhaus, J.; Muller-Stich, B.P.; Wolf, I.; Vetter, M.; Meinzer, H.P.; Koninger, J.; Buchler, M.W.; Gutt, C.N. Development of a Navigation System for Minimally Invasive Esophagectomy. Surg. Endosc. 2008, 22, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Ma, L.F.; Rong, Z.X.; Li, M.D.; Zeng, J.P.; Wang, X.D.; Liao, H.E.; Dong, J.H. Augmented Reality Technology for Preoperative Planning and Intraoperative Navigation during Hepatobiliary Surgery: A Review of Current Methods. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, C.; Plana, A.; Kania, P.; Banerjee, P.P.; Small, S. Usefulness of Three-Dimensional Modeling in Surgical Planning, Resident Training, and Patient Education. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Viglialoro, R.; Esposito, N.; Condino, S.; Cutolo, F.; Guadagni, S.; Gesi, M.; Ferrari, M.; Ferrari, V. Augmented Reality to Improve Surgical Simulation. Lessons Learned Towards the Design of a Hybrid Laparoscopic Simulator for Cholecystectomy. IEEE Trans. Biomed. Eng. 2018, 66, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, M.; Yang, S.; McLeod, G.A. The Use of Artificial Intelligence and Robotics in Regional Anaesthesia. Anaesthesia 2021, 76 (Suppl. S1), 171–181. [Google Scholar] [CrossRef] [PubMed]
- San Martin-Rodriguez, L.; Soto-Ruiz, M.N.; Echeverria-Ganuza, G.; Escalada-Hernandez, P. Augmented Reality for Training Operating Room Scrub Nurses. Med. Educ. 2019, 53, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.H.; Tao, H.S.; Yang, J.; Fang, Z.S.; Cai, W.; Liu, J.; Fan, Y.F. Impact of Three-Dimensional Reconstruction Technique in the Operation Planning of Centrally Located Hepatocellular Carcinoma. J. Am. Coll. Surg. 2015, 220, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Soler, L.; Diana, M.; Mutter, D.; Baumert, T.F.; Habersetzer, F.; Marescaux, J.; Pessaux, P. Trans-Thoracic Minimally Invasive Liver Resection Guided by Augmented Reality. J. Am. Coll. Surg. 2015, 220, e55–e60. [Google Scholar] [CrossRef] [PubMed]
- Mise, Y.; Hasegawa, K.; Satou, S.; Shindoh, J.; Miki, K.; Akamatsu, N.; Arita, J.; Kaneko, J.; Sakamoto, Y.; Kokudo, N. How Has Virtual Hepatectomy Changed the Practice of Liver Surgery?: Experience of 1194 Virtual Hepatectomy before Liver Resection and Living Donor Liver Transplantation. Ann. Surg. 2018, 268, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Taura, K.; Seo, S.; Yasuchika, K.; Nitta, T.; Ogawa, K.; Hatano, E.; Uemoto, S. Usefulness of Operative Planning Based on 3-Dimensional Ct Cholangiography for Biliary Malignancies. Surgery 2015, 158, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Phutane, P.; Buc, E.; Poirot, K.; Ozgur, E.; Pezet, D.; Bartoli, A.; Le Roy, B. Preliminary Trial of Augmented Reality Performed on a Laparoscopic Left Hepatectomy. Surg. Endosc. 2018, 32, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.C.; Volonte, F.; Pugin, F.; Toso, C.; Fusaglia, M.; Gavaghan, K.; Majno, P.E.; Peterhans, M.; Weber, S.; Morel, P. Augmented Environments for the Targeting of Hepatic Lesions during Image-Guided Robotic Liver Surgery. J. Surg. Res. 2013, 184, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kingham, T.P.; Pak, L.M.; Simpson, A.L.; Leung, U.; Doussot, A.; D’Angelica, M.I.; DeMatteo, R.P.; Allen, P.J.; Jarnagin, W.R. 3D Image Guidance Assisted Identification of Colorectal Cancer Liver Metastases Not Seen on Intraoperative Ultrasound: Results from a Prospective Trial. HPB 2018, 20, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ntourakis, D.; Memeo, R.; Soler, L.; Marescaux, J.; Mutter, D.; Pessaux, P. Augmented Reality Guidance for the Resection of Missing Colorectal Liver Metastases: An Initial Experience. World J. Surg. 2016, 40, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Banz, V.M.; Muller, P.C.; Tinguely, P.; Inderbitzin, D.; Ribes, D.; Peterhans, M.; Candinas, D.; Weber, S. Intraoperative Image-Guided Navigation System: Development and Applicability in 65 Patients Undergoing Liver Surgery. Langenbecks Arch. Surg. 2016, 401, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Soler, L.; Agnus, V.; D’Urso, A.; Vix, M.; Dallemagne, B.; Faucher, V.; Roy, C.; Mutter, D.; Marescaux, J.; et al. Prospective Evaluation of Precision Multimodal Gallbladder Surgery Navigation: Virtual Reality, near-Infrared Fluorescence, and X-Ray-Based Intraoperative Cholangiography. Ann. Surg. 2017, 266, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Pessaux, P.; Diana, M.; Soler, L.; Piardi, T.; Mutter, D.; Marescaux, J. Towards Cybernetic Surgery: Robotic and Augmented Reality-Assisted Liver Segmentectomy. Langenbecks Arch. Surg. 2015, 400, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, W.; Yang, J.; Xiang, N.; Zeng, N.; Hu, H.; Jia, F.; Fang, C. Augmented Reality Navigation for Stereoscopic Laparoscopic Anatomical Hepatectomy of Primary Liver Cancer: Preliminary Experience. Front. Oncol. 2021, 11, 663236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zeng, X.; Hu, H.; Xiang, N.; Zeng, N.; Wen, S.; Tian, J.; Yang, J.; Fang, C. Perioperative and Disease-Free Survival Outcomes after Hepatectomy for Centrally Located Hepatocellular Carcinoma Guided by Augmented Reality and Indocyanine Green Fluorescence Imaging: A Single-Center Experience. J. Am. Coll. Surg. 2023, 236, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.; Pulijala, Y. The Application of Virtual Reality and Augmented Reality in Oral & Maxillofacial Surgery. BMC Oral Health 2019, 19, 238. [Google Scholar]
- Jud, L.; Fotouhi, J.; Andronic, O.; Aichmair, A.; Osgood, G.; Navab, N.; Farshad, M. Applicability of Augmented Reality in Orthopedic Surgery—A Systematic Review. BMC Musculoskelet. Disord. 2020, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Meola, A.; Cutolo, F.; Carbone, M.; Cagnazzo, F.; Ferrari, M.; Ferrari, V. Augmented Reality in Neurosurgery: A Systematic Review. Neurosurg. Rev. 2017, 40, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Teatini, A.; de Frutos, J.P.; Eigl, B.; Pelanis, E.; Aghayan, D.L.; Lai, M.; Kumar, R.P.; Palomar, R.; Edwin, B.; Elle, O.J. Influence of Sampling Accuracy on Augmented Reality for Laparoscopic Image-Guided Surgery. Minim. Invasive Ther. Allied Technol. 2021, 30, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kenngott, H.G.; Wagner, M.; Gondan, M.; Nickel, F.; Nolden, M.; Fetzer, A.; Weitz, J.; Fischer, L.; Speidel, S.; Meinzer, H.P.; et al. Real-Time Image Guidance in Laparoscopic Liver Surgery: First Clinical Experience with a Guidance System Based on Intraoperative Ct Imaging. Surg. Endosc. 2014, 28, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yin, D.; Zhang, S.; Xiao, D.; He, B.; Meng, F.; Zhang, Y.; Cai, W.; He, S.; Zhang, W.; et al. Augmented Reality Navigation for Liver Resection with a Stereoscopic Laparoscope. Comput. Methods Programs Biomed. 2020, 187, 105099. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pruitt, K.; Nawawithan, N.; Johnson, B.A.; Gahan, J.; Fei, B. Dense Surface Reconstruction Using a Learning-Based Monocular Vslam Model for Laparoscopic Surgery. In Proceedings of the Medical Imaging 2024: Image-Guided Procedures, Robotic Interventions, and Modeling, San Diego, CA, USA, 18–23 February 2024; p. 12928. [Google Scholar]
- Kanji, F.; Catchpole, K.; Choi, E.; Alfred, M.; Cohen, K.; Shouhed, D.; Anger, J.; Cohen, T. Work-System Interventions in Robotic-Assisted Surgery: A Systematic Review Exploring the Gap between Challenges and Solutions. Surg. Endosc. 2021, 35, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, F.; Siragusa, L.; Zadoroznyj, A.; Laterza, V.; Mangana, O.; Schena, C.A.; Ammendola, M.; Memeo, R.; Bianchi, P.P.; Spinoglio, G.; et al. New Robotic Platforms in General Surgery: What’s the Current Clinical Scenario? Medicina 2023, 59, 1264. [Google Scholar] [CrossRef] [PubMed]
- Randell, R.; Honey, S.; Alvarado, N.; Pearman, A.; Greenhalgh, J.; Long, A.; Gardner, P.; Gill, A.; Jayne, D.; Dowding, D. Embedding Robotic Surgery into Routine Practice and Impacts on Communication and Decision Making: A Review of the Experience of Surgical Teams. Cogn. Technol. Work 2016, 18, 423–437. [Google Scholar] [CrossRef]
- Poulsen, J.L.; Bruun, B.; Oestergaard, D.; Spanager, L. Factors Affecting Workflow in Robot-Assisted Surgery: A Scoping Review. Surg. Endosc. 2022, 36, 8713–8725. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, K.R.; Hallett, E.; Curtis, S.; Mirchi, T.; Souders, C.P.; Anger, J.T. Diagnosing Barriers to Safety and Efficiency in Robotic Surgery. Ergonomics 2018, 61, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Hussein, A.A.; Cavuoto, L.; Sharif, M.; Allers, J.C.; Hinata, N.; Ahmad, B.; Kozlowski, J.D.; Hashmi, Z.; Bisantz, A.; et al. Ambulatory Movements, Team Dynamics and Interactions during Robot-Assisted Surgery. BJU Int. 2016, 118, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Okamoto, J.; Masamune, K.; Muragaki, Y. Robotic Technology in Operating Rooms: A Review. Curr. Robot. Rep. 2021, 2, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hongo, K.; Ogiwara, T.; Nagm, A.; Okamoto, J.; Muragaki, Y.; Lawton, M.; McDermott, M.; Berger, M. Intelligent Surgeon’s Arm Supporting System Iarms in Microscopic Neurosurgery Utilizing Robotic Technology. World Neurosurg. 2018, 119, e661–e665. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.; Schlaefer, A. Feasibility of Touch-Less Control of Operating Room Lights. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Divya, D.S. Hand Gesture Interface for Smart Operation Theatre Lighting. Int. J. Eng. Technol. 2018, 7, 20–23. [Google Scholar] [CrossRef]
- Sandoval, J.; Nouaille, L.; Poisson, G.; Parmantier, Y. Kinematic Design of a Lighting Robotic Arm for Operating Room. In Computational Kinematics, Proceedings of the 7th International Workshop on Computational Kinematics, Futuroscope-Poitiers, France, May 2017; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 44–52. [Google Scholar]
- Gimenez, M.; Gallix, B.; Costamagna, G.; Vauthey, J.N.; Moche, M.; Wakabayashi, G.; Bale, R.; Swanstrom, L.; Futterer, J.; Geller, D.; et al. Definitions of Computer-Assisted Surgery and Intervention, Image-Guided Surgery and Intervention, Hybrid Operating Room, and Guidance Systems: Strasbourg International Consensus Study. Ann. Surg. Open 2020, 1, e021. [Google Scholar] [CrossRef] [PubMed]
- Barstad, R.M.; Fosse, E.; Vatne, K.; Andersen, K.; Tonnessen, T.I.; Svennevig, J.L.; Geiran, O.R. Intraoperative Angiography in Minimally Invasive Direct Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 1997, 64, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Berazaluce, A.M.C.; Hanke, R.E.; von Allmen, D.; Racadio, J.M. The State of the Hybrid Operating Room: Technological Acceleration at the Pinnacle of Collaboration. Curr. Surg. Rep. 2019, 7, 7. [Google Scholar] [CrossRef]
- Narayanam, S.; Gerstle, T.; Amaral, J.; John, P.; Parra, D.; Temple, M.; Connolly, B. Lung Tattooing Combined with Immediate Video-Assisted Thoracoscopic Resection (Ivatr) as a Single Procedure in a Hybrid Room: Our Institutional Experience in a Pediatric Population. Pediatr. Radiol. 2013, 43, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.; Radaelli, A.; Schampaert, S.; van der Bom, I. Cone Beam Ct-Guided Endobronchial Biopsy Assisted by Augmented Fluoroscopy. Chest 2017, 152, A887. [Google Scholar] [CrossRef]
- Jin, H.; Liu, J. Application of the Hybrid Operating Room in Surgery: A Systematic Review. J. Investig. Surg. 2022, 35, 378–389. [Google Scholar] [CrossRef]
- Varu, V.; Greenberg, J.I.; Lee, J.T. Improved Efficiency and Safety for Evar with Utilization of a Hybrid Room. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Odisio, B.C.; Simoneau, E.; Holmes, A.A.; Conrad, C.H.; Vauthey, J.N. Fast-Track Two-Stage Hepatectomy Using a Hybrid Interventional Radiology/Operating Suite as Alternative Option to Associated Liver Partition and Portal Vein Ligation for Staged Hepatectomy Procedure. J. Am. Coll. Surg. 2018, 227, e5–e10. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Odisio, B.C.; Velasco, J.D.; Ninan, E.; Huang, S.Y.; Mahvash, A.; Tzeng, C.D.; Cao, H.S.T.; Gupta, S.; Vauthey, J.N. Fast-Track Two-Stage Hepatectomy by Concurrent Portal Vein Embolization at First-Stage Hepatectomy in Hybrid Interventional Radiology/Operating Suite. Surg. Oncol. 2021, 39, 101648. [Google Scholar] [CrossRef] [PubMed]
- Brouquet, A.; Abdalla, E.K.; Kopetz, S.; Garrett, C.R.; Overman, M.J.; Eng, C.; Andreou, A.; Loyer, E.M.; Madoff, D.C.; Curley, S.A.; et al. High Survival Rate after Two-Stage Resection of Advanced Colorectal Liver Metastases: Response-Based Selection and Complete Resection Define Outcome. J. Clin. Oncol. 2011, 29, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Goumard, C.; Gayet, B.; Fuks, D.; Scatton, O. Laparoscopic Versus Open Two-Stage Hepatectomy for Bilobar Colorectal Liver Metastases: A Bi-Institutional, Propensity Score-Matched Study. Surgery 2019, 166, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Iwata, Y.; Takahashi, H.; Imai, K.; Yokoo, H.; Ishitoya, S.; Ogata, M.; Matsuno, N.; Sumi, Y.; Furukawa, H. Severe Liver Injury with Traumatic Cardiac Arrest Successfully Treated by Damage Control Surgery and Transcatheter Arterial Embolization in the Hybrid Operating Room: A Case Report. Surg. Case Rep. 2021, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Croft, C.A.; Rosenthal, M.D.; Mohr, A.M.; Efron, P.A.; Moore, F.A.; Upchurch, G.R., Jr.; Smith, R.S. Clinical Impact of a Dedicated Trauma Hybrid Operating Room. J. Am. Coll. Surg. 2021, 232, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Belyayev, L.; Herrold, J.A.; Ko, A.; Kundi, R.; DuBose, J.J.; Scalea, T.M.; Morrison, J.J. Endovascular Adjuncts for Hybrid Liver Surgery. J. Trauma Acute Care Surg. 2020, 89, e51–e54. [Google Scholar] [CrossRef] [PubMed]
- Hakoda, H.; Akamatsu, N.; Shibata, E.; Takao, H.; Ichida, A.; Kawaguchi, Y.; Kaneko, J.; Abe, O.; Hasegawa, K. Interventional Treatment for Portal Vein Complications Utilizing a Hybrid Operating Room after Liver Transplantation. HPB 2023, 25, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Kuroda, S.; Chosa, K.; Okada, K.; Tanimine, N.; Tahara, H.; Ohira, M.; Ide, K.; Kobayashi, T.; Ohdan, H. Treatment of Multiple Huge Liver Cysts in a Hybrid Operating Room: A Case Report. Surg. Case Rep. 2021, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- Maker, A.V.; Al Rameni, D.; Prabhakar, N. Combining on-Table Embolization with Immediate Resection to Safely Excise Giant Hepatic Hemangiomas. J. Gastrointest. Surg. 2021, 25, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Hayami, S.; Sonomura, T.; Kawai, M.; Hirono, S.; Okada, K.I.; Tanaka, R.; Yamaue, H. Concomitant Use of Indocyanine Green Fluorescence Imaging and Interventional Radiology for Detection of Liver Segments during Laparoscopic Anatomical Liver Resection: Pilot Feasibility Study. Surg. Laparosc. Endosc. Percutaneous Tech. 2019, 29, 242–246. [Google Scholar] [CrossRef]
- Falkenberg, M.; Rizell, M.; Eilard, M.S.; Regensburger, A.; Razazzian, R.; Kvarnstrom, N. Radiopaque Fiducials Guiding Laparoscopic Resection of Liver Tumors. Surg. Laparosc. Endosc. Percutaneous Tech. 2021, 32, 140–144. [Google Scholar] [CrossRef]
- Ivashchenko, O.V.; Kuhlmann, K.F.D.; van Veen, R.; Pouw, B.; Kok, N.F.M.; Hoetjes, N.J.; Smit, J.N.; Klompenhouwer, E.G.; Nijkamp, J.; Ruers, T.J.M. Cbct-Based Navigation System for Open Liver Surgery: Accurate Guidance toward Mobile and Deformable Targets with a Semi-Rigid Organ Approximation and Electromagnetic Tracking of the Liver. Med. Phys. 2021, 48, 2145–2159. [Google Scholar] [CrossRef]
- Pelanis, E.; Teatini, A.; Eigl, B.; Regensburger, A.; Alzaga, A.; Kumar, R.P.; Rudolph, T.; Aghayan, D.L.; Riediger, C.; Kvarnstrom, N.; et al. Evaluation of a Novel Navigation Platform for Laparoscopic Liver Surgery with Organ Deformation Compensation Using Injected Fiducials. Med. Image Anal. 2021, 69, 101946. [Google Scholar] [CrossRef]
- Peterhans, M.; vom Berg, A.; Dagon, B.; Inderbitzin, D.; Baur, C.; Candinas, D.; Weber, S. A Navigation System for Open Liver Surgery: Design, Workflow and First Clinical Applications. Int. J. Med. Robot. 2011, 7, 7–16. [Google Scholar] [CrossRef]
- Shekhar, R.; Dandekar, O.; Bhat, V.; Philip, M.; Lei, P.; Godinez, C.; Sutton, E.; George, I.; Kavic, S.; Mezrich, R.; et al. Live Augmented Reality: A New Visualization Method for Laparoscopic Surgery Using Continuous Volumetric Computed Tomography. Surg. Endosc. 2010, 24, 1976–1985. [Google Scholar] [CrossRef]
- Soler, L.; Nicolau, S.; Pessaux, P.; Mutter, D.; Marescaux, J. Real-Time 3d Image Reconstruction Guidance in Liver Resection Surgery. Hepatobiliary Surg. Nutr. 2014, 3, 73–81. [Google Scholar]
- Teatini, A.; Pelanis, E.; Aghayan, D.L.; Kumar, R.P.; Palomar, R.; Fretland, A.A.; Edwin, B.; Elle, O.J. The Effect of Intraoperative Imaging on Surgical Navigation for Laparoscopic Liver Resection Surgery. Sci. Rep. 2019, 9, 18687. [Google Scholar] [CrossRef]
- Banz, V.M.; Baechtold, M.; Weber, S.; Peterhans, M.; Inderbitzin, D.; Candinas, D. Computer Planned, Image-Guided Combined Resection and Ablation for Bilobar Colorectal Liver Metastases. World J. Gastroenterol. 2014, 20, 14992–14996. [Google Scholar] [CrossRef]
- Harrison, O.J.; Sarvananthan, S.; Tamburrini, A.; Peebles, C.; Alzetani, A. Image-Guided Combined Ablation and Resection in Thoracic Surgery for the Treatment of Multiple Pulmonary Metastases: A Preliminary Case Series. JTCVS Tech. 2021, 9, 156–162. [Google Scholar] [CrossRef]
- Puijk, R.S.; Nieuwenhuizen, S.; van den Bemd, B.A.T.; Ruarus, A.H.; Geboers, B.; Vroomen, L.; Muglia, R.; de Jong, M.C.; de Vries, J.J.J.; Scheffer, H.J.; et al. Transcatheter Ct Hepatic Arteriography Compared with Conventional Ct Fluoroscopy Guidance in Percutaneous Thermal Ablation to Treat Colorectal Liver Metastases: A Single-Center Comparative Analysis of 2 Historical Cohorts. J. Vasc. Interv. Radiol. 2020, 31, 1772–1783. [Google Scholar] [CrossRef]
- van der Lei, S.; Opperman, J.; Dijkstra, M.; Kors, N.; Boon, R.; van den Bemd, B.A.T.; Timmer, F.E.F.; Nota, I.; van den Bergh, J.E.; de Vries, J.J.J.; et al. The Added Diagnostic Value of Transcatheter Ct Hepatic Arteriography for Intraprocedural Detection of Previously Unknown Colorectal Liver Metastases during Percutaneous Ablation and Impact on the Definitive Treatment Plan. Cardiovasc. Interv. Radiol. 2023, 46, 1257–1266. [Google Scholar] [CrossRef]
- van Tilborg, A.A.; Scheffer, H.J.; Nielsen, K.; van Waesberghe, J.H.; Comans, E.F.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Transcatheter Ct Arterial Portography and Ct Hepatic Arteriography for Liver Tumor Visualization during Percutaneous Ablation. J. Vasc. Interv. Radiol. 2014, 25, 1101–1111.e4. [Google Scholar] [CrossRef]
- Yu, Q.; Knight, G.; Karani, K.; Fergus, J.; Leef, J.; Funaki, B.; Ahmed, O. Real-Time Arteriography-Directed Percutaneous Microwave Ablation for Small or Poorly Characterized Hepatic Lesions Using Hybrid Angio-Ct. Abdom. Radiol. 2022, 47, 1457–1463. [Google Scholar] [CrossRef]
- Prichayudh, S.; Rajruangrabin, J.; Sriussadaporn, S.; Pak-Art, R.; Sriussadaporn, S.; Kritayakirana, K.; Samorn, P.; Narueponjirakul, N.; Uthaipaisanwong, A.; Aimsupanimitr, P.; et al. Trauma Hybrid Operating Room (Thor) Shortened Procedure Time in Abdominopelvic Trauma Patients Requiring Surgery and Interventional Radiology Procedures. Injury 2023, 54, 513–518. [Google Scholar] [CrossRef]
- Attigah, N.; Demirel, S.; Hakimi, M.; Bruijnen, H.; Schoffski, O.; Muller, A.; Geis, U.; Bockler, D. Hybrid Operating Rooms Versus Conventional Operating Rooms: Economic Comparisons in Vascular Surgery Using the Example of Endovascular Aneurysm Repair. Chirurg 2017, 88, 587–594. [Google Scholar] [CrossRef]
- Chen, P.H.; Hsu, H.H.; Yang, S.M.; Tsai, T.M.; Tsou, K.C.; Liao, H.C.; Lin, M.W.; Chen, J.S. Preoperative Dye Localization for Thoracoscopic Lung Surgery: Hybrid Versus Computed Tomography Room. Ann. Thorac. Surg. 2018, 106, 1661–1667. [Google Scholar] [CrossRef]
- Patel, S.; Lindenberg, M.; Rovers, M.M.; van Harten, W.H.; Ruers, T.J.M.; Poot, L.; Retel, V.P.; Grutters, J.P.C. Understanding the Costs of Surgery: A Bottom-up Cost Analysis of Both a Hybrid Operating Room and Conventional Operating Room. Int. J. Health Policy Manag. 2022, 11, 299–307. [Google Scholar] [CrossRef]
- Bazzi, M.; Bergbom, I.; Hellström, M.; Fridh, I.; Ahlberg, K.; Lundgren, S.M. Team Composition and Staff Roles in a Hybrid Operating Room: A Prospective Study Using Video Observations. Nurs. Open 2019, 6, 1245–1253. [Google Scholar] [CrossRef]
- Bazzi, M.; Fridh, I.; Ahlberg, K.; Bergbom, I.; Hellström, M.; Lundèn, M. Collaboration in the Hybrid Operating Room: A Focus Group Study from the Perspective of the Nursing Staff. J. Radiol. Nurs. 2021, 40, 259–267. [Google Scholar] [CrossRef]
- Sood, S.; Mbarika, V.; Jugoo, S.; Dookhy, R.; Doarn, C.R.; Prakash, N.; Merrell, R.C. What Is Telemedicine? A Collection of 104 Peer-Reviewed Perspectives and Theoretical Underpinnings. Telemed. J. e-Health 2007, 13, 573–590. [Google Scholar] [CrossRef]
- Sorensen, M.J.; Bessen, S.; Danford, J.; Fleischer, C.; Wong, S.L. Telemedicine for Surgical Consultations—Pandemic Response or Here to Stay?: A Report of Public Perceptions. Ann. Surg. 2020, 272, e174–e180. [Google Scholar] [CrossRef]
- Johnson, B. Robotic Telesurgery: Benefits Beyond Barriers. BMH Med. J. 2016, 3, 51–54. [Google Scholar]
- Marescaux, J. Code Name: “Lindbergh Operation”. Ann. Chir. 2002, 127, 2–4. [Google Scholar] [CrossRef]
- Minopoulos, G.; Kokkonis, G.; Psannis, K.; Ishibashi, Y. A Survey on Haptic Data over 5g Networks. Int. J. Future Gener. Commun. Netw. 2019, 12, 37–54. [Google Scholar] [CrossRef]
- Pandav, K.; Te, A.G.; Tomer, N.; Nair, S.S.; Tewari, A.K. Leveraging 5g Technology for Robotic Surgery and Cancer Care. Cancer Rep. 2022, 5, e1595. [Google Scholar] [CrossRef]
- Acemoglu, A.; Peretti, G.; Trimarchi, M.; Hysenbelli, J.; Krieglstein, J.; Geraldes, A.; Deshpande, N.; Ceysens, P.M.V.; Caldwell, D.G.; Delsanto, M.; et al. Operating from a Distance: Robotic Vocal Cord 5g Telesurgery on a Cadaver. Ann. Intern. Med. 2020, 173, 940–941. [Google Scholar] [CrossRef]
- Tian, W.; Fan, M.; Zeng, C.; Liu, Y.; He, D.; Zhang, Q. Telerobotic Spinal Surgery Based on 5g Network: The First 12 Cases. Neurospine 2020, 17, 114–120. [Google Scholar] [CrossRef]
- Zheng, B.; Janmohamed, Z.; MacKenzie, C.L. Reaction Times and the Decision-Making Process in Endoscopic Surgery. Surg. Endosc. 2003, 17, 1475–1480. [Google Scholar] [CrossRef]
- Choi, P.J.; Oskouian, R.J.; Tubbs, R.S. Telesurgery: Past, Present, and Future. Cureus 2018, 10, e2716. [Google Scholar] [CrossRef]
- Shenai, M.B.; Tubbs, R.S.; Guthrie, B.L.; Cohen-Gadol, A.A. Virtual Interactive Presence for Real-Time, Long-Distance Surgical Collaboration during Complex Microsurgical Procedures. J. Neurosurg. 2014, 121, 277–284. [Google Scholar] [CrossRef]
- Forbes. T-Mobile: How 5g Will Bring High-Speed Internet to Underserved Communities. Forbes. Available online: https://www.forbes.com/sites/tmobile/2021/04/09/how-5g-will-bring-high-speed-internet-to-underserved-communities/ (accessed on 15 May 2024).
- Maurice, M.J.; Zhu, H.; Kim, S.P.; Abouassaly, R. Robotic Prostatectomy Is Associated with Increased Patient Travel and Treatment Delay. Can. Urol. Assoc. J. 2016, 10, 192. [Google Scholar] [CrossRef]
- Kim, A.H.; Kim, S.P. Surviving Travel or Travelling to Survive: The Association of Travel Distance with Survival in Muscle Invasive Bladder Cancer. Transl. Androl. Urol. 2018, 7 (Suppl. S1), S83. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Bowers, H.E.; Campbell, D.M.; Parikh, P.P.; Woods, R.J. Meeting Increasing Demands for Rural General Surgeons. Am. Surg. 2015, 81, 1195–1200. [Google Scholar] [CrossRef]
- Pollock, J.D.; Love, T.P.; Steffes, B.C.; Thompson, D.C.; Mellinger, J.; Haisch, C. Is It Possible to Train Surgeons for Rural Africa? A Report of a Successful International Program. World J. Surg. 2011, 35, 493–499. [Google Scholar] [CrossRef]
- Walker, J.P. Status of the Rural Surgical Workforce. Surg. Clin. 2020, 100, 869–877. [Google Scholar] [CrossRef]
- Graham, L.A.; Hawn, M.T. Learning Curves and the Challenges of Adopting New Surgical Techniques. JAMA Netw. Open 2019, 2, e1913569. [Google Scholar] [CrossRef]
- Chen, I.A.; Ghazi, A.; Sridhar, A.; Stoyanov, D.; Slack, M.; Kelly, J.D.; Collins, J.W. Evolving Robotic Surgery Training and Improving Patient Safety, with the Integration of Novel Technologies. World J. Urol. 2021, 39, 2883–2893. [Google Scholar] [CrossRef]
- Bilgic, E.; Turkdogan, S.; Watanabe, Y.; Madani, A.; Landry, T.; Lavigne, D.; Feldman, L.S.; Vassiliou, M.C. Effectiveness of Telementoring in Surgery Compared with on-Site Mentoring: A Systematic Review. Surg. Innov. 2017, 24, 379–385. [Google Scholar] [CrossRef]
- Checcucci, E.; Verri, P.; Amparore, D.; Cacciamani, G.E.; Rivas, J.G.; Autorino, R.; Mottrie, A.; Breda, A.; Porpiglia, F. The Future of Robotic Surgery in Urology: From Augmented Reality to the Advent of Metaverse. Ther. Adv. Urol. 2023, 15, 17562872231151853. [Google Scholar] [CrossRef]
- Moor, M.; Banerjee, O.; Abad, Z.S.H.; Krumholz, H.M.; Leskovec, J.; Topol, E.J.; Rajpurkar, P. Foundation Models for Generalist Medical Artificial Intelligence. Nature 2023, 616, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Andras, I.; Mazzone, E.; van Leeuwen, F.W.B.; De Naeyer, G.; van Oosterom, M.N.; Beato, S.; Buckle, T.; O’Sullivan, S.; van Leeuwen, P.J.; Beulens, A.; et al. Artificial Intelligence and Robotics: A Combination That Is Changing the Operating Room. World J. Urol. 2020, 38, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, P.; Patterson, M.; Lahlou, S.; Mesman, J.; Nystrom, P.; Krage, R. Variation and Adaptation: Learning from Success in Patient Safety-Oriented Simulation Training. Adv. Simul. 2017, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.J.; Chen, J.; Gill, I.S. Automated Performance Metrics and Machine Learning Algorithms to Measure Surgeon Performance and Anticipate Clinical Outcomes in Robotic Surgery. JAMA Surg. 2018, 153, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.T.; Crook, B.S.; Lorentz, S.G.; Danilkowicz, R.M.; Lau, B.C.; Taylor, D.C.; Dickens, J.F.; Anakwenze, O.; Klifto, C.S. Evaluation High-Quality of Information from Chatgpt (Artificial Intelligence-Large Language Model) Artificial Intelligence on Shoulder Stabilization Surgery. Arthroscopy 2024, 40, 726–731.e6. [Google Scholar] [CrossRef] [PubMed]
- Singhal, K.; Azizi, S.; Tu, T.; Mahdavi, S.S.; Wei, J.; Chung, H.W.; Scales, N.; Tanwani, A.; Cole-Lewis, H.; Pfohl, S.; et al. Large Language Models Encode Clinical Knowledge. Nature 2023, 620, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Solano, Q.P.; Hayward, L.; Chopra, Z.; Quanstrom, K.; Kendrick, D.; Abbott, K.L.; Kunzmann, M.; Ahle, S.; Schuller, M.; Otles, E.; et al. Natural Language Processing and Assessment of Resident Feedback Quality. J. Surg. Educ. 2021, 78, e72–e77. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Frigerio, I.A.-O.; Spolverato, G.; Croner, R.; Illanes, A.A.-O.; Chouillard, E.A.-O.; Elyan, E. Artificial Intelligence Surgery: How Do We Get to Autonomous Actions in Surgery? Sensors 2021, 21, 5526. [Google Scholar] [CrossRef]
- Saeidi, H.; Opfermann, J.D.; Kam, M.; Wei, S.; Leonard, S.; Hsieh, M.H.; Kang, J.U.; Krieger, A. Autonomous Robotic Laparoscopic Surgery for Intestinal Anastomosis. Sci. Robot. 2022, 7, eabj2908. [Google Scholar] [CrossRef]
- Yang, G.Z.; Cambias, J.; Cleary, K.; Daimler, E.; Drake, J.; Dupont, P.E.; Hata, N.; Kazanzides, P.; Martel, S.; Patel, R.V.; et al. Medical Robotics-Regulatory, Ethical, and Legal Considerations for Increasing Levels of Autonomy. Sci. Robot. 2017, 2, eaam8638. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Nevejans, N.; Allen, C.; Blyth, A.; Leonard, S.; Pagallo, U.; Holzinger, K.; Holzinger, A.; Sajid, M.I.; Ashrafian, H. Legal, Regulatory, and Ethical Frameworks for Development of Standards in Artificial Intelligence (Ai) and Autonomous Robotic Surgery. Int. J. Med. Robot. 2019, 15, e1968. [Google Scholar] [CrossRef] [PubMed]
- Riskin, D.J.; Longaker, M.T.; Gertner, M.; Krummel, T.M. Innovation in Surgery: A Historical Perspective. Ann. Surg. 2006, 244, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Niki, O.; Saira, G.; Arvind, S.; Mike, D. Cyber-Attacks Are a Permanent and Substantial Threat to Health Systems: Education Must Reflect That. Digit. Health 2022, 8, 20552076221104665. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.J.; Ikoma, N.; Lyu, H.; Jackson, G.P.; Landman, A. Protecting Procedural Care-Cybersecurity Considerations for Robotic Surgery. NPJ Digit. Med. 2022, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Price, W.N., 2nd; Cohen, I.G. Privacy in the Age of Medical Big Data. Nat. Med. 2019, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Saceanu, S.; Soare, C.; Surlin, V.; Patrascu, A. Telesurgery and Robotic Surgery: Ethical and Legal Aspect. J. Community Med. Health Educ. 2015, 5. [Google Scholar] [CrossRef]
- Collins, J.W.; Marcus, H.J.; Ghazi, A.; Sridhar, A.; Hashimoto, D.; Hager, G.; Arezzo, A.; Jannin, P.; Maier-Hein, L.; Marz, K.; et al. Ethical Implications of Ai in Robotic Surgical Training: A Delphi Consensus Statement. Eur. Urol. Focus 2022, 8, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kiyasseh, D.; Laca, J.; Haque, T.F.; Otiato, M.; Miles, B.J.; Wagner, C.; Donoho, D.A.; Trinh, Q.D.; Anandkumar, A.; Hung, A.J. Human Visual Explanations Mitigate Bias in Ai-Based Assessment of Surgeon Skills. NPJ Digit. Med. 2023, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.; Geboers, B.; Gouma, D.J.; Goslings, J.C.; Ubbink, D.T. Predictors of Surgical Complications: A Systematic Review. Surgery 2015, 158, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Nori, H.; Lee, Y.T.; Zhang, S.; Carignan, D.; Edgar, R.; Fusi, N.; King, N.; Larson, J.; Li, Y.; Liu, W. Can Generalist Foundation Models Outcompete Special-Purpose Tuning? Case Study in Medicine. arXiv 2023, arXiv:2311.16452. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laterza, V.; Marchegiani, F.; Aisoni, F.; Ammendola, M.; Schena, C.A.; Lavazza, L.; Ravaioli, C.; Carra, M.C.; Costa, V.; De Franceschi, A.; et al. Smart Operating Room in Digestive Surgery: A Narrative Review. Healthcare 2024, 12, 1530. https://doi.org/10.3390/healthcare12151530
Laterza V, Marchegiani F, Aisoni F, Ammendola M, Schena CA, Lavazza L, Ravaioli C, Carra MC, Costa V, De Franceschi A, et al. Smart Operating Room in Digestive Surgery: A Narrative Review. Healthcare. 2024; 12(15):1530. https://doi.org/10.3390/healthcare12151530
Chicago/Turabian StyleLaterza, Vito, Francesco Marchegiani, Filippo Aisoni, Michele Ammendola, Carlo Alberto Schena, Luca Lavazza, Cinzia Ravaioli, Maria Clotilde Carra, Vittore Costa, Alberto De Franceschi, and et al. 2024. "Smart Operating Room in Digestive Surgery: A Narrative Review" Healthcare 12, no. 15: 1530. https://doi.org/10.3390/healthcare12151530






