Abstract
Precision cancer medicine necessitates a personalized treatment plan for each individual patient. Given cancer’s heterogeneity and dynamic nature, the plot of patient-specific signatures composed of multiple cancer circulating biomarkers is useful to reveal the complete tumor landscape for guiding precision medicine. As an emerging new technology, surface-enhanced Raman scattering (SERS) shows the intrinsic advantage of performing multiplexed detection with the extremely narrow Raman spectral line widths. In this review, we first discuss the design principle of SERS nanotags to enable the detection of multiple circulating biomarkers, highlighting the important roles of plasmonic nanostructures and triple bond-modulated Raman reporters. Following this, we detail the use of isotropic and anisotropic nanostructures as SERS enhancement substrates for amplifying Raman signals in multi-biomarker detection. Furthermore, we present the triple bond-modulated molecules as Raman reporters in SERS nanotags to expand the multiplexing capability for biomarker measurements. Finally, we offer critical insights into the challenges and perspectives of SERS nanotags for cancer diagnosis, particularly from the aspect of future clinical transition. It is expected that this review can facilitate the design of more functional SERS nanotags with high sensitivity and multiplexing capability to assist early and accurate cancer screening. We also believe our review will be of interest in the fields of molecular imaging, biomedicine, and analytical chemistry.
1. Introduction
As the leading cause of death worldwide, cancer is responsible for nearly 10 million deaths in 2020 [1]. Fortunately, emerging findings suggested that precision medicine can significantly reduce cancer mortality through introducing timely and effective medical interference [2,3,4,5]. To assist precision medicine, biomarkers circulating in body fluids (e.g., blood or urine) are able to noninvasively provide a complete cancer profile to enable early detection and guide personalized treatment management [6,7,8]. Currently, a variety of cancer circulating biomarkers has been investigated as surrogates to indicate cancer occurrence, progression, and treatment response, including proteins, circulating tumor cells (CTCs), nucleic acids (NAs), and extracellular vehicles (EVs) [9,10,11,12].
Despite the significant role of circulating biomarkers in cancer detection, their practical use for precision medicine is largely challenged by two issues: (1) the extremely low abundance of cancer-associated circulating biomarkers in the presence of large amounts of interference molecules. For instance, only 1–100 CTCs are found in one milliliter of blood with 1–2 million peripheral blood mononuclear cells, in which CTCs may experience further loss during the isolation and purification procedures [13,14], and (2) the inaccurate reflection of cancer status by considering only one relevant biomarker. Accumulating evidence shows that the mere use of prostate-specific antigen (PSA) for prostate cancer screening may not produce an improved survival benefit but comes with overtreatments and life-alerting side effects [15,16]. As such, new technologies that enable highly sensitive, specific, and parallel analysis of multiple circulating biomarkers are expected to assist accurate decision-making [2,17].
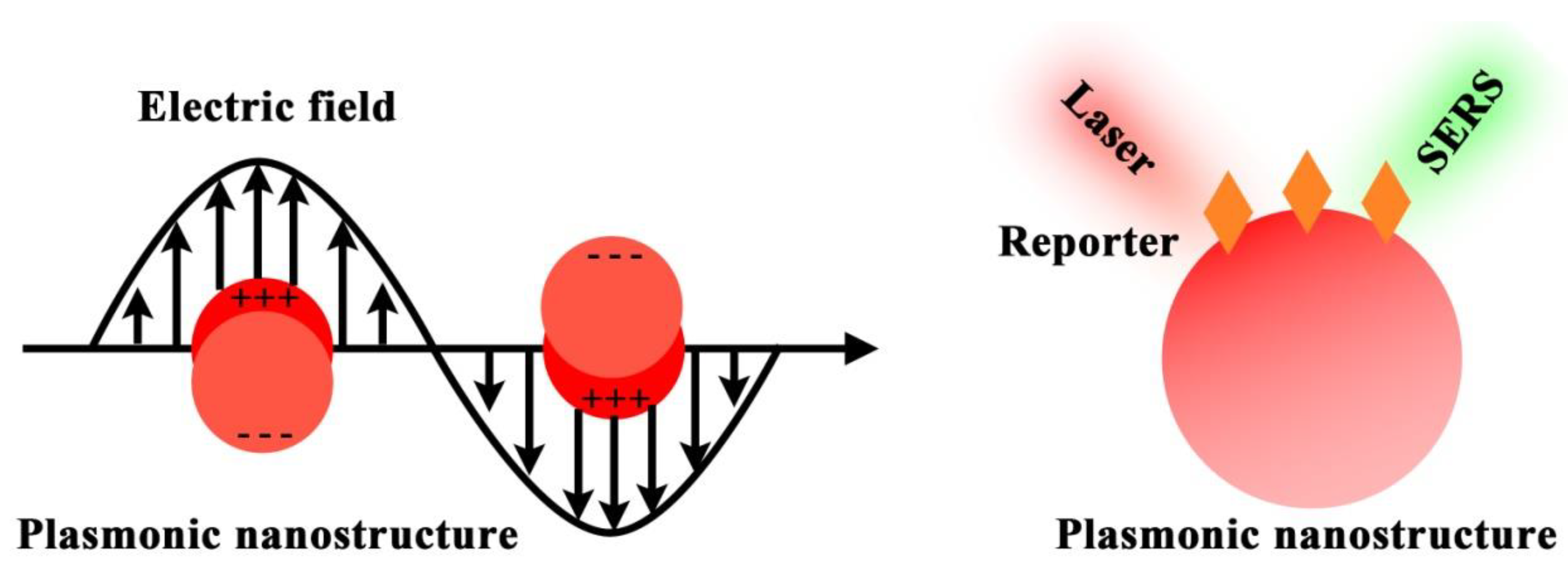
Surface-enhanced Raman scattering (SERS) is an emerging spectroscopic technology that has witnessed rapid developments in the past decade [18]. By integrating with nanotechnology (e.g., noble metal nanoparticles), SERS allows 106–1015 Raman signal amplification and thus sensitive sensing down to single molecules [19]. In addition, SERS possesses extremely narrow Raman spectral line widths (i.e., ~1 nm), which are about 50 times narrower than the commonly used fluorescence bands [19]. The intrinsic narrow Raman peaks particularly benefit multiplexed labeling with the potential to analyze 31 targets in parallel [20]. Taken together, with the advantages of high sensitivity and multiplexing capability, SERS is a good candidate to implement circulating biomarker detection for early and accurate cancer detection.
In this review, we feature the use of the SERS technique, particularly SERS nanotags, to detect cancer circulating biomarkers in a highly sensitive and multiplexed way (Figure 1). We first deliberate on the SERS nanotag design principle. Following that, we focus on the SERS nanotags using isotropic or anisotropic nanostructures as plasmonic nanostructures to enhance Raman signals for highly sensitive biomarker detection. Furthermore, we discuss the SERS nanotags with the use of triple bond-modulated Raman reporters to expand the multiplexing detection. Finally, we provide our insights on the current challenges and outlooks of SERS technique in cancer detection. This review will help researchers in the fields of Raman imaging, nanomedicine, and biomedicine to develop promising Raman agents for cancer early diagnosis and therapy.
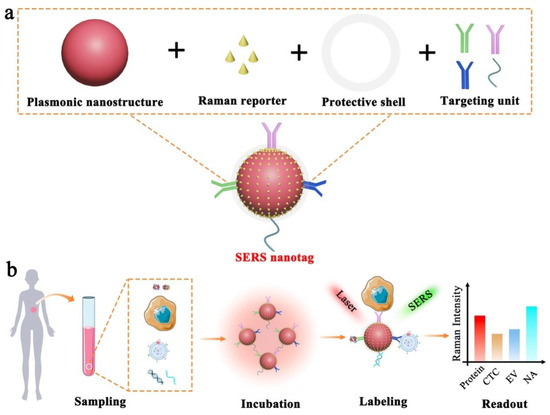
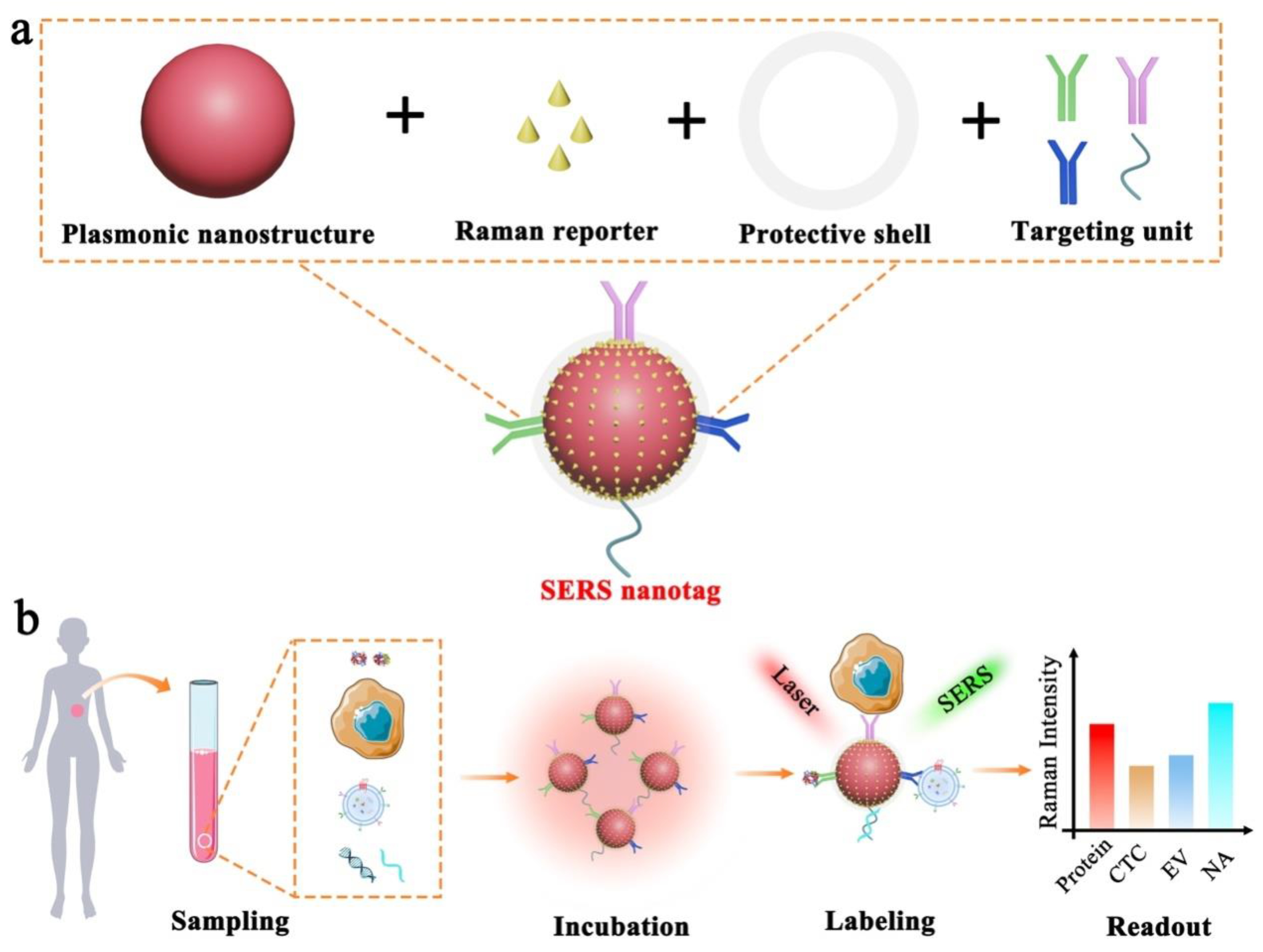
Figure 1.
Schematic illustration of SERS nanotag enabled highly sensitive and multiplexed detection of cancer circulating biomarkers. (a) The design of a typical SERS nanotag with the use of four key components. (b) The application of SERS nanotags in the detection of protein, CTC, EV, and NA.
5. Challenges and Outlooks
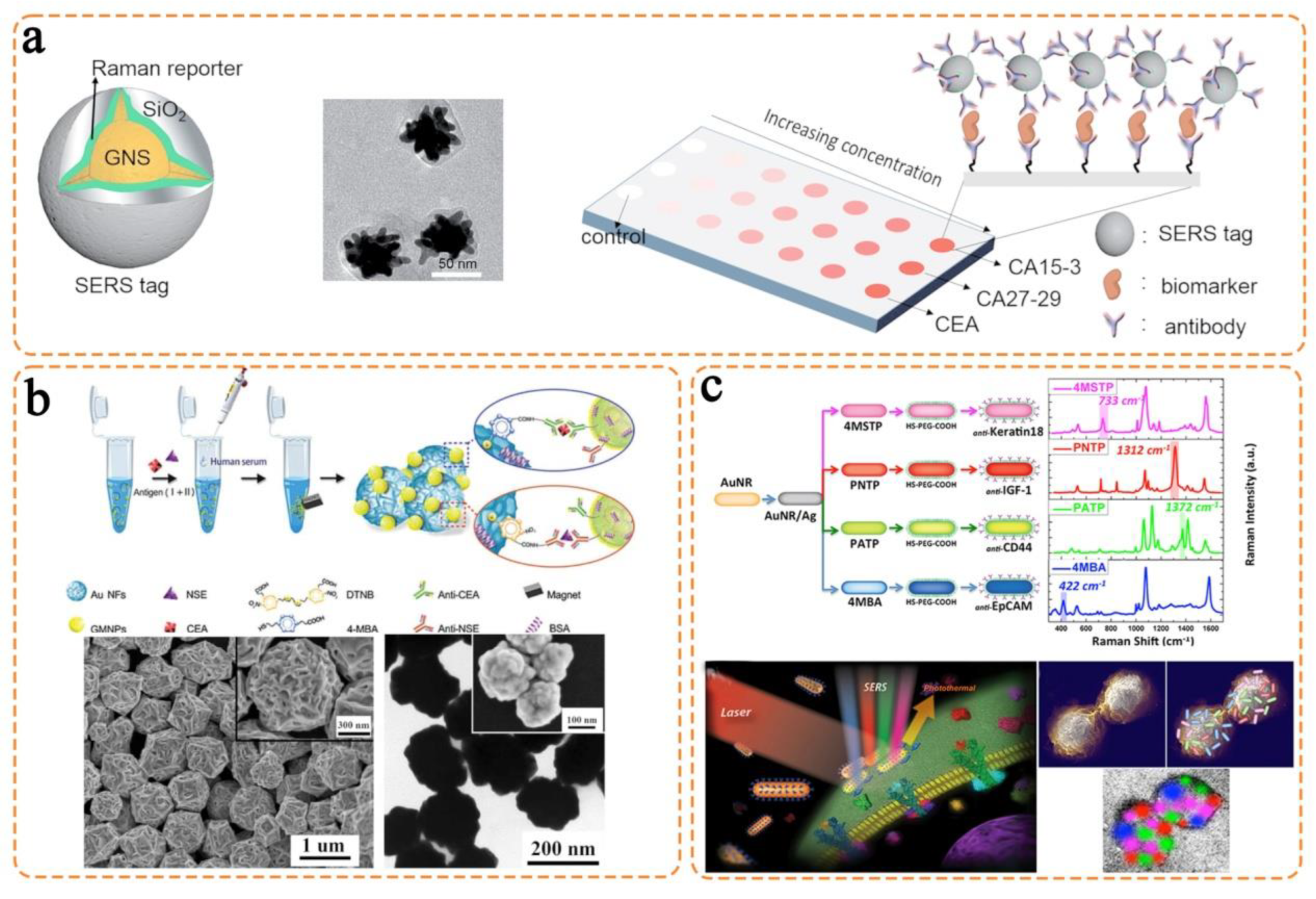
In the preceding parts, we detailed the design principle of SERS nanotags as well as the use of SERS nanotags with different nanostructure morphologies and triple bond-modulated Raman reporters in circulating biomarker detection. Compared with the traditional biochemical assays (e.g., electrochemical and fluorescence tests), the SERS nanotag-enabled biomarker detection demonstrates an outstanding advantage of simultaneously measuring multiple targets due to the extremely narrow Raman linewidths. The representative works using different SERS nanotags are summarized in Table 2. Despite the favorable analytical performance in these studies (e.g., high sensitivity and specificity), the ultimate application of SERS nanotags in cancer detection still faces several challenges, particularly for use in clinical settings. In this section, we provide insight into the possible challenges and outlooks of SERS nanotags in multiplexed detection of circulating biomarkers, aiming to transit this technology into clinical use.

Table 2.
Representative SERS nanotags in circulating biomarker detection.
5.1. Improve the Nanostructure Reproducibility and Stability
To ensure the successful application of SERS nanotags in cancer detection, the first criterion is to produce accurate signal readouts with sufficient reproducibility. As the signal is highly dependent on the electromagnetic field enhancement, the preparation of uniform nanostructures from batch to batch is essential. Furthermore, the freshly prepared nanostructures may have the required stability in the beginning. In practice, the nanostructure is susceptible to aggregate in buffer solutions, which normally happens during the functionalization of Raman reporters or targeting units on nanostructure surfaces [68,69]. The nanostructure aggregation can cause significant variations in SERS signals because of the random “hot spots” produced in the aggregates. Thus, more effective approaches to prepare highly stable nanostructures that can endure multiple steps of functionalization should be investigated. Moreover, the facile one-step functionalization or one-pot synthesis and modification strategies should also be developed to reduce the aggregation issue in the future.
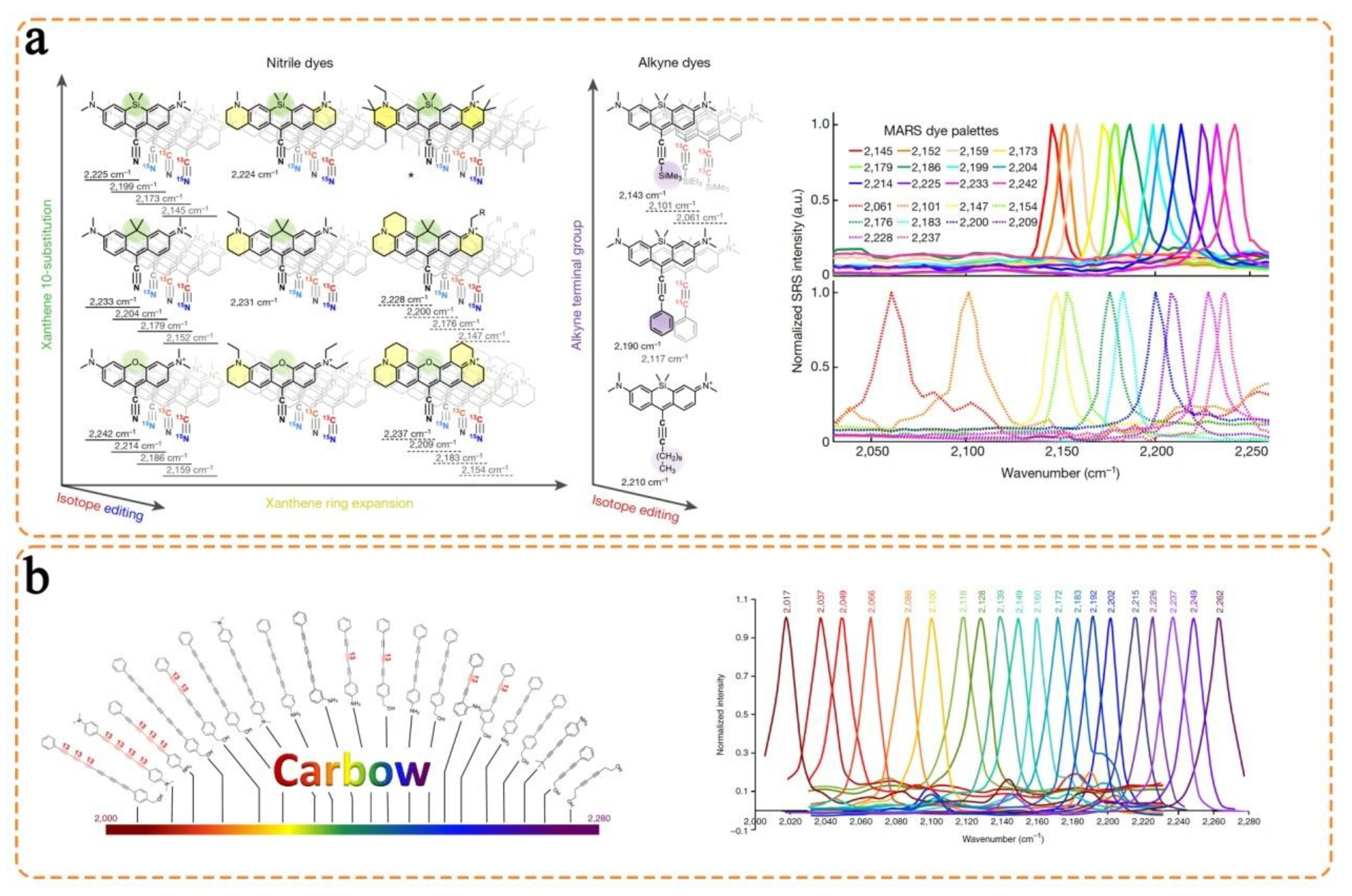
5.2. Explore New Strategies to Tune the Raman Shifts of Triple Bond-Modulated Raman Reporters
Currently, the triple bond-modulated molecules allow an easier separation of Raman peaks from each other in the Raman silent region, which is the major advantage compared to the molecules with Raman signals in the fingerprint region. Although different strategies have been used to tune their Raman shifts (e.g., introducing heavy atoms), the practical synthesis of a series of triple bond-modulated molecules is still difficult. To enable convenient and widespread use of triple bond-modulated Raman reporters, exploring new approaches that allow an easier control of Raman shifts are desired [70,71]. For instance, the test of a library of molecular structures that have flexible vibration and rotation can be a good starting point [72]. The subsequent applications can select the molecules allowing easier synthesis.
5.3. Promote Broad Collaborations
SERS is a technology that involves multidisciplinary knowledge. Specifically, the synthesis of nanostructures relies on material scientists to ensure the controllable morphology and strong Raman signal enhancement [73,74,75]. The achievement of characteristic Raman spectra with non-overlapping signals requires organic synthetic scientists to rationally design novel molecular structures [76,77]. Furthermore, the final application of SERS assay to perform various biomarker detection necessitates researchers from analytical chemistry [78,79]. Taking these thoughts into consideration, the successful SERS application should promote broad collaborations among researchers with different areas of expertise.
5.4. Realizing SERS with Multifunctional Abilities for Multimodal Imaging and Theranostics
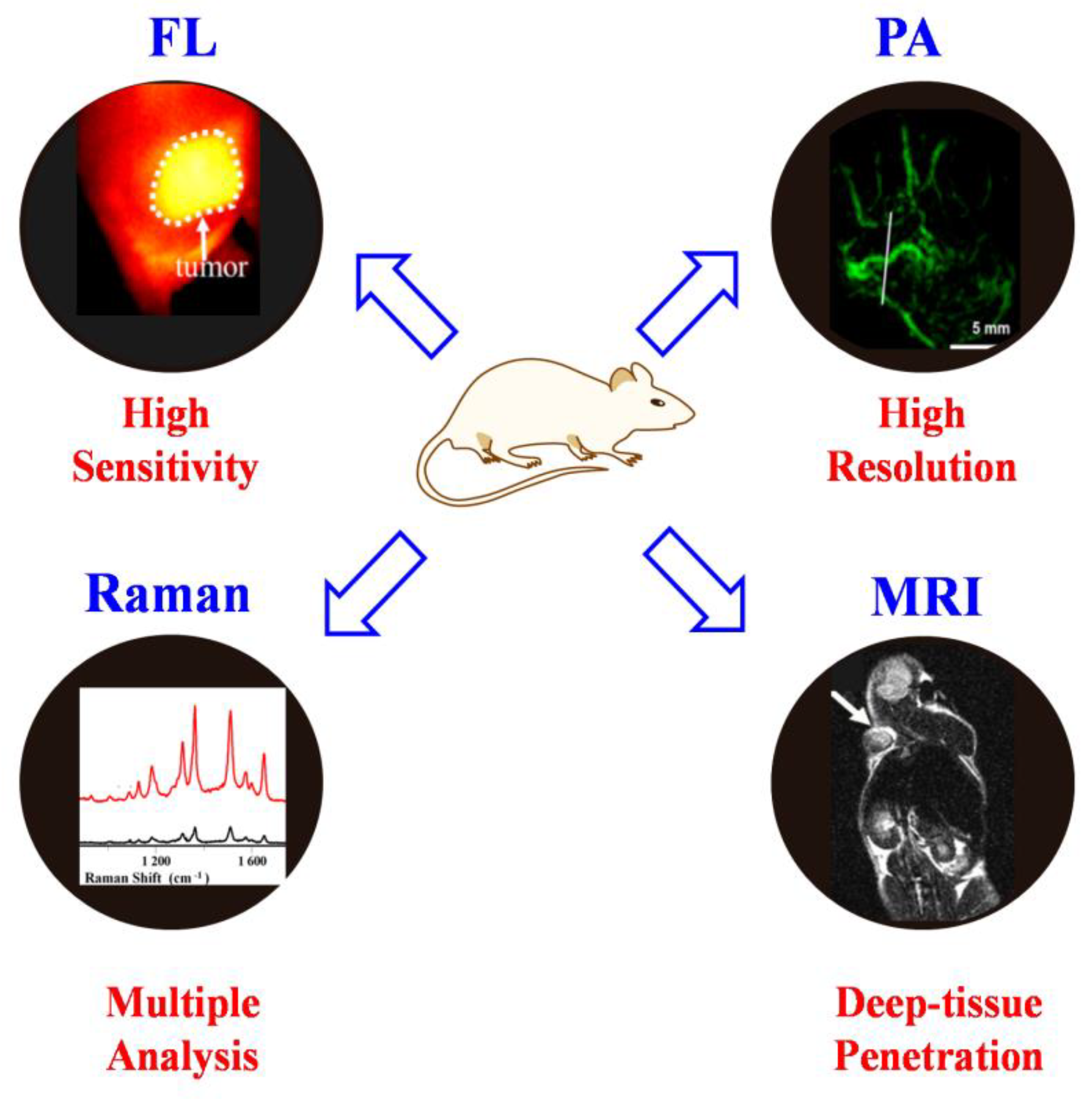
Raman imaging holds the merits of multiple analyses and demonstrates the disadvantages of sensitivity and resolution. It is well known that the frontier fluorescence imaging and photoacoustic imaging modalities, especially in the NIR region, display in vivo imaging in deep tissue with high sensitivity and resolution (Figure 10) [80,81,82]. Therefore, the combination of other molecular imaging modalities with SERS will undoubtedly overcome the limitation of each imaging modality [83,84,85]. On the other hand, the integration of Raman imaging and photo/thermal/chemotherapy modalities could achieve precise image-guided therapy or imaging monitoring of therapeutic performance [86,87,88]. For example, the combination of gold/silver nanoparticles with small-molecule Raman reporters could be easily applied to construct multifunctional probes for in vivo cancer diagnosis and therapy [89,90]. Therefore, many effects should be considered to design novel multifunctional imaging agents based on the SERS for future in vivo theranostics with high-quality imaging figures and therapeutic performance.
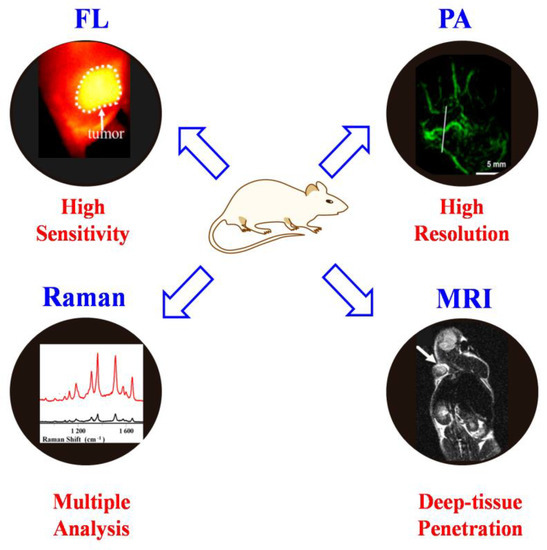
Figure 10.
The advantages of each imaging modality.
5.5. Integration of SERS with Other Imaging or Therapy Modalities toward Precision Medicine
Because of the high sensitivity and intrinsic fingerprint spectrum, SERS nanotags have attracted much attention for in vivo and intraoperative imaging. Recently, in vivo fluorescence imaging and photoacoustic imaging in the second near-infrared (NIR-II) region have demonstrated remarkable advances with better spatial resolution and deeper tissue penetration than in the traditional visible and NIR-I regions [91,92,93]. The development of SERS nanotags at the NIR-II window is therefore highly valuable. The resonant strategy, i.e., the use of resonant substrates and resonant molecules, can be helpful in fabricating bright SERS nanotags at the NIR-II window; however, this strategy has not been demonstrated with sufficient quantitative assessments. Recently, Lin and coworkers reported a quantitative study on the Raman enhancements of the resonant strategy in preparing NIR-II SERS nanotags [94]. By comparing the resonant and nonresonant plasmonic nanorod substrates, the resonant substrates have been shown to provide an enhancement factor of up to four orders of magnitude. When using the resonant Raman reporter molecules, SERS intensities can be further increased by 25–546 times. This work opens a new strategy for fabricating ultrasensitive NIR-II SERS nanotags and provides insights into the design of related plasmonic devices in the future.
5.6. Test the Assay Performance on a Large Cohort of Clinical Samples
The ultimate goal of SERS assay is to shift from fundamental research into clinical use for cancer diagnosis. Admittedly, the current SERS assay reported acceptable analytical performance, with a low limit of detection and wide dynamic detection range. Most of the studies performed testing in buffer solutions or simulated patient samples. However, clinical samples are much more complex than the buffer system, with the existence of a high abundance of interfering molecules, which may lower the analytical performance and even lead to detection failure [95]. Therefore, the test of SERS assays on real samples is essential to allow the transition into clinical use. Though some works may try a few real samples, this is still far behind the required number of samples to achieve the transition to clinical settings. Therefore, our SERS assay can start by testing a relatively large patient cohort (e.g., 50 samples). Gradually, we can increase the recruited patient cohort to progress into a clinical trial.
5.7. Pushing SERS Nanoprobesfrom In Vitro to In Vivo Applications
SERS technology is extremely sensitive and specific, can be multiplexed, and exhibits less photo-bleaching as compared to fluorescence [96]. Therefore, SERS is desirable for the development of non-invasive in vivo diagnostic and imaging tools. Compared with traditional imaging modalities such as MRI, FL, and PA, SERS imaging can provide high-resolution and molecular information for biomarkers at a lower cost. For example, recent research has confirmed that gold nanomaterials such as gold nanoparticles, gold nanostars, and gold nanorods are promising Raman substrates with outstanding surface plasmon resonance effects, adjustable structure, and adequate biocompatibility, making them widely used as SERS nanoprobes for in vivo diagnosis and imaging [97]. Though several types of gold nanomaterial-based SERS nanoprobes have been successfully applied for in vivo applications, the in vivo biocompatibility, sensitivity, and specificity still need to be improved. On the other hand, the SERS technique should also be integrated with other imaging techniques such as fluorescence/photoacoustic imaging. Finally, to speed up the in vivo applications of SERS imaging probes, specific attention should be paid to develop SERS probes in the NIR-II region.
6. Conclusions
As SERS is a powerful technology, in this work, we detailed SERS-based assays for the highly sensitive detection of multiple circulating biomarkers in parallel. In particular, we featured the use of SERS nanotags as the core for biomarker detection. We fully discussed the basic design principle of SERS nanotags, highlighting the four major components (i.e., plasmonic nanostructure, Raman reporter, protective shell, and targeting unit). Following that, we focused on the SERS nanotags that used two types of plasmonic nanostructures and triple bond-modulated Raman reporters in circulating biomarker detection. We also present our opinions on the challenges and outlooks of SERS-based assays in cancer diagnosis. We hope this review can guide the rational design of novel types of SERS nanotags and expend their applications in multiplexed biomarker detection for accurate cancer detection in vitro and in vivo.
Author Contributions
Conceptualization, Y.S. and J.L.; writing—original draft preparation, Z.Z. and R.G.; writing—review and editing, Y.S. and J.L.; supervision, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support from the National Natural Science Foundation of China (22204055, 22022404, 22074050) and the Natural Science Foundation of Hubei Province (2022CFA033); the Fundamental Research Funds for the Central Universities (CCNU22QN007), supported by the Open Project Program of Key Laboratory for Analytical Science of Food Safety and Biology, Ministry of Education (FS2202); the Open Project Program of Hubei Province Key Laboratory of Occupational Hazzard Identification and Control, Wuhan University of Science and Technology (OHIC2022K02); and the Open Project Program of Key Laboratory of Optic-electric Sensing and Analytical Chemistry for Life Science (M2022-5), MOE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| SERS | surface-enhanced Raman scattering | CTCs | circulating tumor cells |
| NAs | nucleic acids | EVs | extracellular vesicles |
| PSA | prostate-specific antigen | BSA | bovine serum albumin |
| LSPR | localized surface plasma resonance | MCSP | melanoma–chondroitin sulfate proteoglycan |
| MCAM | melanoma cell adhesion molecule | EpCAM | epithelial cell adhesion molecule |
| EMT | mesenchymal transition | EHD | electrohydrodynamic |
| CEA | carcinoembryonic antigen | HER2 | human epidermal growth factor receptor 2 |
| AFP | alpha-fetoprotein | GMA | microelectrode array |
| HCR | hybridization chain reaction | CA | cancer antigen |
| SEM | scanning electron microscope | TEM | transmission electron microscope |
| NSE | neuron-specific enolase | IGF | insulin-like growth factor |
| THBS2 | thrombospondin 2 | VCAN | versican |
| TNC | tenascin C | PD-L1 | programmed cell death-ligand 1 |
| MHC | major histocompatibility | MARS | Manhattan Raman scattering |
| SRS | stimulated Raman scattering | MBA | 4-mercaptobenzoic acid |
| TFMBA | 2,3,5,6-tetrafluoro-4-mercaptobenzoic acid | MNBA | 4-mercapto-3-nitro benzoic acid |
| MPY | 4-mercaptopyridine | DTNB | 5,5-dithiobis(2-nitrobenzoic acid) |
| PATP | p-aminothiophenol | PNTP | p-nitrothiophenol |
| 4MSTP | 4-(methylsulfanyl)thiophenol | FGF-2 | fibroblast growth factor 2 |
| G-CSF | granulocyte colony-stimulating factor | AS1411 | oligonucleotide aptamer |
| cRGD | cyclic arginine–glycine–aspartic acid | FL | fluorescence imaging |
| PA | photoacoustic imaging |
References
- World Health Organization (WHO). Cancer. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2022).
- Borrebaeck, C.A.K. Precision siagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.M.; Wee, E.J.H.; Mainwaring, P.N.; Wang, Y.; Trau, M. Toward precision medicine: A cancer molecular subtyping nano-strategy for RNA biomarkers in tumor and urine. Small 2016, 12, 6233–6242. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; Ma, X.; Tuo, W.; Tu, L.; Li, X.; Sun, Y.; Stang, P.J.; Sun, Y. Long wavelength-emissive Ru(II) metallacycle-based photosensitizer assisting in vivo bacterial diagnosis and antibacterial treatment. Proc. Natl. Acad. Sci. 2022, 119, e2209904119. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhong, M.; Ye, F.; Zhang, X. Low-frequency HIFU induced cancer immunotherapy: Tempting challenges and potential opportunities. Cancer Bio. Med. 2019, 16, 714. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472. [Google Scholar] [CrossRef]
- Li, J.; Wuethrich, A.; Dey, S.; Lane, R.E.; Sina, A.A.I.; Wang, J.; Wang, Y.; Puttick, S.; Koo, K.M.; Trau, M. The growing impact of micro/nanomaterial-based systems in precision oncology: Translating “multiomics” technologies. Adv. Funct. Mater. 2020, 30, 1909306. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Yu, G.; Yu, K. Effects of siRNA interference and over-expression of HMGA2 on proliferation and apoptosis of colorectal cancer cells. Int. J. Clin. Exp. Pathol. 2017, 10, 4611. [Google Scholar]
- Li, W.; Wang, H.; Zhao, Z.; Gao, H.; Liu, C.; Zhu, L.; Wang, C.; Yang, Y. Emerging nanotechnologies for liquid biopsy: The detection of circulating tumor cells and extracellular vesicles. Adv. Mater. 2018, 31, 1805344. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhang, Z.; Zheng, F.; Wang, L.; Guo, J.; Zhang, T.; Dai, X.; Zhang, S.; Yang, D.; Kuang, R.; et al. Combining protein and miRNA quantification for bladder cancer analysis. ACS Appl. Mater. Interfaces 2017, 9, 23420–23427. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Li, B.; Situ, B.; Pan, W.; Hu, Y.; An, T.; Yao, S.; Zheng, L. Single-exosome-counting immunoassays for cancer diagnostics. Nano Lett. 2018, 18, 4226–4232. [Google Scholar] [CrossRef]
- Ning, Z.; Gan, J.; Chen, C.; Zhang, D.; Zhang, H. Molecular functions and significance of the MTA family in hormone-independent cancer. Cancer Metast. Rev. 2014, 33, 901. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Chandran, B.K.; Lim, C.T.; Chen, X. Rational design of materials interface for efficient capture of circulating tumor cells. Adv. Sci. 2015, 2, 1500118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Wang, Y.; Trau, M. Simple and rapid colorimetric detection of melanoma circulating tumor cells using bifunctional magnetic nanoparticles. Analyst 2017, 142, 4788–4793. [Google Scholar] [CrossRef]
- Bostwick, D.G. Prostate-specific antigen. Current role in diagnostic pathology of prostate cancer. Am. J. Clin. Pathol. 1994, 102, S31–S37. [Google Scholar] [PubMed]
- Ankerst, D.P.; Thompson, I.M. Sensitivity and specificity of prostate-specific antigen for prostate cancer detection with high rates of biopsy verification. Arch. Ital. Urol. Androl. 2006, 78, 125–129. [Google Scholar]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar]
- Mir-Simon, B.; Reche-Perez, I.; Guerrini, L.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Universal one-pot and scalable synthesis of SERS encoded nanoparticles. Chem. Mater. 2015, 27, 950–958. [Google Scholar] [CrossRef]
- Tan, T.; Tian, C.; Ren, Z.; Yang, J.; Chen, Y.; Sun, L.; Li, Z.; Wu, A.; Yin, J.; Fu, H. LSPR-dependent SERS performance of silver nanoplates with highly stable and broad tunable LSPR prepared through an improved seed-mediated strategy. Phys. Chem. Chem. Phys. 2013, 15, 21034–21042. [Google Scholar] [CrossRef]
- Im, H.; Bantz, K.C.; Lee, S.H.; Johnson, T.W.; Haynes, C.L.; Oh, S.-H. Self-assembled plasmonic nanoring cavity arrays for SERS and LSPR biosensing. Adv. Mater. 2013, 25, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Koo, K.M.; Wang, Y.; Trau, M. Native microRNA targets trigger self-assembly of nanozyme-patterned hollowed nanocuboids with optimal interparticle gaps for plasmonic-activated cancer detection. Small 2019, 15, 1904689. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.Y.; Park, S.-G.; Lee, S.Y.; Jeon, H.C.; Yang, S.-M. Shape control of Ag nanostructures for practical sers substrates. ACS Appl. Mater. Interfaces 2013, 5, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Chikkaraddy, R.; Salmon, A.; Ohadi, H.; de Nijs, B.; Mertens, J.; Carnegie, C.; Bowman, R.W.; Baumberg, J.J. SERS of individual nanoparticles on a mirror: Size does matter, but so does shape. J. Phys. Chem. Lett. 2016, 7, 2264–2269. [Google Scholar] [CrossRef]
- Shen, X.S.; Wang, G.Z.; Hong, X.; Zhu, W. Nanospheres of silver nanoparticles: Agglomeration, surface morphology control and application as SERS substrates. Phys. Chem. Chem. Phys. 2009, 11, 7450–7454. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Z.; Shi, L.; Long, R.; Anzalone, A.V.; Zhang, L.; Hu, F.; Yuste, R.; Cornish, V.W.; Min, W. Super-multiplex vibrational imaging. Nature 2017, 544, 465–470. [Google Scholar] [CrossRef]
- Zheng, X.-S.; Hu, P.; Cui, Y.; Zong, C.; Feng, J.-M.; Wang, X.; Ren, B. Bsa-coated nanoparticles for improved SERS-based intracellular ph sensing. Anal. Chem. 2014, 86, 12250–12257. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Paladini, B.J.; Naczynski, D.J.; Starovoytov, V.; Moghe, P.V.; Fabris, L. Dimeric gold nanoparticle assemblies as tags for SERS-based cancer detection. Adv. Healthc. Mater. 2013, 2, 1370–1376. [Google Scholar] [CrossRef]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: A potential nanoplatform for theranostics. Langmuir 2011, 27, 12186–12190. [Google Scholar] [CrossRef]
- Farahavar, G.; Abolmaali, S.S.; Nejatollahi, F.; Safaie, A.; Javanmardi, S.; Khajeh Zadeh, H.; Yousefi, R.; Nadgaran, H.; Mohammadi-Samani, S.; Tamaddon, A.M.; et al. Single-chain antibody-decorated Au nanocages@liposomal layer nanoprobes for targeted SERS imaging and remote-controlled photothermal therapy of melanoma cancer cells. Mater. Sci. Eng. C 2021, 124, 112086. [Google Scholar] [CrossRef]
- Kumar, A.R.; Shanmugasundaram, K.B.; Li, J.; Zhang, Z.; Ibn Sina, A.A.; Wuethrich, A.; Trau, M. Ultrasensitive melanoma biomarker detection using a microchip sers immunoassay with anisotropic au–ag alloy nanoboxes. RSC Adv. 2020, 10, 28778–28785. [Google Scholar] [CrossRef] [PubMed]
- Farokhinejad, F.; Li, J.; Hugo, L.E.; Howard, C.B.; Wuethrich, A.; Trau, M. Detection of dengue virus 2 with single infected mosquito resolution using yeast affinity bionanofragments and plasmonic sers nanoboxes. Anal. Chem. 2022, 94, 14177–14184. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Cognet, L.; Tamarat, P.; Lounis, B. Observation of intrinsic size effects in the optical response of individual gold nanoparticles. Nano Lett. 2005, 5, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Walkenfort, B.; Yoon, J.H.; Schlucker, S.; Xie, W. Gold and silver nanoparticle monomers are non-sers-active: A negative experimental study with silica-encapsulated raman-reporter-coated metal colloids. Phys. Chem. Chem. Phys. 2015, 17, 21120–21126. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.-I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with sers hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef]
- Willets, K.A. Super-resolution imaging of sers hot spots. Chem. Soc. Rev. 2014, 43, 3854–3864. [Google Scholar] [CrossRef]
- Pearce, A.K.; Wilks, T.R.; Arno, M.C.; O’Reilly, R.K. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nat. Rev. Chem. 2021, 5, 21–45. [Google Scholar] [CrossRef]
- Sajanlal, P.R.; Sreeprasad, T.S.; Samal, A.K.; Pradeep, T. Anisotropic nanomaterials: Structure, growth, assembly, and functions. Nano Rev. 2011, 2, 5883. [Google Scholar] [CrossRef]
- Mulvihill, M.J.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic etching of silver nanoparticles for plasmonic structures capable of single-particlesers. J. Am. Chem. Soc. 2010, 132, 268–274. [Google Scholar] [CrossRef]
- Ye, S.; Benz, F.; Wheeler, M.C.; Oram, J.; Baumberg, J.J.; Cespedes, O.; Christenson, H.K.; Coletta, P.L.; Jeuken, L.J.; Markham, A.F. One-step fabrication of hollow-channel gold nanoflowers with excellent catalytic performance and large single-particle sers activity. Nanoscale 2016, 8, 14932–14942. [Google Scholar] [CrossRef]
- Tran, V.; Thiel, C.; Svejda, J.T.; Jalali, M.; Walkenfort, B.; Erni, D.; Schlücker, S. Probing the sers brightness of individual au nanoparticles, hollow au/ag nanoshells, au nanostars and au core/au satellite particles: Single-particle experiments and computer simulations. Nanoscale 2018, 10, 21721–21731. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Kang, H.; Yang, J.-K.; Jo, A.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H. Ag shell–au satellite hetero-nanostructure for ultra-sensitive, reproducible, and homogeneous nirsers activity. ACS Appl. Mater. Interfaces 2014, 6, 11859–11863. [Google Scholar] [CrossRef] [PubMed]
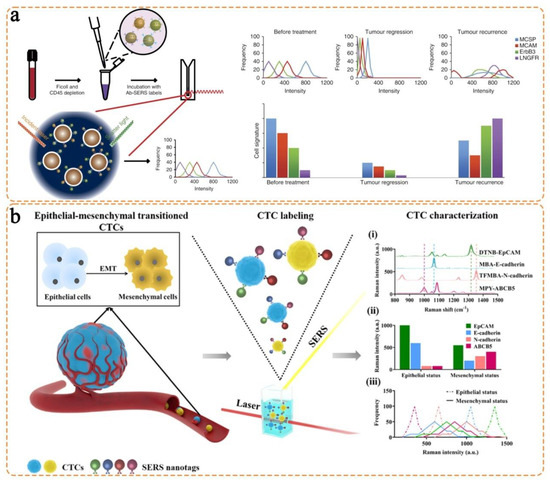
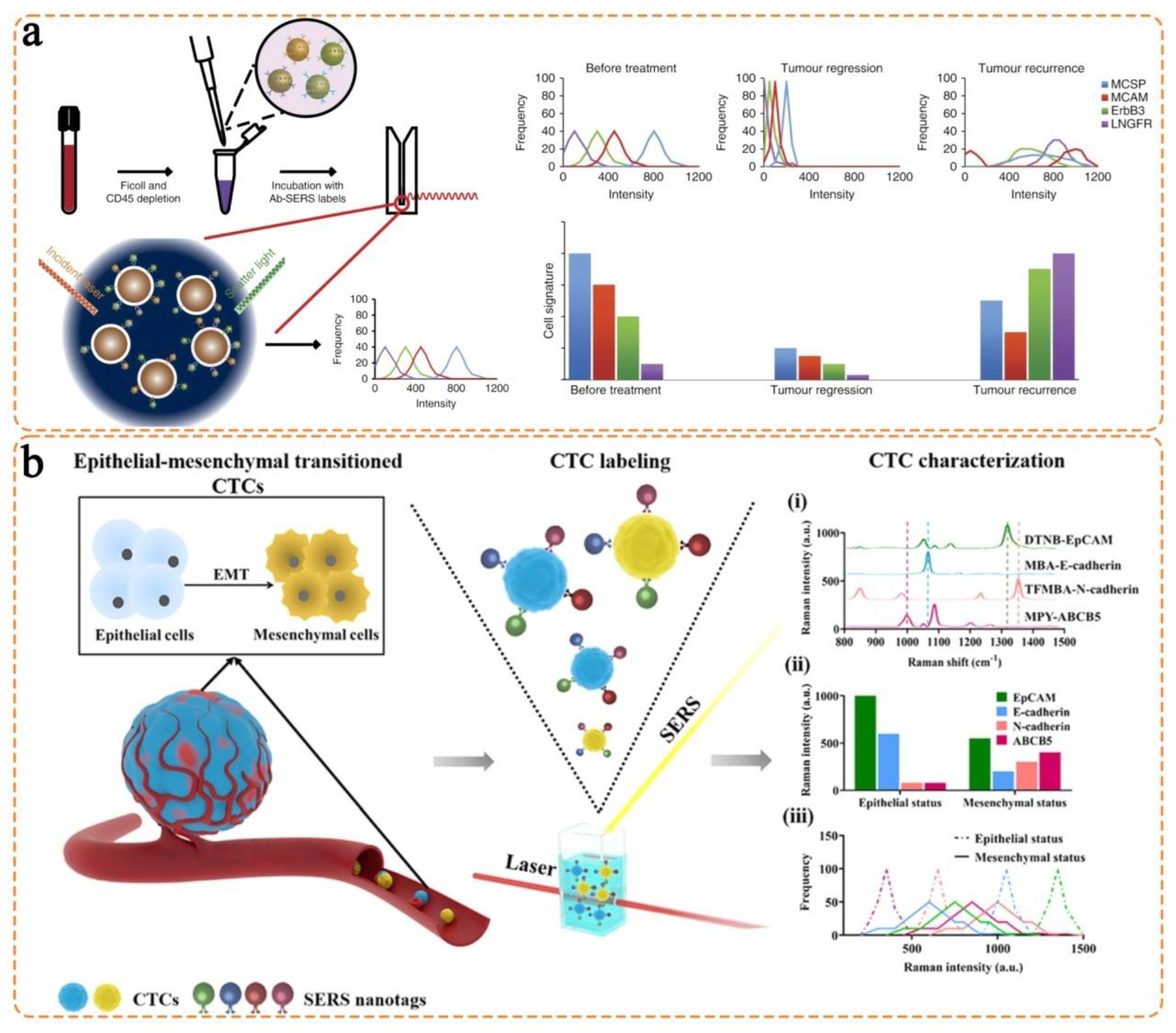
- Tsao, S.C.-H.; Wang, J.; Wang, Y.; Behren, A.; Cebon, J.; Trau, M. Characterising the phenotypic evolution of circulating tumour cells during treatment. Nat. Commun. 2018, 9, 1482. [Google Scholar] [CrossRef]
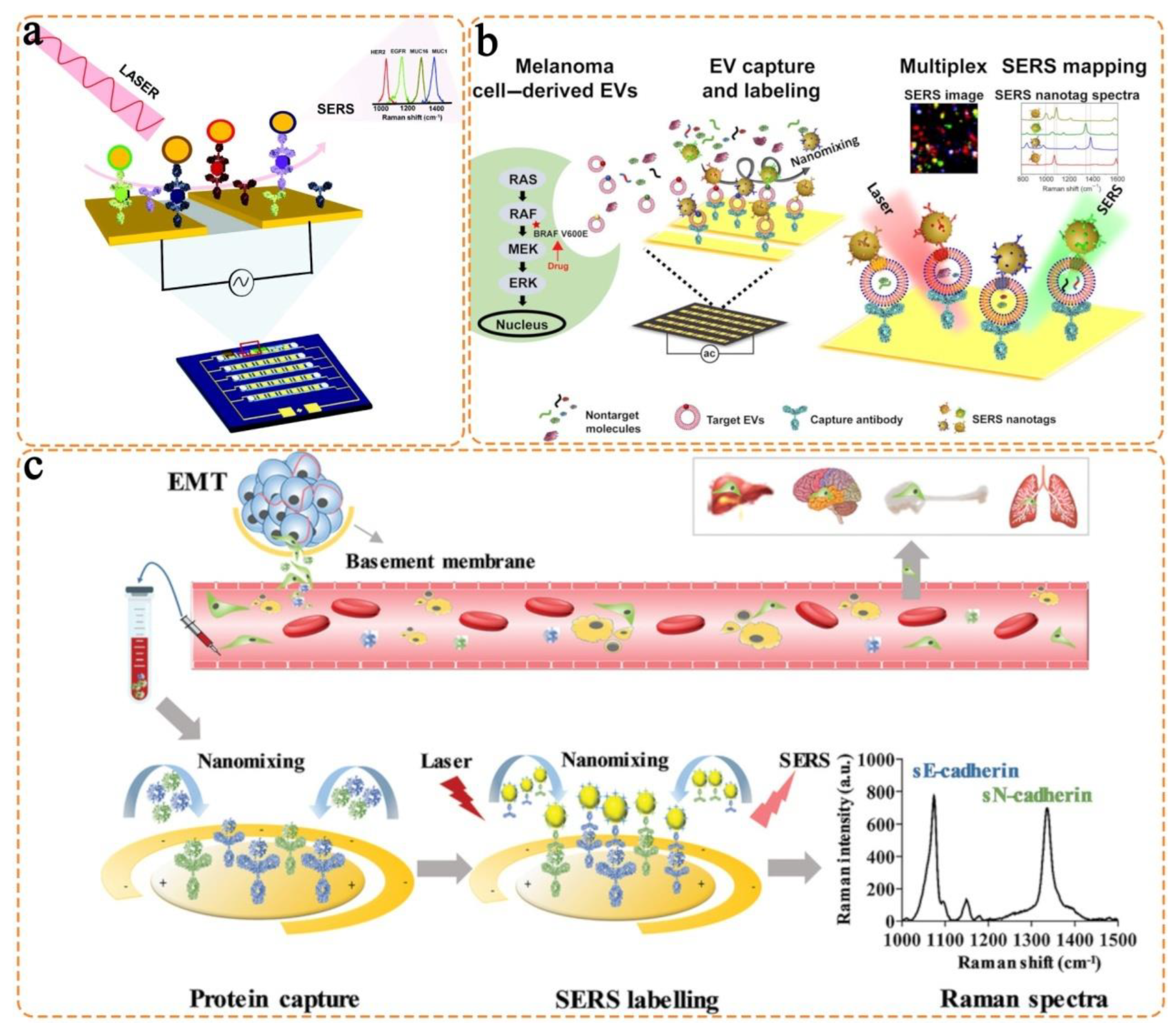
- Zhang, Z.; Wuethrich, A.; Wang, J.; Korbie, D.; Lin, L.L.; Trau, M. Dynamic monitoring of emt in ctcs as an indicator of cancer metastasis. Anal. Chem. 2021, 93, 16787–16795. [Google Scholar] [CrossRef] [PubMed]
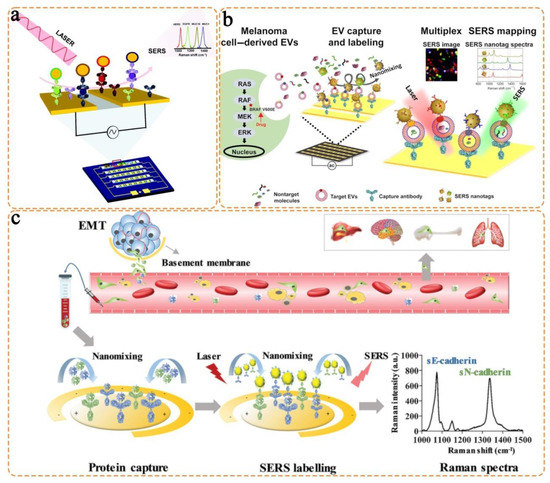
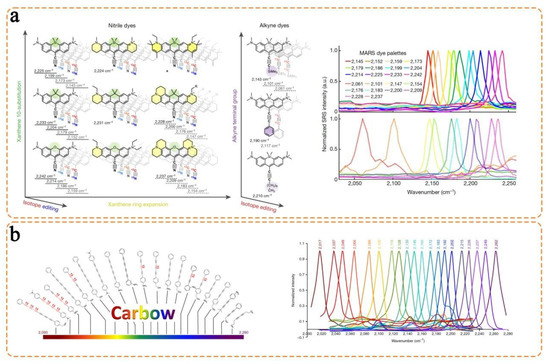
- Kamil Reza, K.; Wang, J.; Vaidyanathan, R.; Dey, S.; Wang, Y.; Trau, M. Electrohydrodynamic-induced SERS immunoassay for extensive multiplexed biomarker sensing. Small 2017, 13, 1602902. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Shanmugasundaram, K.B.; Yeo, B.; Möller, A.; Wuethrich, A.; Lin, L.L.; Trau, M. Tracking drug-induced epithelial–mesenchymal transition in breast cancer by a microfluidic surface-enhanced Raman spectroscopy immunoassay. Small 2020, 16, 1905614. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wuethrich, A.; Sina, A.A.I.; Lane, R.E.; Lin, L.L.; Wang, Y.; Cebon, J.; Behren, A.; Trau, M. Tracking extracellular vesicle phenotypic changes enables treatment monitoring in melanoma. Sci. Adv. 2020, 6, eaax3223. [Google Scholar] [CrossRef] [PubMed]
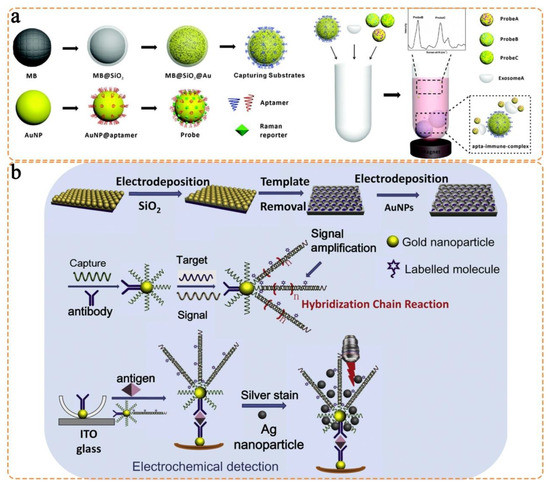
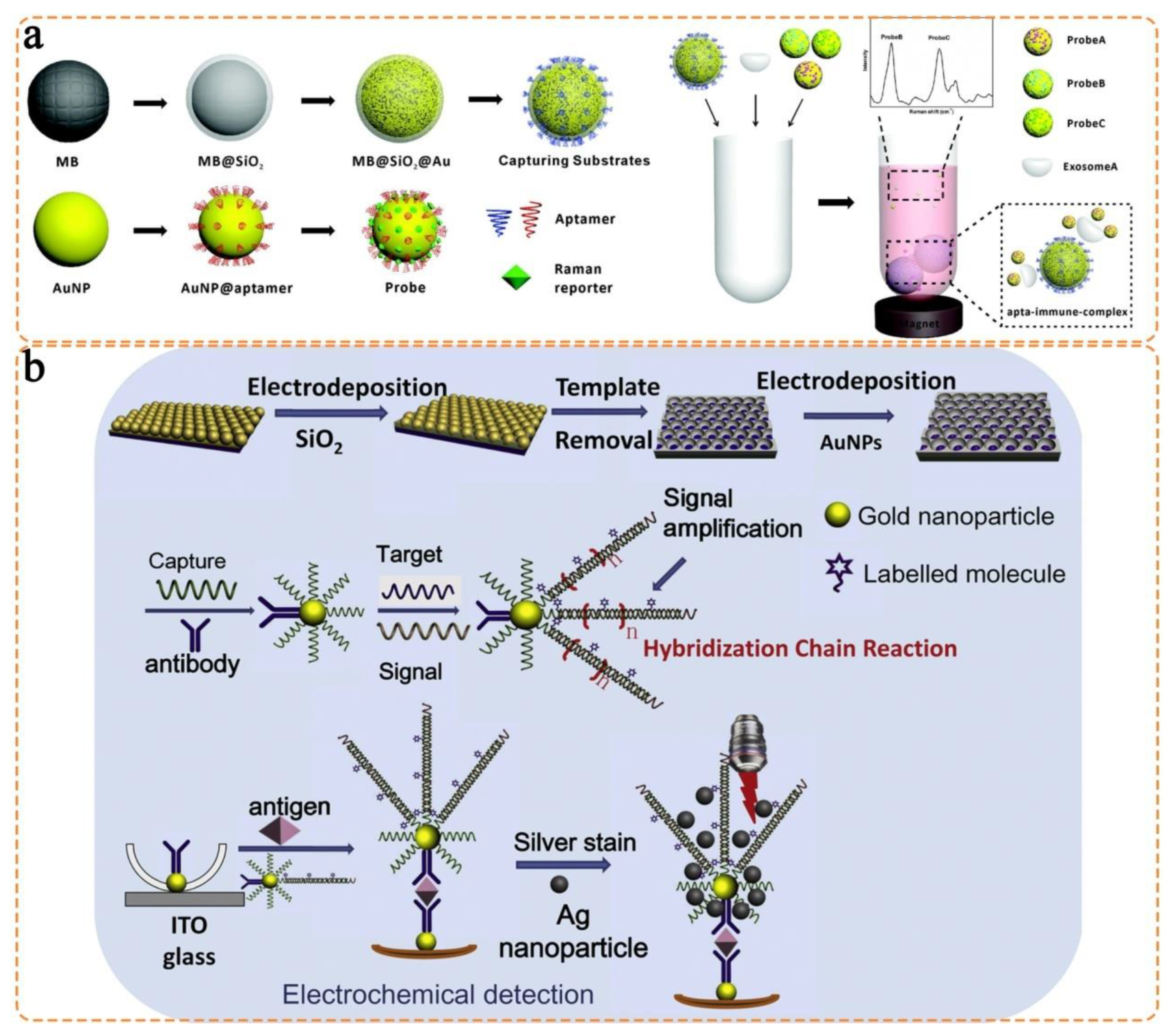
- Wang, Z.; Zong, S.; Wang, Y.; Li, N.; Li, L.; Lu, J.; Wang, Z.; Chen, B.; Cui, Y. Screening and multiple detection of cancer exosomes using an SERS-based method. Nanoscale 2018, 10, 9053–9062. [Google Scholar] [CrossRef]
- Gu, X.; Wang, K.; Qiu, J.; Wang, Y.; Tian, S.; He, Z.; Zong, R.; Kraatz, H.-B. Enhanced electrochemical and SERS signals by self-assembled gold microelectrode arrays: A dual readout platform for multiplex immumoassay of tumor biomarkers. Sens. Actuators B Chem. 2021, 334, 129674. [Google Scholar] [CrossRef]
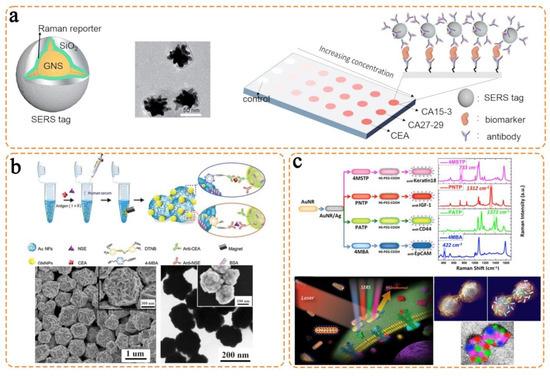
- Li, M.; Kang, J.W.; Sukumar, S.; Dasari, R.R.; Barman, I. Multiplexed detection of serological cancer markers with plasmon-enhanced Raman spectro-immunoassay. Chem. Sci. 2015, 6, 3906–3914. [Google Scholar] [CrossRef]
- Song, C.; Yang, Y.; Yang, B.; Min, L.; Wang, L. Combination assay of lung cancer associated serum markers using surface-enhanced Raman spectroscopy. J. Mater. Chem. B 2016, 4, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Nima, Z.A.; Mahmood, M.; Xu, Y.; Mustafa, T.; Watanabe, F.; Nedosekin, D.A.; Juratli, M.A.; Fahmi, T.; Galanzha, E.I.; Nolan, J.P.; et al. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. Sci. Rep. 2014, 4, 4752. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Wang, J.; Maksymov, I.S.; Greentree, A.D.; Hu, J.; Shen, A.; Wang, Y.; Trau, M. Facile one-pot synthesis of nanodot-decorated gold–silver alloy nanoboxes for single-particle surface-enhanced Raman scattering activity. ACS Appl. Mater. Interfaces 2018, 10, 32526–32535. [Google Scholar] [CrossRef] [PubMed]
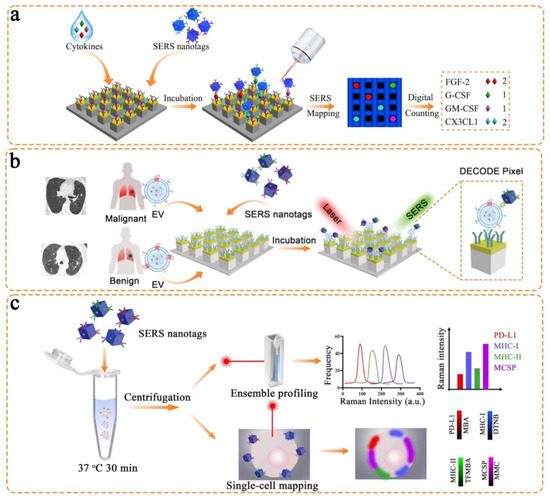
- Li, J.; Wuethrich, A.; Sina, A.A.I.; Cheng, H.-H.; Wang, Y.; Behren, A.; Mainwaring, P.N.; Trau, M. A digital single-molecule nanopillar SERS platform for predicting and monitoring immune toxicities in immunotherapy. Nat. Commun. 2021, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sina, A.A.I.; Antaw, F.; Fielding, D.; Möller, A.; Lobb, R.; Wuethrich, A.; Trau, M. Digital decoding of single extracellular vesicle phenotype differentiates early malignant and benign lung lesions. Adv. Sci. 2022, 10, 2204207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wuethrich, A.; Zhang, Z.; Wang, J.; Lin, L.L.; Behren, A.; Wang, Y.; Trau, M. SERS multiplex profiling of melanoma circulating tumor cells for predicting the response to immune checkpoint blockade therapy. Anal. Chem. 2022, 94, 14573–14582. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Grewal, Y.S.; Howard, C.B.; Raftery, L.J.; Mahler, S.; Wang, Y.; Trau, M. Multiplexed SERS detection of soluble cancer protein biomarkers with gold–silver alloy nanoboxes and nanoyeast single-chain variable fragments. Anal. Chem. 2018, 90, 10377–10384. [Google Scholar] [CrossRef]
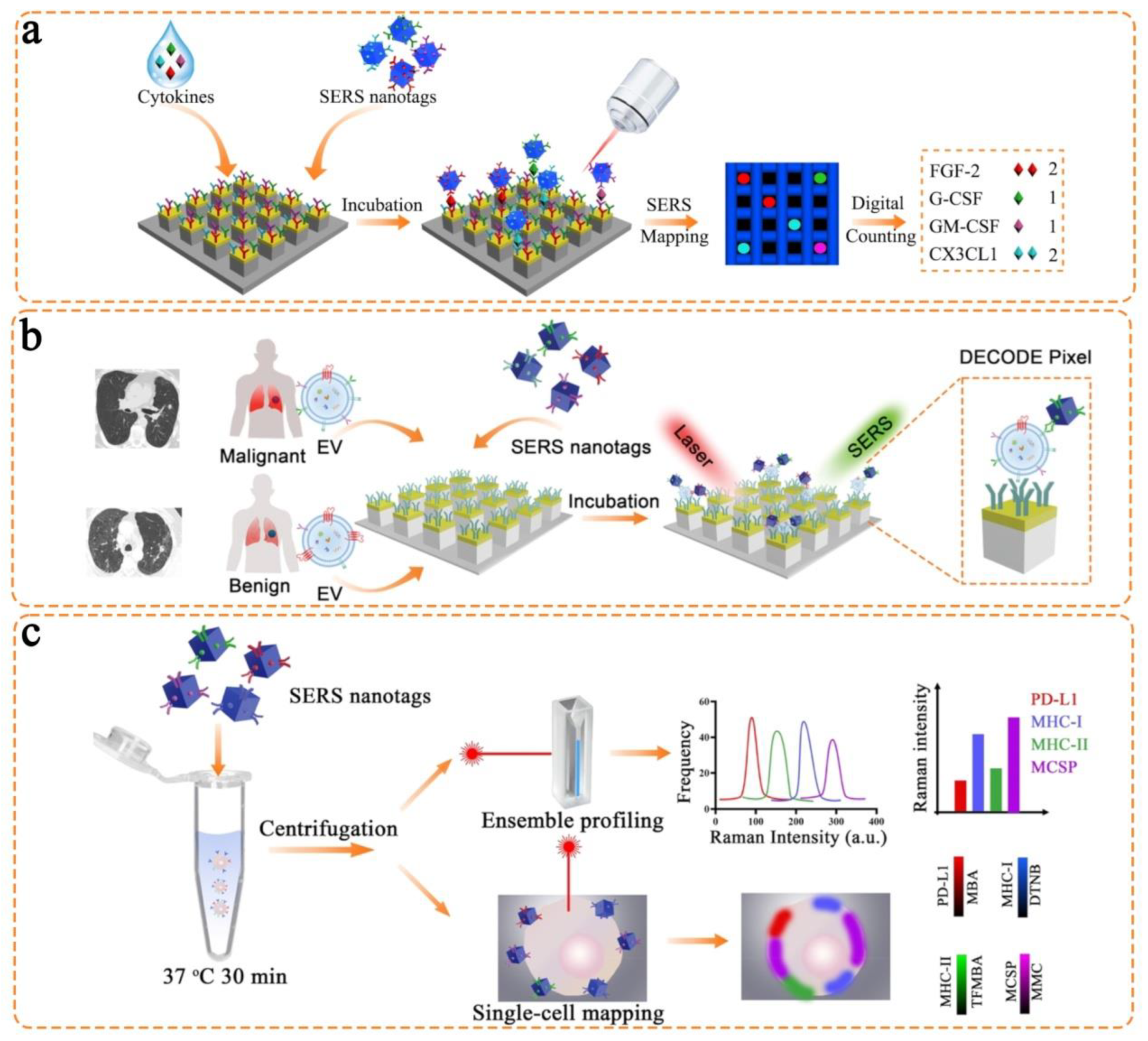
- Miao, Y.; Qian, N.; Shi, L.; Hu, F.; Min, W. 9-Cyanopyronin probe palette for super-multiplexed vibrational imaging. Nat. Commun. 2021, 12, 4518. [Google Scholar] [CrossRef]
- Hu, F.; Zeng, C.; Long, R.; Miao, Y.; Wei, L.; Xu, Q.; Min, W. Supermultiplexed optical imaging and barcoding with engineered polyynes. Nat. Methods 2018, 15, 194–200. [Google Scholar] [CrossRef]
- Hu, F.; Shi, L.; Min, W. Biological imaging of chemical bonds by stimulated Raman scattering microscopy. Nat. Methods 2019, 16, 830–842. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Z.; Qian, N.; Wei, S.; Hu, F.; Min, W. Multiplexed live-cell profiling with Raman probes. Nat. Commun. 2021, 12, 3405. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wei, M.; Miao, Y.; Qian, N.; Shi, L.; Singer, R.A.; Benninger, R.K.P.; Min, W. Highly-multiplexed volumetric mapping with Raman dye imaging and tissue clearing. Nat. Biotechnol. 2022, 40, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, J.-Q.; Zhang, X.-G.; Wu, D.-Y.; Shen, A.-G.; Hu, J.-M. Alkyne-modulated surface-enhanced Raman scattering-palette for optical interference-free and multiplex cellular imaging. Anal. Chem. 2016, 88, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-R.; Wang, L.-H.; Ren, J.-Q.; Bai, X.-W.; Zeng, L.-W.; Shen, A.-G.; Hu, J.-M. Accurate clinical diagnosis of liver cancer based on simultaneous detection of ternary specific antigens by magnetic induced mixing surface-enhanced Raman scattering emissions. Anal. Chem. 2019, 91, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
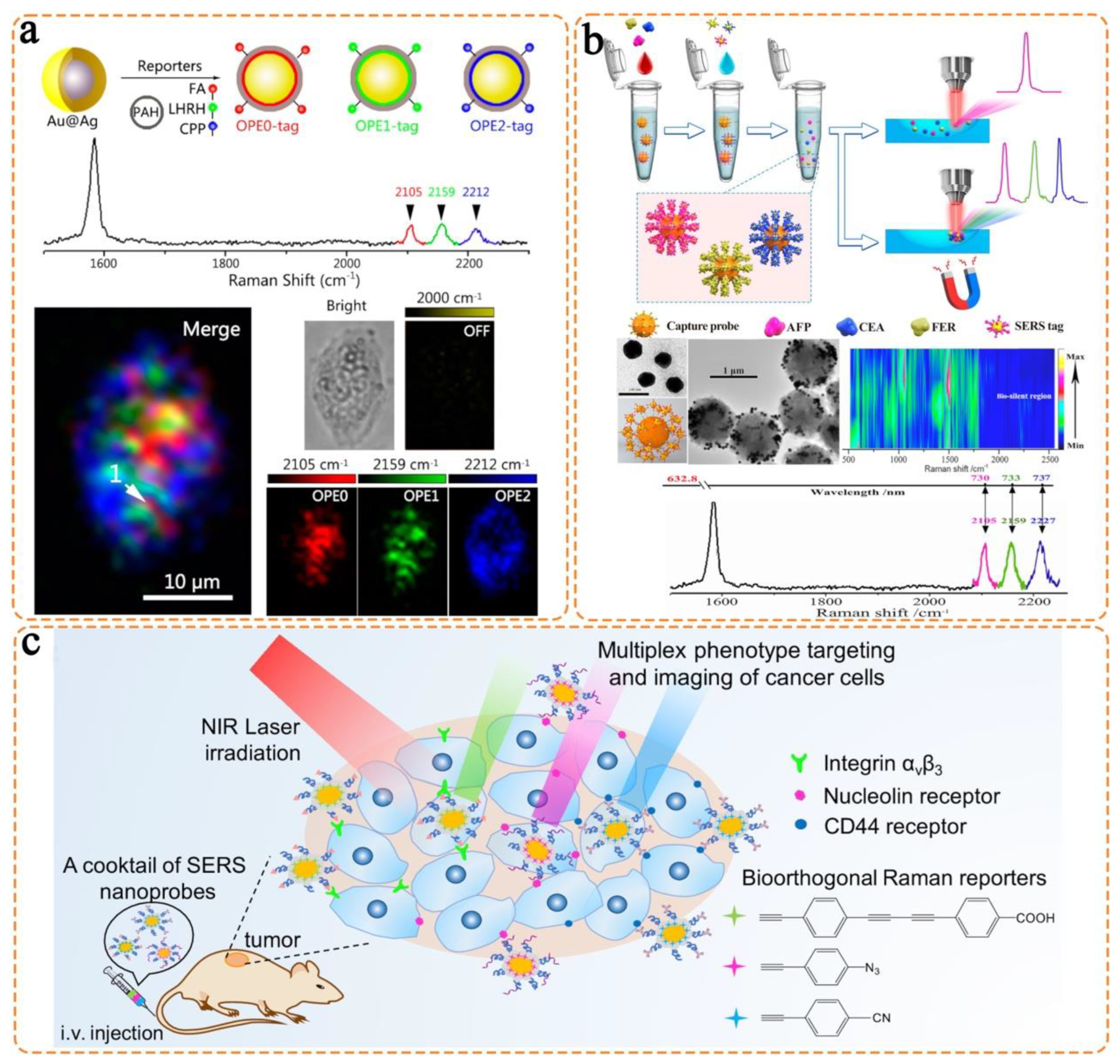
- Wang, J.; Liang, D.; Feng, J.; Tang, X. Multicolor cocktail for breast cancer multiplex phenotype targeting and diagnosis using bioorthogonal surface-enhanced Raman scattering nanoprobes. Anal. Chem. 2019, 91, 11045–11054. [Google Scholar] [CrossRef]
- Li, M.; Wu, J.; Ma, M.; Feng, Z.; Mi, Z.; Rong, P.; Liu, D. Alkyne- and nitrile-anchored gold nanoparticles for multiplex SERS imaging of biomarkers in cancer cells and tissues. Nanotheranostics 2019, 3, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sun, Y.; Cao, Y.; Kang, S.H. Plasmonic nanostructure-based bioimaging and detection techniques at the single-cell level. Trend Anal. Chem. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, Z.; Li, Q.; Wang, R.; Yu, F. Toward Sensitive and Reliable Surface-Enhanced Raman Scattering Imaging: From Rational Design to Biomedical Applications. ACS Sens. 2012, 6, 3912–3932. [Google Scholar] [CrossRef] [PubMed]
- Eremina, O.E.; Czaja, A.T.; Fernando, A.; Aron, A.; Eremin, D.B.; Zavaleta, C. Expanding the Multiplexing Capabilities of Raman Imaging to Reveal Highly Specific Molecular Expression and Enable Spatial Profiling. ACS Nano 2022, 16, 10341–10353. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, R.; Yang, B.; Tian, T.; Zhang, K.; Liu, B. A Rational Designed Bioorthogonal Surface-Enhanced Raman Scattering Nanoprobe for Quantitatively Visualizing Endogenous Hydrogen Sulfide in Single Living Cells. ACS Sens. 2022, 7, 893–899. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, X.; Wu, G.; Cheng, L. Controlled Self-Assembly of Metallacycle -Bridged Gold Nanoparticles for Surface-Enhanced Raman Scattering. Chem. Eur. J. 2020, 26, 11695–11700. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gong, K.; Liu, Y.; Zhang, L. Strategies and Challenges of Identifying Nanoplastics in Environment by Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2023, 57, 25–43. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, W.; Yang, X.; Liu, Q.; Cheng, L. Supramolecular metallacycle-assisted interfacial self-assembly: A promising method of fabricating gold nanoparticle monolayers with precise interparticle spacing for tunable SERS activity. Tetrahedron Lett. 2022, 94, 153716. [Google Scholar] [CrossRef]
- Morsby, J.J.; Thimes, R.L.; Olson, J.E.; McGarraugh, H.H.; Payne, J.N.; Camden, J.P.; Smith, B.D. Enzyme Sensing Using 2-Mercaptopyridine-Carbonitrile Reporters and Surface-Enhanced Raman Scattering. ACS Omega 2022, 7, 6419–6426. [Google Scholar] [CrossRef]
- Li, M.; Tian, S.; Meng, F.; Yin, M.; Yue, Q.; Wang, S.; Bu, W.; Luo, L. Continuously Multiplexed Ultrastrong Raman Probes by Precise Isotopic Polymer Backbone Doping for Multidimensional Information Storage and Encryption. Nano Lett. 2022, 22, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Plou, J.; Valera, P.S.; Garcia, I.; de Albuquerque, C.D.L.; Carracedo, A.; Liz-Marzan, L.M. Prospects of Surface-Enhanced Raman Spectroscopy for Biomarker Monitoring toward Precision Medicine. ACS Photonics 2022, 9, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Ye, J. Boosting the Brightness of Thiolated Surface-Enhanced Raman Scattering Nanoprobes by Maximal Utilization of the Three-Dimensional Volume of Electromagnetic Fields. J. Phy. Chem. Lett. 2022, 13, 6496–6502. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, H.; Hu, Z.; Fan, Y.; Guan, X.; Feng, W.; Liu, Z.; Sun, Y. Rigidity Bridging Flexibility to Harmonize Three Excited-State Deactivation Pathways for NIR-II-Fluorescent-Imaging-Guided Phototherapy. Adv. Healthc. Mater. 2021, 10, 2101003. [Google Scholar] [CrossRef]
- Yang, H.; Tu, L.; Li, J.; Bai, S.; Hu, Z.; Yin, P.; Lin, H.; Yu, Q.; Zhu, H.; Sun, Y. Deep and precise lighting-up/combat diseases through sonodynamic agents integratiing molecular imaging and therapy modalities. Corrdin. Chem. Rev. 2022, 453, 214333. [Google Scholar]
- Sun, Y.; Ding, F.; Chen, Z.; Zhang, R.; Li, C.; Xu, Y.; Zhang, Y.; Ni, R.; Li, X.; Yang, G.; et al. Melanin-dot-mediat4ed delivery of metallacycle for NIR-II/photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. Proc. Nat. Acad. Sci. USA 2019, 116, 16729–16735. [Google Scholar] [CrossRef] [PubMed]
- Tuo, W.; Xu, Y.; Fan, Y.; Li, J.; Qiu, M.; Xiong, X.; Li, X.; Sun, Y. Biomedical applications of Pt(II) metallacycle/metallacage-based agents From mono-chemotherapy to versatile imaging contrasts and theranostic platforms. Coordin. Chem. Rev. 2021, 443, 214017. [Google Scholar] [CrossRef]
- Li, Q.; Ge, X.; Ye, J.; Li, Z.; Su, L.; Wu, Y.; Yang, H.; Song, J. Dual Ratiometric SERS and Photoacoustic Core-Satelite Nanoprobe for Quantitatively Visualizing Hydrogen Peroxide in Inflammation and Cancer. Angew. Chem. Int. Ed. 2021, 60, 7323–7332. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.; Tu, L.; Choi, M.; Fan, Y.; Chen, X.; Sessler, J.L.; Kim, J.S.; Sun, Y. Rationally designed Ru(II)-metallacycle chemo-phototheranostic that emits beyond 1000 nm. Chem. Sci. 2022, 13, 6541–6549. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; An, J.; Ma, X.; Yang, J.; Luo, L.; Deng, Y.; Kim, J.S.; Sun, Y. Construction of a 980 nm laser-activated Pt(II) metallacycle nanosystem for efficient and safe photo-induced bacteria sterilization. Sci. China Chem. 2023, 66, 155–163. [Google Scholar] [CrossRef]
- Xu, Y.; Tuo, W.; Yang, L.; Sun, Y.; Li, C.; Chen, X.; Yang, W.; Yang, G.; Stang, P.J.; Sun, Y. Design of a Metallacycle-Based Supramolecular Photosensitizer for In Vivo Image-Guided Photodynamic Inactivation of Bacteria. Angew. Chem. Int. Ed. 2022, 61, e202110048. [Google Scholar]
- Tu, L.; Li, C.; Liu, C.; Bai, S.; Yang, J.; Zhang, X.; Xu, L.; Xiong, X.; Sun, Y. Rationally designed Ru(II) metallacycles with tunable imidazole ligands for synergistical chemo-phototherapy of cancer. Chem. Commun. 2022, 58, 9068–9071. [Google Scholar] [CrossRef]
- Henry, A.; Sharma, B.; Cardinal, M.F.; Kurouski, D.; van Duyne, R.P. Surface-Enhanced Raman Spectroscopy Biosensing: In Vivo Diagnosics and Multimodal Imaging. Anal. Chem. 2016, 88, 6638–6647. [Google Scholar] [CrossRef]
- Chen, Z.; Mas, J.; Forbes, L.H.; Andrews, M.R.; Dholakia, K. Depth-resolved multimodal imaging: Wavelength modulated spatially offset Raman spectroscopy with optical coherence tomography. J. Biophotonics 2018, 11, e201700129. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zhang, Y.; Guan, X.; Mei, L.; Feng, H.; Li, J.; Tu, L.; Feng, G.; Deng, G.; et al. Construction of Long-Wavelength Emissive Organic Nanosonosensitizer Targeting Mitochondria for Precise and Efficient In Vivo Sonotherapy. Adv. Funct. Mater. 2022, 32, 2207259. [Google Scholar] [CrossRef]
- Fan, Y.; Li, C.; Bai, S.; Ma, X.; Yang, J.; Guan, X.; Sun, Y. NIR-II Emissive Ru(II) Metallacycle Aaaisting Fluorescence Imaging and Cancer Therapy. Small 2022, 18, 2201625. [Google Scholar] [CrossRef]
- Li, C.; Guan, X.; Zhang, X.; Zhou, D.; Son, S.; Xu, Y.; Deng, M.; Guo, Z.; Sun, Y.; Kim, J.S. NIR-II bioimaging of small molecule fluorophores: From basic research to clinical applications. Biosen. Bioelec. 2022, 216, 114620. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, Y.; Zhu, S.; Ye, J.; Lin, L. Resonant Strategy in Designing NIR-II SERS Nanotages: A Quantitative Study. J. Phys. Chem. C. 2022, 126, 12575–12581. [Google Scholar] [CrossRef]
- Merone, G.M.; Tartaglia, A.; Locatelli, M.; D’Ovidio, C.; Rosato, E.; de Grazia, U.; Santavenere, F.; Rossi, S.; Savini, F. Analytical chemistry in the 21st century: Challenges, solutions, and future perspectives of complex matrices quantitative analyses in biological/clinical field. Analytica 2020, 1, 44–59. [Google Scholar] [CrossRef]
- Wen, C.; Wang, L.; Liu, L.; Shen, X.; Chen, H. Surface-enhanced Raman Probes Based on Gold Nanomaterials for in vivo Diagnosis and Imaging. Chem. Asian J. 2022, 17, e202200014. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Pan, Y.; Wang, S.; Wang, X.; Zhao, X.; Ren, W.; Lu, G.; Wu, A. Raman Reporter-Coupled Agcore@Aushell Nanostars for in Vivo Improved Surface Enhanced Raman Scattering Imaging and Near-infrared-Triggered Photothermal Therapy in Breast Cancers. ACS Appl. Mater. Interfaces 2015, 7, 16781–16791. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).