SnO2 Nanowire/MoS2 Nanosheet Composite Gas Sensor in Self-Heating Mode for Selective and ppb-Level Detection of NO2 Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
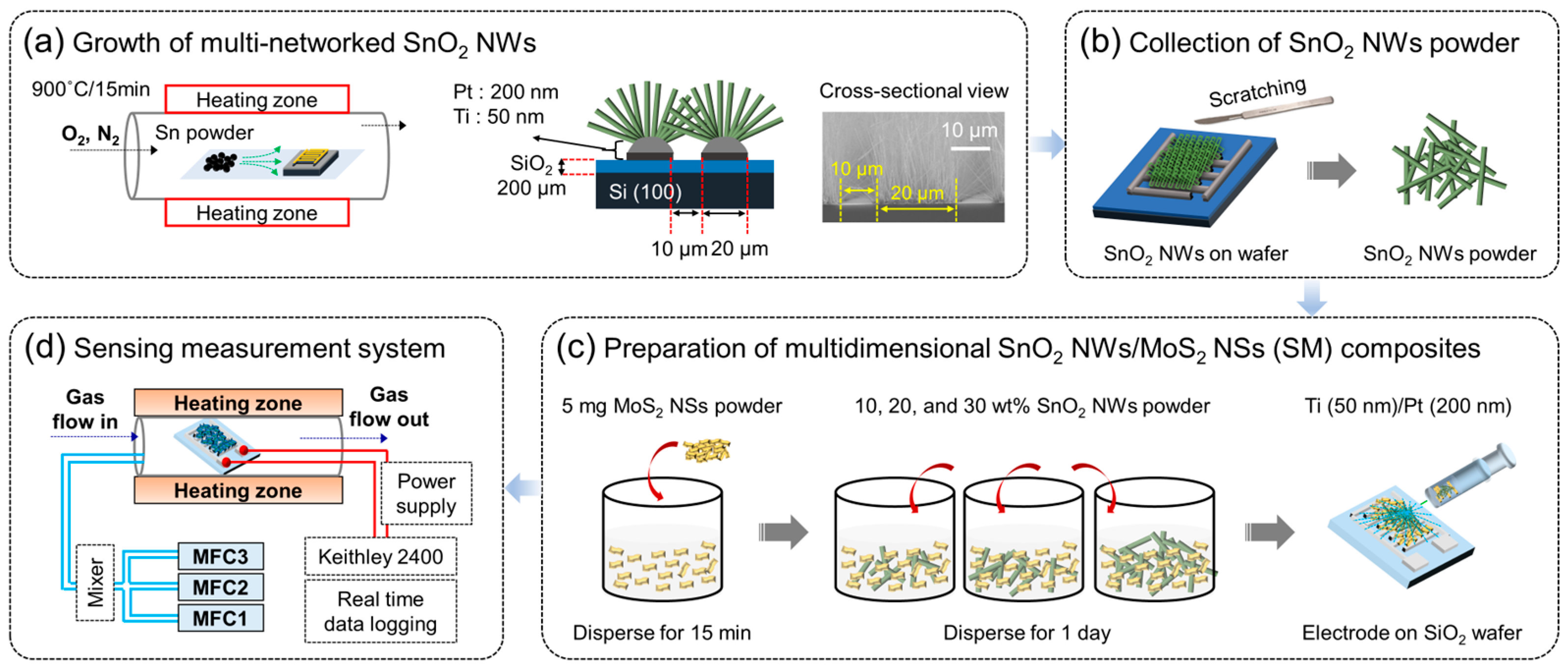
2.2. Synthesis of SnO2 NWs and SnO2 NWs/MoS2 NS Composite
2.3. Characterizations
2.4. Gas Sensing Tests
3. Results and Discussion
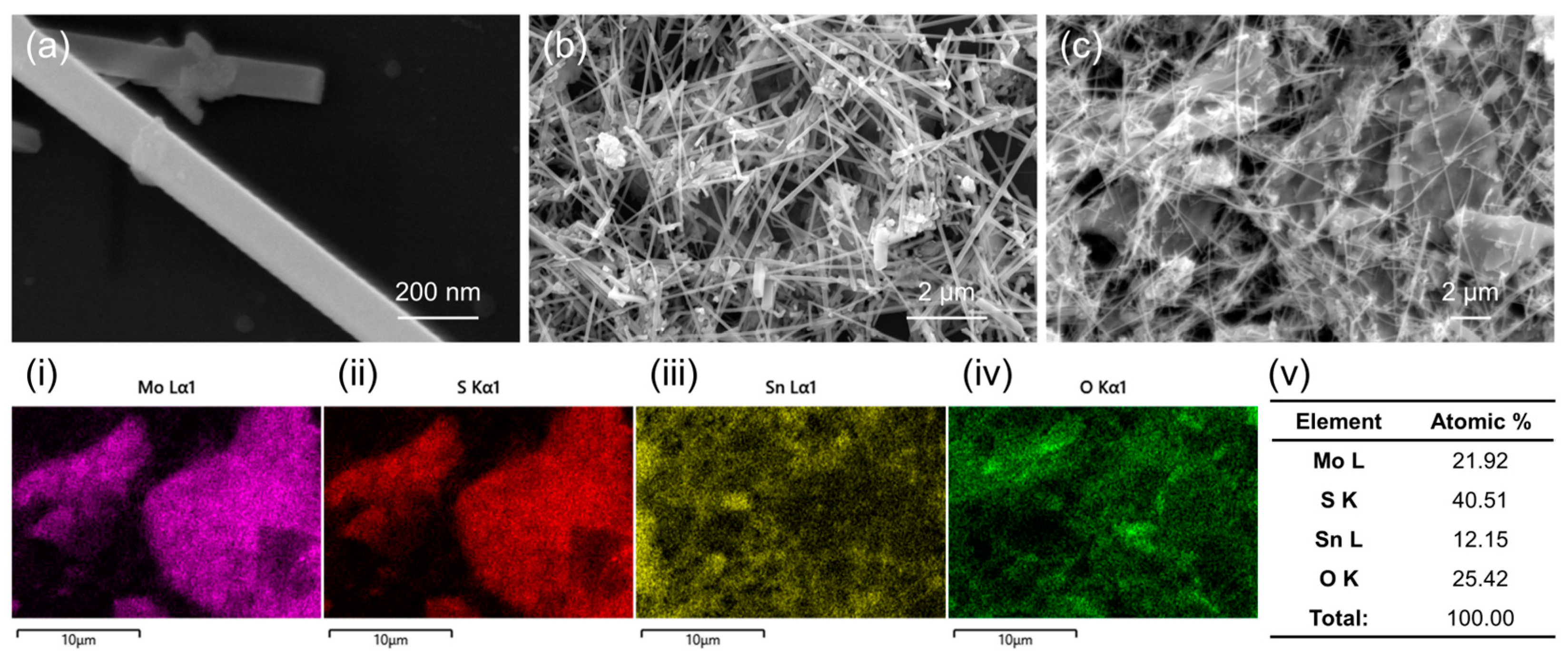
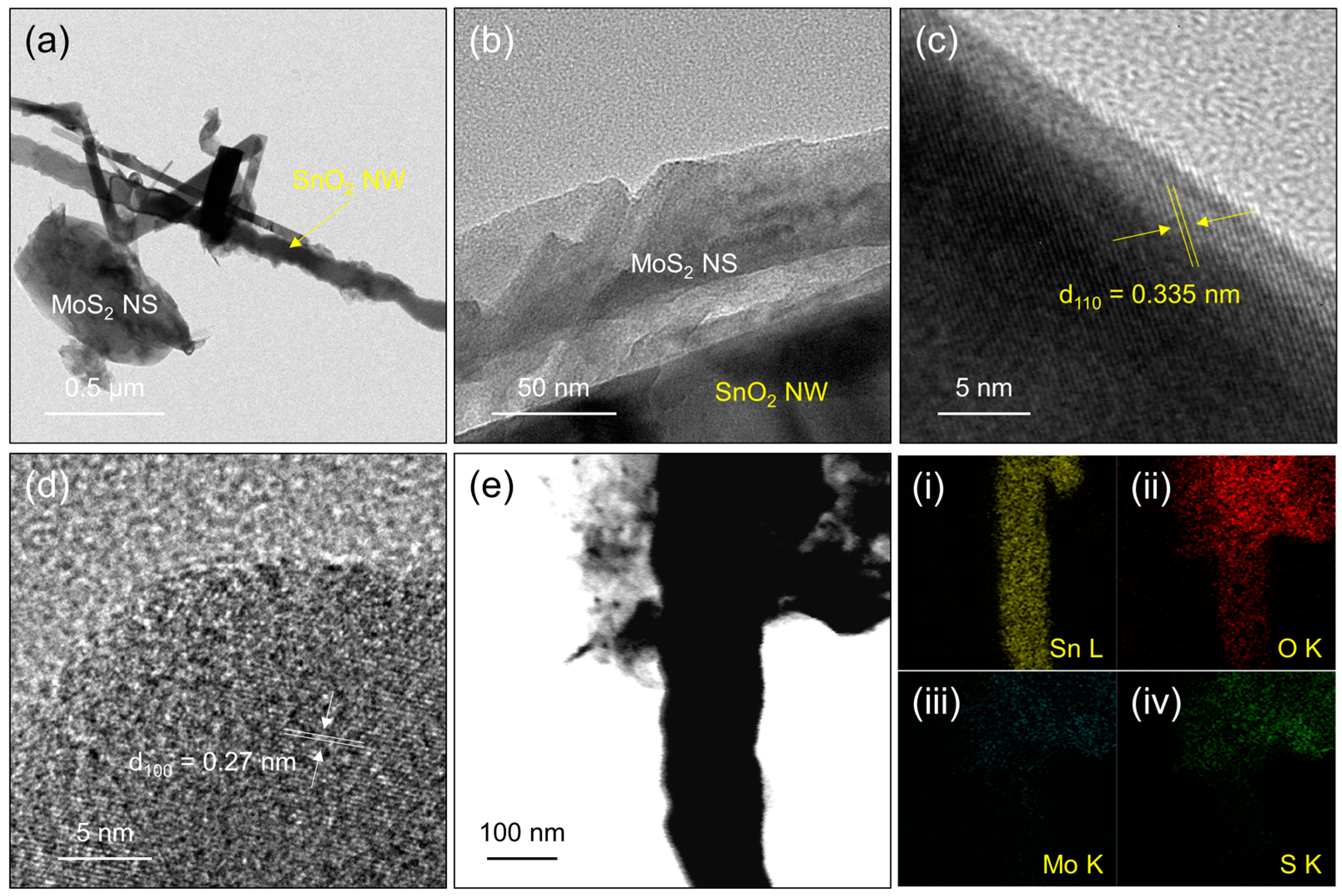
3.1. Characterization Studies
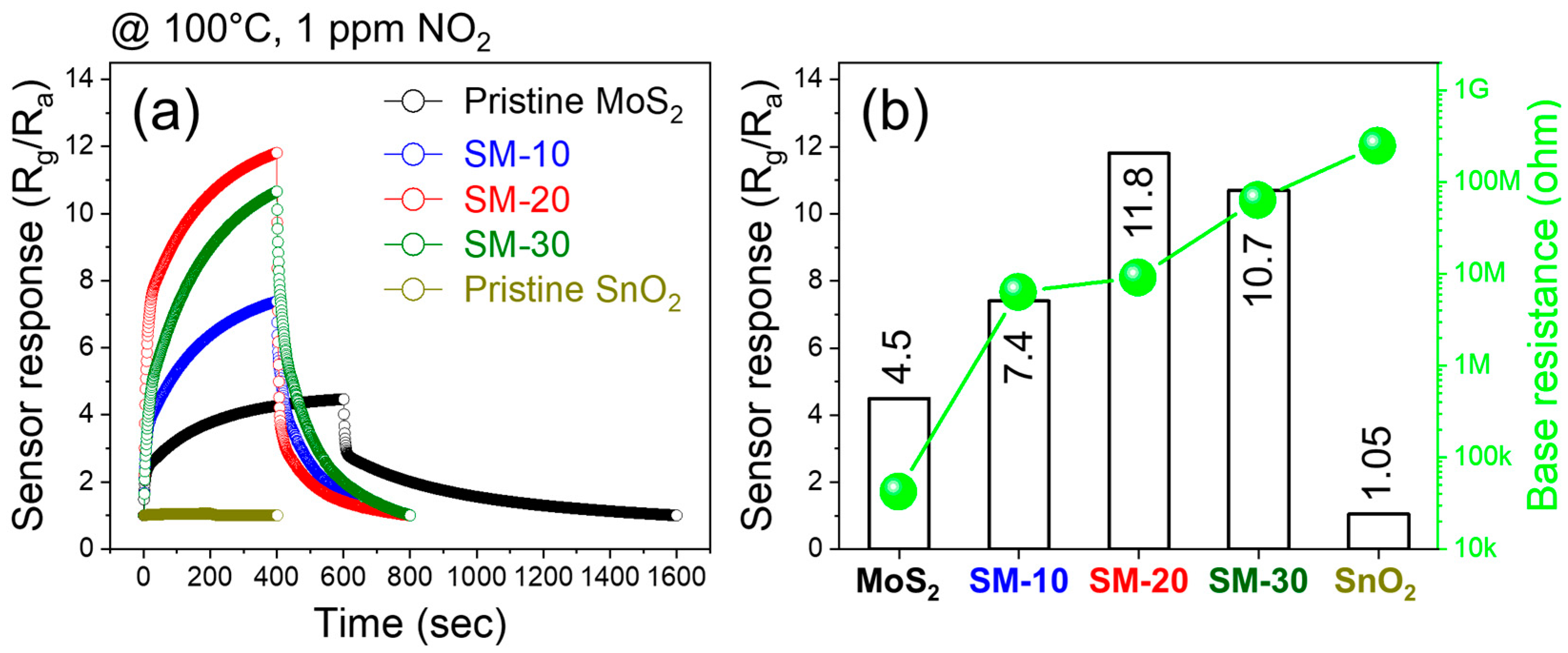
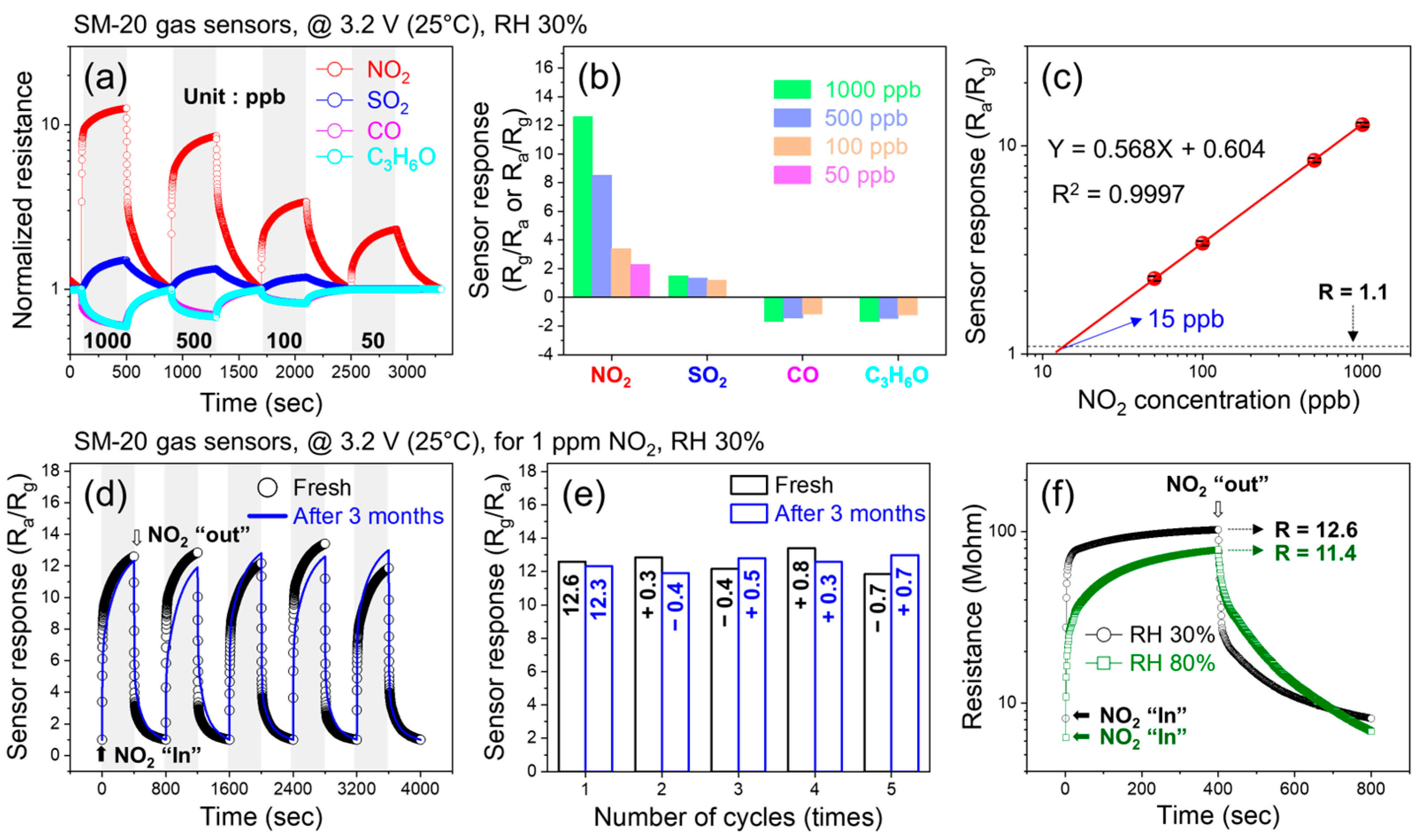
3.2. Gas Sensing Studies
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Zhang, L.; Zhang, C.; Chen, Z.; Li, J. Impact of NO2 Emissions from Household Heating Systems with Wall-Mounted Gas Stoves on Indoor and Ambient Air Quality in Chinese Urban Areas. Sci. Total Environ. 2024, 908, 168075. [Google Scholar] [CrossRef]
- Jion, M.M.M.F.; Jannat, J.N.; Mia, M.Y.; Ali, M.A.; Islam, M.S.; Ibrahim, S.M.; Pal, S.C.; Islam, A.; Sarker, A.; Malafaia, G.; et al. A Critical Review and Prospect of NO2 and SO2 Pollution over Asia: Hotspots, Trends, and Sources. Sci. Total Environ. 2023, 876, 162851. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Feng, C.; Guo, H.; Li, X.; Liu, W.; Feng, Y.; Liu, K.; Chen, D.; Zheng, Y.; Guo, F. UV-Activated CuO Nanospheres Modified with rGO Nanosheets for Ppb-Level Detection of NO2 Gas at Room Temperature. Sens. Actuators B Chem. 2023, 393, 134195. [Google Scholar] [CrossRef]
- Peng, H.; Yang, J.; Lin, C.; Qi, L.; Li, L.; Shi, K. Gas-Sensitive Performance of Metal-Organic Framework-Derived CuO NPs/Ti3C2TX MXene Heterostructures for Efficient NO2 Detection at Room Temperature. J. Alloys Compd. 2024, 980, 173657. [Google Scholar] [CrossRef]
- Gao, J.; Yin, Y.; Guo, Y.; Jia, L.; Xia, F.; Liu, C.; Hou, M.; Wang, F. Synthesis of Ti3C2Tx Nanosheets / ZnO Nanowires Composite Material for NO2 Gas Sensing. Arab. J. Chem. 2024, 17, 105776. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, H.; Zhang, Y.; Han, D.; Cheng, Y.; Jian, A.; Sang, S. ZnO/GaN n-n Heterojunction Porous Nanosheets for Ppb-Level NO2 Gas Sensors. Sens. Actuators B Chem. 2023, 396, 134629. [Google Scholar] [CrossRef]
- Brophy, R.E.; Junker, B.; Fakhri, E.A.; Árnason, H.Ö.; Svavarsson, H.G.; Weimar, U.; Bârsan, N.; Manolescu, A. Ultra Responsive NO2 Silicon Nanowires Gas Sensor. Sens. Actuators B Chem. 2024, 410, 135648. [Google Scholar] [CrossRef]
- Shin, K.Y.; Mirzaei, A.; Oum, W.; Kim, E.B.; Kim, H.M.; Moon, S.; Kim, S.S.; Kim, H.W. Enhanced NO2 Gas Response of ZnO–Ti3C2Tx MXene Nanocomposites by Microwave Irradiation. Sens. Actuators B Chem. 2024, 409, 135605. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Gorban, Y.M.; Averin, A.A.; Gorobtsov, P.Y.; Simonenko, N.P.; Lebedinskii, Y.Y.; Simonenko, E.P.; Kuznetsov, N.T. Obtaining of ZnO/Fe2O3 Thin Nanostructured Films by AACVD for Detection of Ppb-Concentrations of NO2 as a Biomarker of Lung Infections. Biosensors 2023, 13, 445. [Google Scholar] [CrossRef]
- Qin, H.; Xie, J.; Xu, H.; Li, Y.; Cao, Y. Green Solid-State Chemical Synthesis and Excellent Xylene-Detecting Behaviors of Y-Doped α-MoO3 Nanoarrays. Mater. Res. Bull. 2017, 93, 256–263. [Google Scholar] [CrossRef]
- Hussain, S.; Amu-Darko, J.N.O.; Wang, M.; Alothman, A.A.; Ouladsmane, M.; Aldossari, S.A.; Khan, M.S.; Qiao, G.; Liu, G. CuO-Decorated MOF Derived ZnO Polyhedral Nanostructures for Exceptional H2S Gas Detection. Chemosphere 2023, 317, 137827. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Mohan, J.; Lakshmy, S.; Thomas, S.; Chakraborty, B.; Thomas, S.; Kalarikkal, N. A Review of the Synthesis, Properties, and Applications of 2D Transition Metal Dichalcogenides and Their Heterostructures. Mater. Chem. Phys. 2023, 297, 127332. [Google Scholar] [CrossRef]
- Krishna, S.; Choi, S.H.; Kim, S.M.; Kim, K.K. Sapphire Substrates for Large-Area 2D Transition Metal Dichalcogenides Synthesis: A Brief Review. Curr. Appl. Phys. 2024, 59, 208–213. [Google Scholar] [CrossRef]
- Guan, L.; Chen, Z.; Liu, Y.; Wang, R.; Yan, K.; Xu, Z.; Li, J.; Liu, Z.; Li, J.; Liu, H. Engineering Sulfur-Rich MoS2 Adsorbent with Abundant Unsaturated Coordination Sulfur Sites for Gaseous Mercury Capture from High-Concentration SO2 Smelting Flue Gas. Chem. Eng. J. 2024, 483, 149122. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, K.; Qin, G.; Gao, B.; Zhang, T.; Huang, X.; Zhou, Y. Phase Engineering on MoS2 to Realize Dielectric Gene Engineering for Enhancing Microwave Absorbing Performance. Adv. Funct. Mater. 2024, 34, 2316338. [Google Scholar] [CrossRef]
- Yang, D.-H.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Co-Decoration of WS2 Nanosheets with Both Au and In2O3-Nanoparticles for Attaining the CO Sensing in Self-Heating Mode. Appl. Surf. Sci. 2023, 635, 157790. [Google Scholar] [CrossRef]
- Wang, T.; Liu, G.; Zhang, D.; Wang, D.; Chen, F.; Guo, J. Fabrication and Properties of Room Temperature Ammonia Gas Sensor Based on SnO2 Modified WSe2 Nanosheets Heterojunctions. Appl. Surf. Sci. 2022, 597, 153564. [Google Scholar] [CrossRef]
- Pramanik, M.; Jana, B.; Ghatak, A.; Das, K. Improvement in Efficiency of MoS2 Nanoflower Based Ethylene Gas Sensor on Transition Metal Doping: An Experimental and Theoretical Investigation. Mater. Chem. Phys. 2024, 314, 128892. [Google Scholar] [CrossRef]
- Kodan, S.; Kumar, A.; Sanger, A.; Arora, A.; Malik, V.K.; Chandra, R. Vertically Aligned MoSe2-WS2 Nanoworms Heterojunction towards Room Temperature NO2 Gas Sensors. Sens. Actuators B Chem. 2024, 407, 135481. [Google Scholar] [CrossRef]
- Kumar, S.; Mirzaei, A.; Kumar, A.; Lee, M.H.; Ghahremani, Z.; Kim, T.-U.; Kim, J.-Y.; Kwoka, M.; Kumar, M.; Kim, S.S.; et al. Nanoparticles Anchored Strategy to Develop 2D MoS2 and MoSe2 Based Room Temperature Chemiresistive Gas Sensors. Coord. Chem. Rev. 2024, 503, 215657. [Google Scholar] [CrossRef]
- Chen, P.; Hu, J.; Yin, M.; Bai, W.; Chen, X.; Zhang, Y. MoS2 Nanoflowers Decorated with Au Nanoparticles for Visible-Light-Enhanced Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 5981–5991. [Google Scholar] [CrossRef]
- Burman, D.; Raha, H.; Manna, B.; Pramanik, P.; Guha, P.K. Substitutional Doping of MoS2 for Superior Gas-Sensing Applications: A Proof of Concept. ACS Sens. 2021, 6, 3398–3408. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Vasseghian, Y.; Doan, V.D.; Nguyen, T.T.T.; Vo, T.-T.T.; Do, H.H.; Vu, K.B.; Vu, Q.H.; Lam, T.D.; Tran, V.A. Flexible and High-Sensitivity Sensor Based on Ti3C2–MoS2 MXene Composite for the Detection of Toxic Gases. Chemosphere 2022, 291, 133025. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sattigeri, R.M.; Kumar, S.; Jha, P.K.; Sharma, S. Superior Room-Temperature Ammonia Sensing Using a Hydrothermally Synthesized MoS2/SnO2 Composite. ACS Omega 2021, 6, 11602–11613. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale Metal Oxide-Based Heterojunctions for Gas Sensing: A Review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Hassun, H.K.; Hussein, B.H.; Salman, E.M.T.; Shaban, A.H. Photoelectric Properties of SnO2: Ag/P–Si Heterojunction Photodetector. Energy Rep. 2020, 6, 46–54. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide Semiconductor Gas Sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T.; Yao, L.; Xiao, X.; Wang, Y. SnO2 Nanostructured Materials Used as Gas Sensors for the Detection of Hazardous and Flammable Gases: A Review. Nano Mater. Sci. 2022, 4, 339–350. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultra-Fast Response and Highly Selectivity Hydrogen Gas Sensor Based on Pd/SnO2 Nanoparticles. Int. J. Hydrogen Energy 2022, 47, 3157–3169. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, M.S.; Jung, H.; Choe, Y.-S.; Kim, W.; Song, Y.G.; Kang, C.-Y.; Lee, H.-S.; Lee, W. Selective C2H2 Detection with High Sensitivity Using SnO2 Nanorod Based Gas Sensors Integrated with a Gas Chromatography. Sens. Actuators B Chem. 2020, 307, 127598. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, J. Highly Sensitive Ethanol Gas Sensors Based on Co-Doped SnO2 Nanobelts and Pure SnO2 Nanobelts. Phys. E Low-Dimens. Syst. Nanostructures 2023, 147, 115604. [Google Scholar] [CrossRef]
- Su, P.; Li, W.; Zhang, J.; Xie, X. Chemiresistive Gas Sensor Based on Electrospun Hollow SnO2 Nanotubes for Detecting NO at the Ppb Level. Vacuum 2022, 199, 110961. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Hung, C.M.; Toan, N.V.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. One-Step Fabrication of SnO2 Porous Nanofiber Gas Sensors for Sub-Ppm H2S Detection. Sens. Actuators Phys. 2020, 303, 111722. [Google Scholar] [CrossRef]
- Domènech-Gil, G.; Samà, J.; Fàbrega, C.; Gràcia, I.; Cané, C.; Barth, S.; Romano-Rodríguez, A. Highly Sensitive SnO2 Nanowire Network Gas Sensors. Sens. Actuators B Chem. 2023, 383, 133545. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Yuan, Z.; Liu, B.; Zhao, Q.; Huang, Q.; Li, Z.; Zeng, W.; Duan, Z.; Tai, H. Synergistic Effect of Electron Scattering and Space Charge Transfer Enabled Unprecedented Room Temperature NO2 Sensing Response of SnO2. Small 2023, 19, 2303631. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, W.; Li, X.; Liu, Y.; Liang, Y.; Chen, B.; Yang, Y.; Luo, X.; Xu, K.; Yuan, C. Light-Assisted Room Temperature Gas Sensing Performance and Mechanism of Direct Z-Scheme MoS2/SnO2 Crystal Faceted Heterojunctions. J. Hazard. Mater. 2022, 436, 129246. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Song, P.; Zhang, S.; Yang, Z.; Wang, Q. Dispersed SnO2 Nanoparticles on MoS2 Nanosheets for Superior Gas-Sensing Performances to Ethanol. RSC Adv. 2015, 5, 79593–79599. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Hu, K.; Li, Y.; Zeng, W.; Zeng, L. Hierarchical Composites of MoS2 Nanoflower Anchored on SnO2 Nanofiber for Methane Sensing. Ceram. Int. 2019, 45, 22981–22986. [Google Scholar] [CrossRef]
- Bai, J.; Shen, Y.; Zhao, S.; Chen, Y.; Li, G.; Han, C.; Wei, D.; Yuan, Z.; Meng, F. Flower-like MoS2 Hierarchical Architectures Assembled by 2D Nanosheets Sensitized with SnO2 Quantum Dots for High-Performance NH3 Sensing at Room Temperature. Sens. Actuators B Chem. 2022, 353, 131191. [Google Scholar] [CrossRef]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.; Wang, J.; Sun, B.; Zhang, Y.; Wang, R.; Shi, K. Thin-Layered MoS2 Nanoflakes Vertically Grown on SnO2 Nanotubes as Highly Effective Room-Temperature NO2 Gas Sensor. J. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C.; et al. The Gas Sensor Utilizing Polyaniline/MoS2 Nanosheets/SnO2 Nanotubes for the Room Temperature Detection of Ammonia. Sens. Actuators B Chem. 2021, 332, 129444. [Google Scholar] [CrossRef]
- Wang, W.; Zhen, Y.; Zhang, J.; Li, Y.; Zhong, H.; Jia, Z.; Xiong, Y.; Xue, Q.; Yan, Y.; Alharbi, N.S.; et al. SnO2 Nanoparticles-Modified 3D-Multilayer MoS2 Nanosheets for Ammonia Gas Sensing at Room Temperature. Sens. Actuators B Chem. 2020, 321, 128471. [Google Scholar] [CrossRef]
- Viet, N.N.; Thong, L.V.; Dang, T.K.; Phuoc, P.H.; Chien, N.H.; Hung, C.M.; Hoa, N.D.; Duy, N.V.; Toan, N.V.; Son, N.T.; et al. MoS2 Nanosheets-Decorated SnO2 Nanofibers for Enhanced SO2 Gas Sensing Performance and Classification of CO, NH3 and H2 Gases. Anal. Chim. Acta 2021, 1167, 338576. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Chen, Y.; Wang, S.; Wang, X.; Jiang, H.; Ma, S.; Yuan, F.; Zhang, Q. N-n Heterogeneous MoS2/SnO2 Nanotubes and The Excellent Triethylamine (TEA) Sensing Performances. Mater. Lett. 2022, 311, 131522. [Google Scholar] [CrossRef]
- Han, Y.; Ma, Y.; Liu, Y.; Xu, S.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Construction of MoS2/SnO2 Heterostructures for Sensitive NO2 Detection at Room Temperature. Appl. Surf. Sci. 2019, 493, 613–619. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, M.H.; Pawar, K.K.; Bharath, S.P.; Kim, T.-U.; Kim, J.-Y.; Kim, S.S.; Kim, H.W. Metal Oxide Nanowires Grown by a Vapor–Liquid–Solid Growth Mechanism for Resistive Gas-Sensing Applications: An Overview. Materials 2023, 16, 6233. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, S.S.; Park, C. Pt Nanoparticle Decoration on Femtosecond Laser-Irradiated SnO2 Nanowires for Enhancing C7H8 Gas Sensing. Sens. Actuators B Chem. 2023, 379, 133279. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Li, J.; Duan, Z.; Yang, Y.; Yuan, Z.; Jiang, Y.; Tai, H. Pd-Decorated ZnO Hexagonal Microdiscs for NH3 Sensor. Chemosensors 2024, 12, 43. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Li, Q. Controlled Fabrication of SnO2 Arrays of Well-Aligned Nanotubes and Nanowires. Nanoscale 2010, 2, 2104–2108. [Google Scholar] [CrossRef]
- Mao, H.; Fu, Y.; Yang, H.; Deng, Z.; Sun, Y.; Liu, D.; Wu, Q.; Ma, T.; Song, X.-M. Ultrathin 1T-MoS2 Nanoplates Induced by Quaternary Ammonium-Type Ionic Liquids on Polypyrrole/Graphene Oxide Nanosheets and Its Irreversible Crystal Phase Transition during Electrocatalytic Nitrogen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 25189–25199. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, Z.; Yang, Y.; Tang, T.; Cheng, Y.F.; Xu, K.; Xie, H.G.; Chen, Y.L.; Cheng, L.; Tao, X.W.; et al. 3D Substoichiometric MoO3−x/EGaln Framework for Room Temperature NH3 Gas Sensing. J. Alloys Compd. 2023, 939, 168690. [Google Scholar] [CrossRef]
- Hingangavkar, G.M.; Kadam, S.A.; Ma, Y.-R.; Bandgar, S.S.; Mulik, R.N.; Patil, V.B. MoS2-GO Hybrid Sensor: A Discerning Approach for Detecting Harmful H2S Gas at Room Temperature. Chem. Eng. J. 2023, 472, 144789. [Google Scholar] [CrossRef]
- Rodrigues, J.; Jain, S.; Shimpi, N.G. Performance of 1D Tin (Sn) Decorated Spherical Shape ZnO Nanostructures as an Acetone Gas Sensor for Room and High Temperature. Mater. Sci. Eng. B 2023, 288, 116199. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of Zinc Oxide Semiconductors-Graphene Nanocomposites by Microwave Irradiation for Application to Gas Sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Ma, X.; Cai, X.; Yuan, M.; Qu, Y.; Tan, Y.; Chen, F. Self-Powered and Flexible Gas Sensor Using Defect-Engineered WS2/G Heterostructure. Sens. Actuators B Chem. 2022, 371, 132523. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, Y.; Chung, H.-S.; Kim, A.R.; Kwon, J.-D.; Park, J.; Kim, Y.L.; Kwon, S.-H.; Hahm, M.G.; Cho, B. Effect of Nb Doping on Chemical Sensing Performance of Two-Dimensional Layered MoSe2. ACS Appl. Mater. Interfaces 2017, 9, 3817–3823. [Google Scholar] [CrossRef]
- Guo, R.; Han, Y.; Su, C.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z. Ultrasensitive Room Temperature NO2 Sensors Based on Liquid Phase Exfoliated WSe2 Nanosheets. Sens. Actuators B Chem. 2019, 300, 127013. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhao, C.; Han, T.; Fei, T.; Liu, S.; Lu, G. Rational Synthesis of Molybdenum Disulfide Nanoparticles Decorated Reduced Graphene Oxide Hybrids and Their Application for High-Performance NO2 Sensing. Sens. Actuators B Chem. 2018, 260, 508–518. [Google Scholar] [CrossRef]
- Ko, K.Y.; Park, K.; Lee, S.; Kim, Y.; Woo, W.J.; Kim, D.; Song, J.-G.; Park, J.; Kim, H. Recovery Improvement for Large-Area Tungsten Diselenide Gas Sensors. ACS Appl. Mater. Interfaces 2018, 10, 23910–23917. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Umar, A.; Wang, Y.; Li, H.; Zhou, G. Three-Dimensional Crumpled Graphene-Based Nanosheets with Ultrahigh NO2 Gas Sensibility. ACS Appl. Mater. Interfaces 2017, 9, 11819–11827. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 Nanoparticles-Reduced Graphene Oxide Nanocomposites for NO2 Sensing at Low Operating Temperature. Sens. Actuators B Chem. 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Shen, T.; Sun, J. Flexible Resistive NO2 Gas Sensor of SnO2@Ti3C2Tx MXene for Room Temperature Application. Ceram. Int. 2024, 50, 2459–2466. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Song, Y.; Shen, T.; Sun, J. Facile Solvothermal Synthesis of ZnO/Ti3C2Tx MXene Nanocomposites for NO2 Detection at Low Working Temperature. Sens. Actuators B Chem. 2022, 367, 132025. [Google Scholar] [CrossRef]
- Yan, H.; Chu, L.; Li, Z.; Sun, C.; Shi, Y.; Ma, J. 2H-MoS2/Ti3C2Tx MXene Composites for Enhanced NO2 Gas Sensing Properties at Room Temperature. Sens. Actuators Rep. 2022, 4, 100103. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tri, N.N.; Noh, J.S. Improved NO2 Gas Sensing Performance of 2D MoS2/Ti3C2Tx MXene Nanocomposite. Appl. Surf. Sci. 2022, 604, 154624. [Google Scholar] [CrossRef]
- Guo, F.; Feng, C.; Zhang, Z.; Zhang, L.; Xu, C.; Zhang, C.; Lin, S.; Wu, H.; Zhang, B.; Tabusi, A.; et al. A Room-Temperature NO2 Sensor Based on Ti3C2TX MXene Modified with Sphere-Like CuO. Sens. Actuators B Chem. 2023, 375, 132885. [Google Scholar] [CrossRef]
- Premkumar, V.K.; Vishnuraj, R.; Sheena, T.S.; Yang, X.; Pullithadathil, B.; Zhang, C.; Wu, Z. Influence of ZnO Hexagonal Pyramid Nanostructures for Highly Sensitive and Selective NO2 Gas Sensor. J. Alloys Compd. 2024, 994, 174625. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low Power-Consumption CO Gas Sensors Based on Au-Functionalized SnO2-ZnO Core-Shell Nanowires. Sens. Actuators B Chem. 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Babar, B.M.; Pisal, K.B.; Mujawar, S.H.; Patil, V.L.; Kadam, L.D.; Pawar, U.T.; Kadam, P.M.; Patil, P.S. Concentration Modulated Vanadium Oxide Nanostructures for NO2 Gas Sensing. Sens. Actuators B Chem. 2022, 351, 130947. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. A Low Temperature Operated NO2 Gas Sensor Based on TeO2/SnO2 p–n Heterointerface. Sens. Actuators B Chem. 2013, 176, 875–883. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Kwon, Y.J.; Kim, S.S.; Kim, T.W.; Kim, H.W. Selective NO2 Sensor Based on Bi2O3 Branched SnO2 Nanowires. Sens. Actuators B Chem. 2018, 274, 356–369. [Google Scholar] [CrossRef]


| Sensing Material | T (°C) | Conc. (ppb) | Response (Rg/Ra) or (Ra/Rg) | τRes/τRec | Ref. |
|---|---|---|---|---|---|
| SnO2 NWs (20 wt%)-MoS2 NSs composite gas sensor | RT, (3.2 V) | 1000 | 12.6 | 268/63 s | This work |
| WS2/Graphene heterostructure | RT | 50 | 6.0 | 110/168 s | [55] |
| Nb-MoSe2 | 150 | 3000 | 8.0 | 30/30 min | [56] |
| WSe2 nanosheets | RT | 1000 | 8.21 | 50/1050 s | [57] |
| MoS2-rGO heterojunction | 160 | 3000 | 1.24 | 8/20 s | [58] |
| Trilayer WSe2 film | RT | 10,000 | 2.8 | 960/600 s | [59] |
| 3D crumpled reduced graphene oxide nanosheets | RT | 1000 | 1.5 | 500/3000 s | [60] |
| SnO2-rGO nanocomposites | 50 | 500 | 1.5 | 400/300 s | [61] |
| SnO2/Ti3C2Tx nanocomposite | RT | 300 | 78.2% [ΔR/Ra (%)] | 54/400 s | [62] |
| ZnO/Ti3C2Tx nanocomposite | 160 | 8 | 3.6 | 254/~380 | [63] |
| MoS2/MXene nanocomposite | RT | 100 | 65.6% [ΔR/Ra (%)] | ~700/~900 | [64] |
| MoS2/Ti3C2Tx nanocomposite | RT | 20 | 65.6% [ΔR/Ra (%)] | 525/155 | [65] |
| Ti3C2Tx/CuO nanocomposite | RT | 100 | 59% [ΔR/Ra (%)] | ~100/~100 | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Mirzaei, A.; Kim, J.-H. SnO2 Nanowire/MoS2 Nanosheet Composite Gas Sensor in Self-Heating Mode for Selective and ppb-Level Detection of NO2 Gas. Chemosensors 2024, 12, 107. https://doi.org/10.3390/chemosensors12060107
Kim J-Y, Mirzaei A, Kim J-H. SnO2 Nanowire/MoS2 Nanosheet Composite Gas Sensor in Self-Heating Mode for Selective and ppb-Level Detection of NO2 Gas. Chemosensors. 2024; 12(6):107. https://doi.org/10.3390/chemosensors12060107
Chicago/Turabian StyleKim, Jin-Young, Ali Mirzaei, and Jae-Hun Kim. 2024. "SnO2 Nanowire/MoS2 Nanosheet Composite Gas Sensor in Self-Heating Mode for Selective and ppb-Level Detection of NO2 Gas" Chemosensors 12, no. 6: 107. https://doi.org/10.3390/chemosensors12060107








