Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. PEDOT-PAH Films’ Preparation
2.3. SPR Measurements
2.4. Electrochemical Measurements
2.5. Urine Sample
3. Results and Discussions
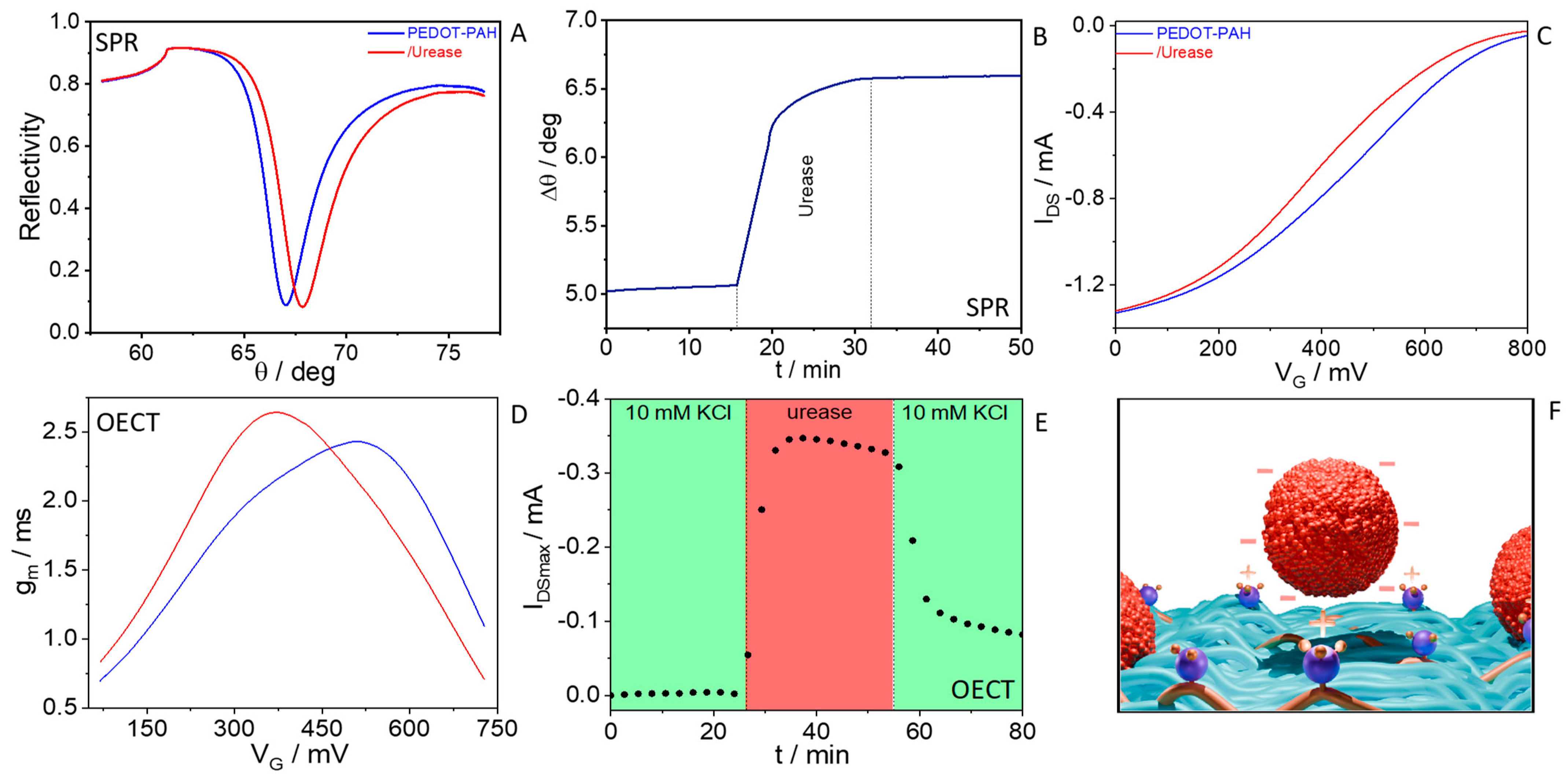
3.1. Urease Electrostatic Integration to PEDOT-PAH Films and Monitoring with OECTs
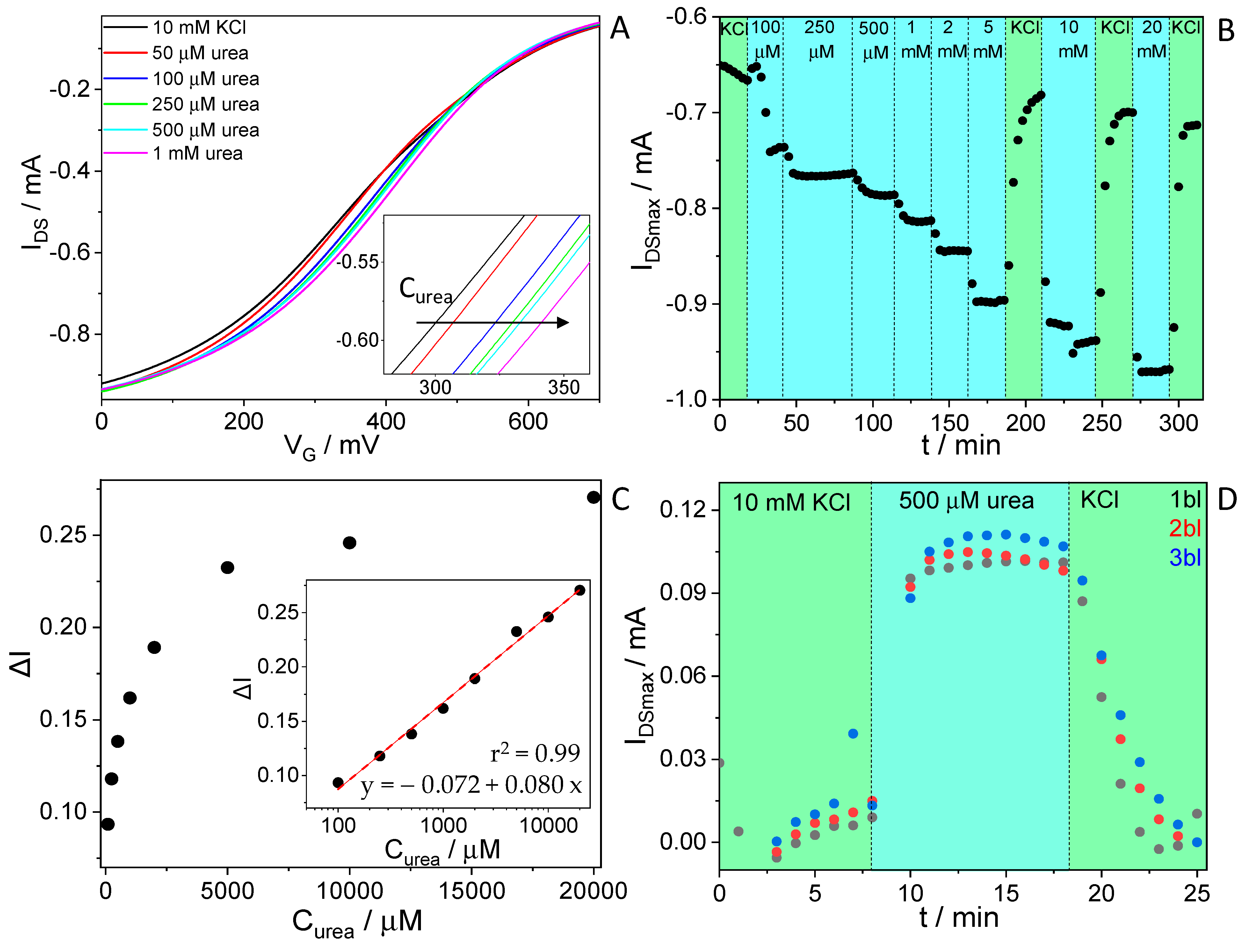
3.2. Urea Sensing with OECTs
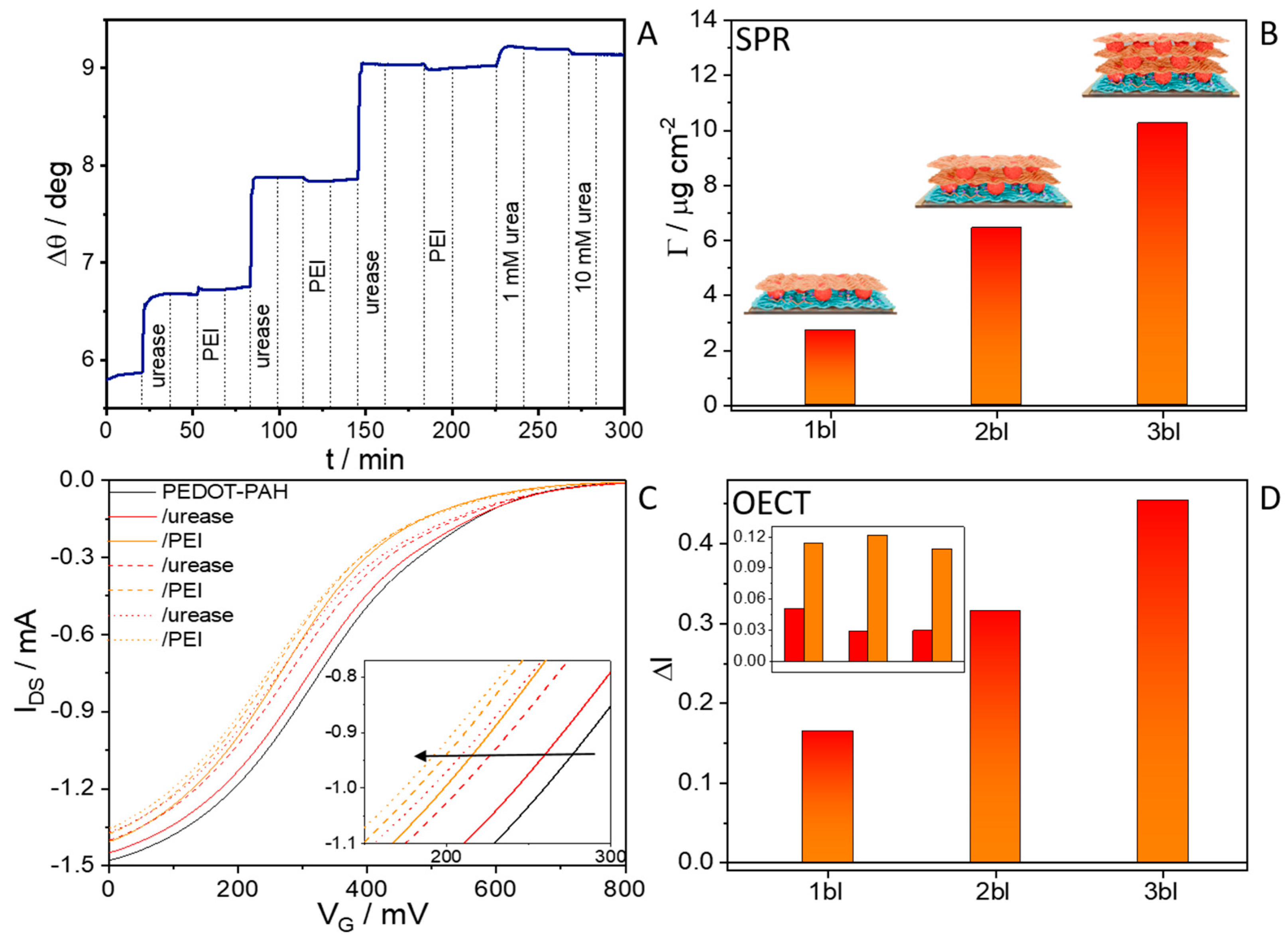
3.3. PEI Integration and Multilayer Assembly
3.4. Effect of PEI Integration on the Biosensor Stability and Effect of the Addition of Multiple Urease/PEI Bilayers on the Sensing Response
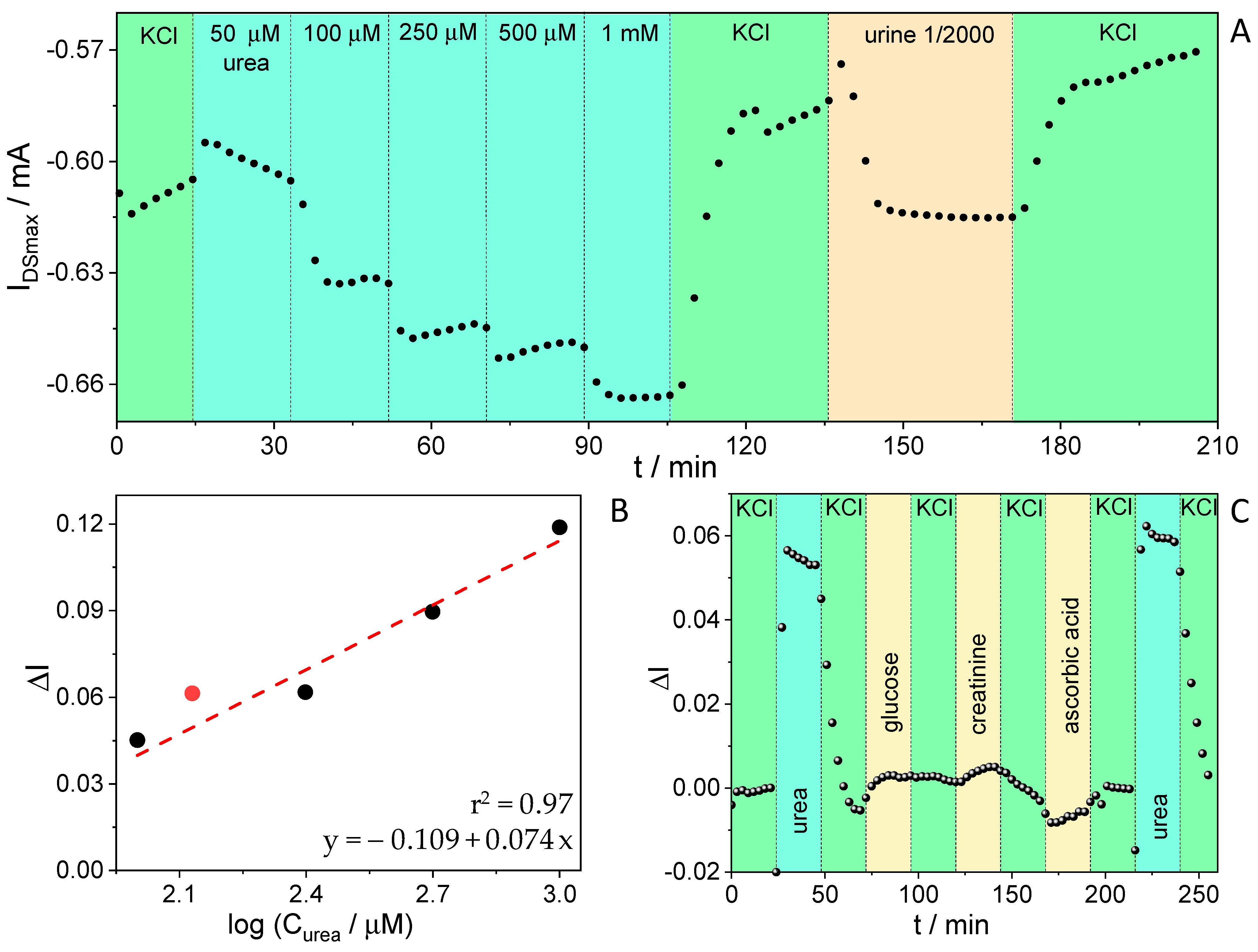
3.5. Urea Sensing from Real Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Marks, A.; Griggs, S.; Gasparini, N.; Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 2022, 9, 2102039. [Google Scholar] [CrossRef]
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized Organic Thin Film Transistors for Biosensing. Acc. Chem. Res. 2019, 52, 277–287. [Google Scholar] [CrossRef]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Druet, V.; Saleh, A.; Inal, S. Organic Bioelectronic Devices for Metabolite Sensing. Chem. Rev. 2022, 122, 4581–4635. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-State and Transient Behavior of Organic Electrochemical Transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Vurro, F.; Marchetti, E.; Bettelli, M.; Manfrini, L.; Finco, A.; Sportolaro, C.; Coppedè, N.; Palermo, N.; Tommasini, M.G.; Zappettini, A.; et al. Application of the OECT-Based In Vivo Biosensor Bioristor in Fruit Tree Monitoring to Improve Agricultural Sustainability. Chemosensors 2023, 11, 374. [Google Scholar] [CrossRef]
- Diacci, C.; Abedi, T.; Lee, J.W.; Gabrielsson, E.O.; Berggren, M.; Simon, D.T.; Niittylä, T.; Stavrinidou, E. Diurnal in vivo xylem sap glucose and sucrose monitoring using implantable organic electrochemical transistor sensors. iScience 2021, 24, 101966. [Google Scholar] [CrossRef]
- Tseng, H.; Chen, Y.; Zhang, P.; Hsiao, Y. Additive Blending Effects on PEDOT:PSS Composite Films for Wearable Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2024, 16, 13384–13398. [Google Scholar] [CrossRef]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable Sensor System for Detection of Lactate in Sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Wang, Y.; Zhang, Y.; Ding, X.; Zhong, W.; Wang, D.; Wang, W.; Liu, Q.; Liu, K.; Li, M.; et al. Wearable Fiber-Based Organic Electrochemical Transistors as a Platform for Highly Sensitive Dopamine Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 13105–13113. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.G.; Lo Fiego, A.; Patrick, I.; Creamer, A.; Stevens, M.M. Organic Bioelectronics: Using Highly Conjugated Polymers to Interface with Biomolecules, Cells, and Tissues in the Human Body. Adv. Mater. Technol. 2020, 5, 2000384. [Google Scholar] [CrossRef]
- Kim, C.-H.; Azimi, M.; Fan, J.; Nagarajan, H.; Wang, M.; Cicoira, F. All-printed and stretchable organic electrochemical transistors using a hydrogel electrolyte. Nanoscale 2023, 15, 3263–3272. [Google Scholar] [CrossRef]
- Wang, L.; Yue, X.; Sun, Q.; Zhang, L.; Ren, G.; Lu, G.; Yu, H.-D.; Huang, W. Flexible organic electrochemical transistors for chemical and biological sensing. Nano Res. 2022, 15, 2433–2464. [Google Scholar] [CrossRef]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 2020, 19, 13–26. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Marks, A.; Luscombe, C.K. Molecular Design Strategies toward Improvement of Charge Injection and Ionic Conduction in Organic Mixed Ionic-Electronic Conductors for Organic Electrochemical Transistors. Chem. Rev. 2022, 122, 4325–4355. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, G.E.; Azzaroni, O.; Knoll, W.; Marmisollé, W.A. Functionalization Strategies of PEDOT and PEDOT:PSS Films for Organic Bioelectronics Applications. Chemosensors 2021, 9, 212. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [PubMed]
- Bidinger, S.L.; Han, S.; Malliaras, G.G.; Hasan, T. Highly stable PEDOT:PSS electrochemical transistors. Appl. Phys. Lett. 2022, 120, 073302. [Google Scholar] [CrossRef]
- Carli, S.; Bianchi, M.; Di Lauro, M.; Prato, M.; Toma, A.; Leoncini, M.; De Salvo, A.; Murgia, M.; Fadiga, L.; Biscarini, F. Multifunctionally-doped PEDOT for organic electrochemical transistors. Front. Mater. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Colucci, R.; Feitosa, B.d.A.; Faria, G.C. Impact of Ionic Species on the Performance of Pedot:PSS-Based Organic Electrochemical Transistors. Adv. Electron. Mater. 2023, 10, 2300235. [Google Scholar] [CrossRef]
- Sappia, L.D.; Pascual, B.S.; Azzaroni, O.; Marmisollé, W. PEDOT-Based Stackable Paper Electrodes for Metal-Free Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 9283–9293. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Scotto, J.; Allegretto, J.A.; Piccinini, E.; Cantillo, A.L.; Knoll, W.; Azzaroni, O.; Marmisollé, W.A. Layer-by-Layer Assembly Monitored by PEDOT-Polyamine-Based Organic Electrochemical Transistors. ACS Appl. Electron. Mater. 2022, 4, 5953–5962. [Google Scholar] [CrossRef]
- Montero-Jimenez, M.; Amante, F.L.; Fenoy, G.E.; Scotto, J.; Azzaroni, O.; Marmisolle, W.A. PEDOT-Polyamine-Based Organic Electrochemical Transistors for Monitoring Protein Binding. Biosensors 2023, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, G.E.; von Bilderling, C.; Knoll, W.; Azzaroni, O.; Marmisollé, W.A. PEDOT:Tosylate-Polyamine-Based Organic Electrochemical Transistors for High-Performance Bioelectronics. Adv. Electron. Mater. 2021, 7, 2100059. [Google Scholar] [CrossRef]
- Sappia, L.D.; Piccinini, E.; von Binderling, C.; Knoll, W.; Marmisollé, W.; Azzaroni, O. PEDOT-polyamine composite films for bioelectrochemical platforms—Flexible and easy to derivatize. Mater. Sci. Eng. C 2020, 109, 110575. [Google Scholar] [CrossRef] [PubMed]
- Sappia, L.D.; Piccinini, E.; Marmisollé, W.; Santilli, N.; Maza, E.; Moya, S.; Battaglini, F.; Madrid, R.E.; Azzaroni, O. Integration of Biorecognition Elements on PEDOT Platforms through Supramolecular Interactions. Adv. Mater. Interfaces 2017, 4, 1700502. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible Organic Electrochemical Transistors for Highly Selective Enzyme Biosensors and Used for Saliva Testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, N.; Yang, A.; Law, H.K.; Li, L.; Yan, F. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef]
- Tang, H.; Yan, F.; Lin, P.; Xu, J.; Chan, H.L.W. Highly Sensitive Glucose Biosensors Based on Organic Electrochemical Transistors Using Platinum Gate Electrodes Modified with Enzyme and Nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Huang, C.-H.; Parvez, K.; Worsley, R.; Mattana, G.; Piro, B.; Noël, V.; Casiraghi, C.; Briand, D. All-Inkjet-Printed Graphene-Gated Organic Electrochemical Transistors on Polymeric Foil as Highly Sensitive Enzymatic Biosensors. ACS Appl. Nano Mater. 2022, 5, 1664–1673. [Google Scholar] [CrossRef]
- Diacci, C.; Lee, J.W.; Janson, P.; Dufil, G.; Méhes, G.; Berggren, M.; Simon, D.T.; Stavrinidou, E. Real-Time Monitoring of Glucose Export from Isolated Chloroplasts Using an Organic Electrochemical Transistor. Adv. Mater. Technol. 2020, 5, 1900262. [Google Scholar] [CrossRef]
- Galliani, M.; Diacci, C.; Berto, M.; Sensi, M.; Beni, V.; Berggren, M.; Borsari, M.; Simon, D.T.; Biscarini, F.; Bortolotti, C.A. Flexible Printed Organic Electrochemical Transistors for the Detection of Uric Acid in Artificial Wound Exudate. Adv. Mater. Interfaces 2020, 7, 2001218. [Google Scholar] [CrossRef]
- Braendlein, M.; Pappa, A.; Ferro, M.; Lopresti, A.; Acquaviva, C.; Mamessier, E.; Malliaras, G.G.; Owens, R.M. Lactate Detection in Tumor Cell Cultures Using Organic Transistor Circuits. Adv. Mater. 2017, 29, 1605744. [Google Scholar] [CrossRef]
- Montero-Jimenez, M.; Lugli-Arroyo, J.; Fenoy, G.E.; Piccinini, E.; Knoll, W.; Marmisollé, W.A.; Azzaroni, O. Transduction of Amine–Phosphate Supramolecular Interactions and Biosensing of Acetylcholine through PEDOT-Polyamine Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Piccinini, E.; Bliem, C.; Reiner-Rozman, C.; Battaglini, F.; Azzaroni, O.; Knoll, W. Enzyme-polyelectrolyte multilayer assemblies on reduced graphene oxide field-effect transistors for biosensing applications. Biosens. Bioelectron. 2017, 92, 661–667. [Google Scholar] [CrossRef]
- Pérez-Mitta, G.; Peinetti, A.S.; Cortez, M.L.; Toimil-Molares, M.E.; Trautmann, C.; Azzaroni, O. Highly Sensitive Biosensing with Solid-State Nanopores Displaying Enzymatically Reconfigurable Rectification Properties. Nano Lett. 2018, 18, 3303–3310. [Google Scholar] [CrossRef]
- Berto, M.; Diacci, C.; Theuer, L.; Di Lauro, M.; Simon, D.T.; Berggren, M.; Biscarini, F.; Beni, V.; Bortolotti, C.A. Label free urea biosensor based on organic electrochemical transistors. Flex. Print. Electron. 2018, 3, 024001. [Google Scholar] [CrossRef]
- Shalileh, F.; Sabahi, H.; Dadmehr, M.; Hosseini, M. Sensing approaches toward detection of urea adulteration in milk. Microchem. J. 2023, 193, 108990. [Google Scholar] [CrossRef]
- Dhawan, G.; Sumana, G.; Malhotra, B.D. Recent developments in urea biosensors. Biochem. Eng. J. 2009, 44, 42–52. [Google Scholar] [CrossRef]
- Berger, M.; Sehlmeyer, M.; Sellering, F.; Röhrich, H.; Perl, T.; Mansour, H.; Baasner, D.; Zimmermann, S. In-line monitoring of electrolytes and urea during continuous renal replacement therapy. J. Sens. Sens. Syst. 2020, 9, 251–262. [Google Scholar] [CrossRef]
- Derin, E.; Inci, F. Advances in Biosensor Technologies for Acute Kidney Injury. ACS Sens. 2022, 7, 358–385. [Google Scholar] [CrossRef]
- Liu, L.; Mo, H.; Wei, S.; Raftery, D. Quantitative analysis of urea in human urine and serum by 1H nuclear magnetic resonance. Analyst 2012, 137, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Vadgama, P. Analytical Reviews in Clinical Biochemistry: The Estimation of Urea. Ann. Clin. Biochem. Int. J. Lab. Med. 1992, 29, 245–264. [Google Scholar] [CrossRef]
- SINGH, M.; VERMA, N.; GARG, A.; REDHU, N. Urea biosensors. Sens. Actuators B Chem. 2008, 134, 345–351. [Google Scholar] [CrossRef]
- Quadrini, L.; Laschi, S.; Ciccone, C.; Catelani, F.; Palchetti, I. Electrochemical methods for the determination of urea: Current trends and future perspective. TrAC Trends Anal. Chem. 2023, 168, 117345. [Google Scholar] [CrossRef]
- Botewad, S.N.; Gaikwad, D.K.; Girhe, N.B.; Thorat, H.N.; Pawar, P.P. Urea biosensors: A comprehensive review. Biotechnol. Appl. Biochem. 2023, 70, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Jakhar, S.; Narwal, V. Determination of urea with special emphasis on biosensors: A review. Biosens. Bioelectron. 2019, 123, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, M.R.; Shaban, S.M.; Elbalaawy, A.Y.; Hafez, E.; Shin, J.; Cho, S.-Y.; Kim, D.-H. Fluorometric and Colorimetric Hybrid Carbon-Dot Nanosensors for Dual Monitoring of Urea. ACS Appl. Nano Mater. 2023, 6, 7992–8003. [Google Scholar] [CrossRef]
- Sharma, S.; Mishra, S.K. Exploiting the Advantages of Ag/ITO/Enzyme Trapped Gel Layers to Develop a Highly Sensitive and Selective Fiber Optic Plasmonic Urea Sensor. Chemosensors 2023, 11, 421. [Google Scholar] [CrossRef]
- Cheng, L.; Zheng, W.; Zhang, Y.-N.; Li, X.; Zhao, Y. Highly Sensitive Fiber-Optic SPR Urea Sensor Based on ZIF-8/Urease. IEEE Trans. Instrum. Meas. 2023, 72, 7002807. [Google Scholar] [CrossRef]
- Yang, Y.; Yoon, S.G.; Shin, C.; Jin, H.; Lee, W.H.; Park, J.; Kim, Y.S. Ionovoltaic urea sensor. Nano Energy 2019, 57, 195–201. [Google Scholar] [CrossRef]
- Ortega Vega, M.R.; Luo, Y.; Werheid, M.; Weidinger, I.; Senkovska, I.; Grothe, J.; Kaskel, S. Ni-based metal-organic framework sensor material for urea detection: Mechanistic insights and performance. Electrochim. Acta 2024, 477, 143748. [Google Scholar] [CrossRef]
- Jaworska, E.; Maksymiuk, K.; Michalska, A. Carbon Nanotubes-Based Potentiometric Bio-Sensors for Determination of Urea. Chemosensors 2015, 3, 200–210. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Rosso Cagnani, G.; Gomes, N.O.; Raymundo-Pereira, P.A.; Machado, S.A.S.; Gutierrez, M.A.; Krieger, J.E.; Oliveira, O.N. Wearable potentiometric biosensor for analysis of urea in sweat. Biosens. Bioelectron. 2023, 223, 114994. [Google Scholar] [CrossRef] [PubMed]
- Khataee, S.; Dehghan, G.; Shaghaghi, Z.; Khataee, A. An enzyme-free sensor based on La-doped CoFe-layered double hydroxide decorated on reduced graphene oxide for sensitive electrochemical detection of urea. Microchim. Acta 2024, 191, 152. [Google Scholar] [CrossRef] [PubMed]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Demirbas, E. A novel comparative study for electrochemical urea biosensor design: Effect of different ferrite nanoparticles (MFe2O4, M: Cu, Co, Ni, Zn) in urease immobilized composite system. Bioelectrochemistry 2023, 149, 108324. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Bubnova, O.; Jafari, M.J.; Brooke, R.; Liu, X.; Gabrielsson, R.; Ederth, T.; Evans, D.R.; Andreasen, J.W.; Fahlman, M.; et al. Acido-basic control of the thermoelectric properties of poly(3,4-ethylenedioxythiophene)tosylate (PEDOT-Tos) thin films. J. Mater. Chem. C 2015, 3, 10616–10623. [Google Scholar] [CrossRef] [PubMed]
- Mitraka, E.; Kergoat, L.; Khan, Z.U.; Fabiano, S.; Douhéret, O.; Leclère, P.; Nilsson, M.; Andersson Ersman, P.; Gustafsson, G.; Lazzaroni, R.; et al. Solution processed liquid metal-conducting polymer hybrid thin films as electrochemical pH-threshold indicators. J. Mater. Chem. C 2015, 3, 7604–7611. [Google Scholar] [CrossRef]
- Buth, F.; Donner, A.; Sachsenhauser, M.; Stutzmann, M.; Garrido, J.A. Biofunctional Electrolyte-Gated Organic Field-Effect Transistors. Adv. Mater. 2012, 24, 4511–4517. [Google Scholar] [CrossRef]
- Song, J.; Jańczewski, D.; Ma, Y.; Hempenius, M.; Xu, J.; Vancso, G.J. Disassembly of redox responsive poly(ferrocenylsilane) multilayers: The effect of blocking layers, supporting electrolyte and polyion molar mass. J. Colloid Interface Sci. 2013, 405, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Diforti, J.F.; Piccinini, E.; Allegretto, J.A.; von Bilderling, C.; Marmisollé, W.A.; Azzaroni, O. Empowering Bioelectronics with Supramolecular Nanoarchitectonics: PEDOT-Based Organic Electrochemical Transistors with Tunable Electronic Properties. ACS Appl. Electron. Mater. 2024, 6, 1211–1222. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Y.; Zhu, R.; Chen, Y.; Liu, X.; Xu, J.; Li, M.; Wang, D. Urea Detection of Electrochemical Transistor Sensors based on Polyanline (PANI)/MWCNT/Cotton Yarns. Electroanalysis 2021, 33, 2406–2416. [Google Scholar] [CrossRef]
- Werkmeister, F.X.; Koide, T.; Nickel, B.A. Ammonia sensing for enzymatic urea detection using organic field effect transistors and a semipermeable membrane. J. Mater. Chem. B 2016, 4, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sant, W.; Temple-Boyer, P.; Chanié, E.; Launay, J.; Martinez, A. On-line monitoring of urea using enzymatic field effect transistors. Sens. Actuators B Chem. 2011, 160, 59–64. [Google Scholar] [CrossRef]
- Marchenko, S.V.; Kucherenko, I.S.; Hereshko, A.N.; Panasiuk, I.V.; Soldatkin, O.O.; El’skaya, A.V.; Soldatkin, A.P. Application of potentiometric biosensor based on recombinant urease for urea determination in blood serum and hemodialyzate. Sens. Actuators B Chem. 2015, 207, 981–986. [Google Scholar] [CrossRef]
- Öndeş, B.; Akpınar, F.; Uygun, M.; Muti, M.; Aktaş Uygun, D. High stability potentiometric urea biosensor based on enzyme attached nanoparticles. Microchem. J. 2021, 160, 105667. [Google Scholar] [CrossRef]
- Mitchell, K.M. Acetylcholine and Choline Amperometric Enzyme Sensors Characterized In Vitro and In Vivo. Anal. Chem. 2004, 76, 1098–1106. [Google Scholar] [CrossRef]


| System | LOD | Operational Range | Sample | Reference |
|---|---|---|---|---|
| PANI/MWCNT FECT | 1 nm | 1 nM–1 mM | Saliva | Wang et al. (2021) [64] |
| PEDOT:PSS OECT | 1 μM | 1 μM–1 mM | Aqueous solutions | Berto et al. (2018) [39] |
| Graphene FET | 1 μM | 1–1000 μM | Aqueous solutions | Piccinini et al. (2017) [37] |
| OFETs | - | 0.75–7.5 mM | Aqueous solutions | Werkmeister et al. (2016) [65] |
| pH-sensitive chemical FET | - | 0.5–30 mM | Dialysate solutions | Sant et al. (2011) [66] |
| pH-sensitive FET | 100 μM | 0.5–40 mM | Hemodialysate and blood serum | Marchenko et al. (2015) [67] |
| pH-sensitive urease-OECT | 100 μΜ | 0.1–20 mM | Aqueous solutions | This work |
| pH-sensitive urease-OECT | 100 μM | 0.1–1 mM | Diluted urine | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neyra Recky, J.R.; Montero-Jimenez, M.; Scotto, J.; Azzaroni, O.; Marmisollé, W.A. Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing. Chemosensors 2024, 12, 124. https://doi.org/10.3390/chemosensors12070124
Neyra Recky JR, Montero-Jimenez M, Scotto J, Azzaroni O, Marmisollé WA. Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing. Chemosensors. 2024; 12(7):124. https://doi.org/10.3390/chemosensors12070124
Chicago/Turabian StyleNeyra Recky, Jael R., Marjorie Montero-Jimenez, Juliana Scotto, Omar Azzaroni, and Waldemar A. Marmisollé. 2024. "Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing" Chemosensors 12, no. 7: 124. https://doi.org/10.3390/chemosensors12070124
APA StyleNeyra Recky, J. R., Montero-Jimenez, M., Scotto, J., Azzaroni, O., & Marmisollé, W. A. (2024). Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing. Chemosensors, 12(7), 124. https://doi.org/10.3390/chemosensors12070124







