Organelle Targeting Self-Assembled Fluorescent Probe for Anticancer Treatment
Abstract
1. Introduction
2. Key Milestones of Organelle Targeting Self-Assembled Fluorescent Probe in Cancer Treatment
2.1. Pyrene-Based Monomer
2.2. NIR-Based Monomer
2.3. NBD Based Monomer
2.4. FITC Based Monomer
2.5. NDI Based Monomer
2.6. AIE Based Monomer
3. Conclusions and Future Outlook
- (i)
- Most of the mitochondria targeting units are positive so it may induce undesirable interaction with the negatively charged biomolecule inside the complex cellular environments.
- (ii)
- Sometimes nonspecific accumulation of the precursor molecule inside the cellular organelle may happen which leads to unexpected self-assembly and causes the breakdown of cellular immune systems.
- (iii)
- Off-target accumulation also leads to the toxicity of the probe molecule for the normal cells.
- (iv)
- Many fluorescent probes face the aggregation caused-quenching effect (ACQ) or photobleaching after self-assembly and thus desired activity of the probe cannot be achieved.
Author Contributions
Funding
Conflicts of Interest
References
- Ntziachristos, V. Fluorescence molecular imaging. Annu. Rev. Biomed. Eng. 2006, 8, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Quang, D.T. Calixarene-Derived Fluorescent Probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Dodani, S.; Chang, C. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Huo, F.; Cheng, F.; Zhu, X.; Mafireyi, T.; Strongin, R.M.; Yin, C. Correction: Functional synthetic probes for selective targeting and multi-analyte detection and imaging. Chem. Soc. Rev. 2019, 48, 4336–4337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhong, Y.; He, C.; Fu, L.; Huang, Q.; Kuang, Y.; Yi, X.; Zeng, W.; Zhong, H.; Yang, M. Recent advances in organic fluorescent probes for tumor related enzyme detection. Dye. Pigm. 2022, 204, 110386. [Google Scholar] [CrossRef]
- Balleza, E.; Kim, J.; Cluzel, P. Systematic characterization of maturation time of fluorescent proteins in living cells. Nat. Methods 2018, 15, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Wang, D.; Pollard, A.C.; Chen, Z.G.; Egap, E. Triblock near-infrared fluorescent polymer semiconductor nanoparticles for targeted imaging. J. Mater. Chem. C 2017, 5, 5685–5692. [Google Scholar] [CrossRef]
- Bucevičius, J.; Gerasimaitė, R.; Kiszka, K.A.; Pradhan, S.; Kostiuk, G.; Koenen, T.; Lukinavičius, G. A general highly efficient synthesis of biocompatible rhodamine dyes and probes for live-cell multicolor nanoscopy. Nat. Commun. 2023, 14, 1306. [Google Scholar] [CrossRef]
- Fuente-Herreruela, D.D.L.; Gónzalez-Charro, V.; Almendro-Vedia, V.G.; Morán, M.; Martín, M.Á.; Lillo, M.P.; Natale, P.; López-Montero, I. Rhodamine-based sensor for real-time imaging of mitochondrial ATP in living fibroblasts. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 999–1006. [Google Scholar] [CrossRef]
- Karakuş, E. A rhodamine based fluorescent chemodosimeter for the selective and sensitive detection of copper (II) ions in aqueous media and living cells. J. Mol. Struct. 2021, 1224, 129037. [Google Scholar] [CrossRef]
- Sun, S.; Wu, X.; Huang, Y.; Jiang, Q.; Zhu, S.; Sun, S. Visual detection of Cu2+ in high-copper feed based on a fluorescent derivative of rhodamine B. Microchem. J. 2021, 171, 106858. [Google Scholar] [CrossRef]
- Okoh, M.P.; Hunter, J.L.; Corrie, J.E.T.; Webb, M.R. A Biosensor for Inorganic Phosphate Using a Rhodamine-Labeled Phosphate Binding Protein. Biochemistry 2006, 45, 14764. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Patra, S.; Basu, S.; Mukherjee, P.; Mukhopadhyay, D. Inorganic phosphate nanorods are a novel fluorescent label in cell biology. J. Nanobiotechnol. 2006, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Ma, X.; Yang, J.; Jiang, M.; Peng, C.; Ma, Z.; Yu, H.; Li, Y. A New Ratiometric Design Strategy Based on Modulation of π-Conjugation Unit for Developing Fluorescent Probe and Imaging of Cellular Peroxynitrite. Anal. Chem. 2022, 94, 4763. [Google Scholar] [CrossRef]
- Li, Z.; Liang, P.Z.; Ren, T.-B.; Yuan, L.; Zhang, X.-B. Orderly Self-Assembly of Organic Fluorophores for Sensing and Imaging. Angew. Chem. Int. Ed. 2023, 62, e202305742. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Huang, C.; Zhao, S.; Zhu, S.; Liu, R.; Zhu, H. Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules 2023, 28, 2997. [Google Scholar] [CrossRef]
- Ding, F.; Fan, Y.; Sun, Y.; Zhang, F. Beyond 1000 nm Emission Wavelength: Recent Advances in Organic and Inorganic Emitters for Deep-Tissue Molecular Imaging. Adv. Healthc. Mater. 2019, 8, e1900260. [Google Scholar] [CrossRef]
- Li, K.; Xu, S.; Xiong, M.; Huan, S.-Y.; Yuan, L.; Zhang, X.-B. Molecular engineering of organic-based agents for in situ bioimaging and phototherapeutics. Chem. Soc. Rev. 2021, 50, 11766–11784. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Kim, D.; Hasan, M.S.; Lim, C.; Seu, M.-S.; Ryu, J.-H. Intracellular Chemical Reaction-Induced Self-Assembly for the Construction of Artificial Architecture and Its Functions. Adv. NanoBiomed Res. 2024, 4, 2300137. [Google Scholar] [CrossRef]
- Okamoto, K.; Chithra, P.; Richards, G.J.; Hill, J.P.; Ariga, K. Self-Assembly of Optical Molecules with Supramolecular Concepts. Int. J. Mol. Sci. 2009, 10, 1950–1966. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, Y.; Lin, X.; Huo, Y.; Zhang, H.; Wang, H. Structure-Based Programming of Supramolecular Assemblies in Living Cells for Selective Cancer Cell Inhibition. Angew. Chem. Int. Ed. 2021, 60, 21807–21816. [Google Scholar] [CrossRef]
- Huang, F.; Anslyn, E.V. Introduction: Supramolecular Chemistry. Chem. Rev. 2015, 115, 6999–7000. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.; Peseka, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Li, X.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. Self-Assembly, Structural Transformation, and Guest-Binding Properties of Supramolecular Assemblies with Triangular Metal-Metal Bonded Units. J. Am. Chem. Soc. 2020, 142, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.E.; Stepanenko, V.; Würthner, F. Fluorescent J-Aggregates of Core-Substituted Perylene Bisimides: Studies on Structure−Property Relationship, Nucleation−Elongation Mechanism, and Sergeants-and-Soldiers Principle. J. Am. Chem. Soc. 2009, 131, 6719–6732. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.E.; Stepanenko, V.; Würthner, F. Supramolecular Construction of Fluorescent J-Aggregates Based on Hydrogen-Bonded Perylene Dyes. Angew. Chem. Int. Ed. 2007, 46, 5541–5544. [Google Scholar] [CrossRef]
- Song, X.; Zhang, R.; Liang, C.; Chen, Q.; Gong, H.; Liu, Z. Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials 2015, 57, 84–92. [Google Scholar] [CrossRef]
- Cai, K.; Xie, J.; Zhang, D.; Shi, W.; Yan, Q.; Zhao, D. Concurrent Cooperative J-Aggregates and Anticooperative H-Aggregates. J. Am. Chem. Soc. 2018, 140, 5764–5773. [Google Scholar] [CrossRef]
- Li, Z.; Liang, P.Z.; Xu, L.; Zhang, X.-X.; Li, K.; Wu, Q.; Lou, X.-F.; Ren, T.-B.; Yuan, L.; Zhang, X.-B. In situ orderly self-assembly strategy affording NIR-II-J-aggregates for in vivo imaging and surgical navigation. Nat. Commun. 2023, 14, 1843. [Google Scholar] [CrossRef]
- Sivagnanam, S.; Das, K.; Basak, M.; Mahata, T.; Stewart, A.; Maity, B.; Das, P. Self-assembled dipeptide based fluorescent nanoparticles as a platform for developing cellular imaging probes and targeted drug delivery chaperones. Nanoscale Adv. 2022, 4, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wu, G.; Tian, X.; Liu, Z. Smart fluorescent probes for in situ imaging of enzyme activity: Design strategies and applications. Future Med. Chem. 2018, 10, 2729–2744. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, J.; Chen, M.; Yang, Z. Self-assembling small molecules for the detection of important analytes. Chem. Soc. Rev. 2014, 43, 7257–7266. [Google Scholar] [CrossRef] [PubMed]
- Hai, Z.; Liang, C. Intracellular Self-Assembly of Nanoprobes for Molecular Imaging. Adv. Biosyst. 2018, 2, 1800108. [Google Scholar] [CrossRef]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, X.; Yin, H.; Ge, Z.; Wang, Y.; Chu, Z.; Raabova, H.; Vavra, J.; Cigler, P.; Liu, R.; et al. Anchored but not internalized: Shape dependent endocytosis of nanodiamond. Sci. Rep. 2017, 7, 46462. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Jin, X.; Shankar, P.; Lee, J.H.; Jo, K.; Lim, K.I. Molecular-Level Interactions between Engineered Materials and Cells. Int. J. Mol. Sci. 2019, 20, 4142. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Basilion, J.P. Photodynamic Therapy: Targeting Cancer Biomarkers for the Treatment of Cancers. Cancers 2021, 13, 2992. [Google Scholar] [CrossRef]
- Elsyed, A.F.N.; Wong, G.-L.; Ameen, M.; Wu, M.-W.; Chang, C.-C. Tunable Fluorescence via Self-Assembled Switching of AIE-Active Micelle-like Nanoaggregates. Int. J. Mol. Sci. 2023, 24, 9941. [Google Scholar] [CrossRef]
- Cheung, S.; O’Shea, D.F. Directed self-assembly of fluorescence responsive nanoparticles and their use for real-time surface and cellular imaging. Nat. Commun. 2017, 8, 1885. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Yu, H.; Song, J.; Liu, S.; Li, G. Pyrene-Based Self-Assembling Peptide for Ratiometric Detection of Heparin. ChemBioChem 2023, 24, e202200652. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Roy, S.; Ullah, Z.; Gu, J.; Huang, H.; Yu, C.; Wang, X.; Wang, H.; Zhang, Y.; et al. Development of Polymethine Dyes for NIR-II Fluorescence Imaging and Therapy. Adv. Healthc. Mater. 2024, 13, 2304506. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Harikumar, K.G.; Rawat, S.S.; Das, S.; Chakraborty, T.K.; Chattopadhyay, A. Environment-Sensitive Fluorescence of 7-Nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)-Labeled Ligands for Serotonin Receptors. Molecules 2021, 26, 3848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mizoguchi, T.; Kuribara, T.; Nakajima, M.; Iwata, M.; Sakamoto, Y.; Nakamura, H.; Murayama, T.; Nemoto, T.; Itoh, M. Py3-FITC: A new fluorescent probe for live cell imaging of collagen-rich tissues and ionocytes. Open Biol. 2021, 11, 200241. [Google Scholar] [CrossRef] [PubMed]
- Bhusanur, D.I.; More, K.S.; Al Kobaisi, M.; Singh, P.K.; Bhosale, S.V.; Bhosale, S.V. Synthesis, Photophysical Properties and Self-Assembly of a Tetraphenylethylene-Naphthalene Diimide Donor-Acceptor Molecule. Chem. Asian J. 2024, 19, e202301046. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, G.-Q.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Palanikumar, L.; Go, E.M.; Kim, I.; Kang, M.G.; Lee, S.; Park, S.; Choi, H.; Kim, C.; Jin, S.-M.; et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Jeong, K.; Go, E.M.; Cho, Y.; Lee, S.; Jin, S.; Hwang, S.-W.; Jang, J.H.; Kang, C.S.; Bang, W.-Y.; et al. Heterochiral Assembly of Amphiphilic Peptides inside the Mitochondria for Supramolecular Cancer Therapeutics. ACS Nano 2019, 13, 11022. [Google Scholar] [CrossRef]
- Jeena, M.T.; Jin, S.; Jana, B.; Ryu, J.-H. Enzyme-instructed morphology transformation of mitochondria-targeting peptide for the selective eradication of osteosarcoma. RSC Chem. Biol. 2022, 3, 1416–1421. [Google Scholar] [CrossRef]
- Jeena, M.T.; Jin, S.; Jeong, K.; Cho, Y.; Kim, J.C.; Lee, J.H.; Lee, S.; Hwang, S.-W.; Kwak, S.K.; Kim, S.; et al. Cancer-Selective Supramolecular Chemotherapy by Disassembly-Assembly Approach. Adv. Funct. Mater. 2022, 32, 2208098. [Google Scholar] [CrossRef]
- Zhu, M.; Xing, P.; Zhou, Y.; Gong, L.; Zhang, J.; Qi, D.; Bian, Y.; Du, H.; Jiang, J. Lysosome-targeting ratiometric fluorescent pH probes based on long-wavelength BODIPY. J. Mater. Chem. B 2018, 6, 4422. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.C.; Das, R.S.; Chatterjee, T.; Bhattacharyya, M.; Guha, S. Supramolecular β-Sheet Forming Peptide Conjugated with Near-Infrared Chromophore for Selective Targeting, Imaging, and Dysfunction of Mitochondria. Bioconjug. Chem. 2020, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Thomas, A.P.; Kim, S.; Lee, I.S.; Choi, H.; Jin, S.; Park, S.A.; Min, S.K.; Kim, C.; Ryu, J.-H. Self-Assembly of Mitochondria-Targeted Photosensitizer to Increase Photostability and Photodynamic Therapeutic Efficacy in Hypoxia. Chem. Eur. J. 2020, 26, 10695. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cao, Y.; Zhu, X.; Yuan, B.; He, L.; Wu, A.; Li, J. Self-Assembly of Organelle-Localized Neuropeptides Triggers Intrinsic Apoptosis against Breast Cancer. Adv. Healthc. Mater. 2023, 12, 2300265. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Peltier, R.; Zhang, M.; Song, D.; Huang, H.; Chen, G.; Chen, Y.; Zhou, F.; Hao, Q.; Bian, L.; et al. Desuccinylation-Triggered Peptide Self-Assembly: Live Cell Imaging of SIRT5 Activity and Mitochondrial Activity Modulation. J. Am. Chem. Soc. 2020, 142, 18150–18159. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, J.; Wang, H.; Zhou, N.; Yang, D.; Green, D.R.; Xu, B. Enzymatic Cleavage of Branched Peptides for Targeting Mitochondria. J. Am. Chem. Soc. 2018, 140, 1215. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhang, Q.; Hong, P.; Xu, B. A Self-Assembling Probe for Imaging the States of Golgi Apparatus in Live Single Cells. Bioconjug. Chem. 2022, 33, 1983. [Google Scholar] [CrossRef]
- Choi, H.; Park, G.; Shin, E.; Shin, S.W.; Jana, B.; Jin, S.; Kim, S.; Wang, H.; Kwak, S.K.; Xu, B.; et al. Intramitochondrial co-assembly between ATP and nucleopeptides induces cancer cell apoptosis. Chem. Sci. 2022, 13, 6197–6204. [Google Scholar] [CrossRef]
- Kim, S.; Chae, J.-B.; Kim, D.; Park, C.-W.; Sim, Y.; Lee, H.; Park, G.; Lee, J.; Hong, S.; Jana, B.; et al. Supramolecular Senolytics via Intracellular Oligomerization of Peptides in Response to Elevated Reactive Oxygen Species Levels in Aging Cells. J. Am. Chem. Soc. 2023, 145, 21991–22008. [Google Scholar] [CrossRef]
- Jana, B.; Jin, S.; Go, E.M.; Cho, Y.; Kim, D.; Kim, S.; Kwak, S.K.; Ryu, J.-H. Intra-Lysosomal Peptide Assembly for the High Selectivity Index against Cancer. J. Am. Chem. Soc. 2023, 145, 18414–18431. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Jana, B.; Ryu, J.-H. Intra-mitochondrial reaction for cancer cell imaging and anti-cancer therapy by aggregation-induced emission. RSC Adv. 2020, 10, 43383. [Google Scholar] [CrossRef]
- Ji, X.; Wang, N.; Wang, J.; Wang, T.; Huang, X.; Hao, H. Non-destructive real-time monitoring and investigation of the self-assembly process using fluorescent probes. Chem. Sci. 2024, 15, 3800–3830. [Google Scholar] [CrossRef] [PubMed]
- Agafontsev, A.M.; Shumilova, T.A.; Oshchepkov, A.S.; Hampel, F.; Kataev, E.A. Ratiometric Detection of ATP by Fluorescent Cyclophanes with Bellows-Type Sensing Mechanism. Chem. Eur. J. 2020, 26, 9991. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Venkatesan, P.; Thirumalaivasan, N.; Wu, S.-P.; Sun, K.-W. Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging. Chemosensors 2023, 11, 115. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, D.; Lu, P.; Yang, X.; Liao, D.; Zhang, Y.; Zhang, M.; Yu, C.; Yam, V.W.W. Nucleic Acid-Induced Aggregation and Pyrene Excimer Formation. Org. Lett. 2009, 11, 4302–4305. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; Marchi, E.D.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Özalp, V.C.; Pedersen, T.R.; Nielsen, L.J.; Olsen, L.F. Time-resolved Measurements of Intracellular ATP in the Yeast Saccharomyces cerevisiae using a New Type of Nanobiosensor. J. Biol. Chem. 2010, 285, 37579–37588. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Chen, X. In Response to Precision Medicine: Current Subcellular Targeting Strategies for Cancer Therapy. Adv. Mater. 2023, 35, 2209529. [Google Scholar] [CrossRef]
- Mills, E.; Kelly, B.; O’Neill, L. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Sweetwyne, M.T.; Pippin, J.W.; Eng, D.G.; Hudkins, K.L.; Chiao, Y.A.; Campbell, M.D.; Marcinek, D.J.; Alpers, C.E.; Szeto, H.H.; Rabinovitch, P.S.; et al. The mitochondrial-targeted peptide, SS-31, improves glomerular architecture in mice of advanced age. Kidney Int. 2017, 91, 1126–1145. [Google Scholar] [CrossRef] [PubMed]
- Sibrian-Vazquez, M.; Nesterova, I.V.; Jensen, T.J.; Vicente, M.G.H. Mitochondria Targeting by Guanidine– and Biguanidine–Porphyrin Photosensitizers. Bioconjug. Chem. 2008, 19, 705. [Google Scholar] [CrossRef]
- Jeena, M.T.; Kim, S.; Jin, S.; Ryu, J.-H. Recent Progress in Mitochondria-Targeted Drug and Drug-Free Agents for Cancer Therapy. Cancers 2020, 12, 4. [Google Scholar] [CrossRef]
- Shi, J.; Du, X.; Yuan, D.; Zhou, J.; Zhou, N.; Huang, Y.; Xu, B. D-Amino Acids Modulate the Cellular Response of Enzymatic-Instructed Supramolecular Nanofibers of Small Peptides. Biomacromolecules 2014, 15, 3559. [Google Scholar] [CrossRef] [PubMed]
- Seia, M.; Zisman, E. Different roles of D-amino acids in immune phenomena. FASEB J. 1997, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Lee, S.; Barui, A.K.; Jin, S.; Cho, Y.; Hwang, S.-W.; Kim, S.; Ryu, J.-H. Intra-mitochondrial self-assembly to overcome the intracellular enzymatic degradation of l-peptides. Chem. Commun. 2020, 56, 6265. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.; Park, G.; Choi, H.; Ryu, J.-H. Spatiotemporal Self-Assembly of Peptide Amphiphiles by Carbonic Anhydrase IX-Targeting Induces Cancer-Lysosomal Membrane Disruption. JACS Au 2022, 2, 2539–2547. [Google Scholar] [CrossRef]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 2018, 4, 27. [Google Scholar] [CrossRef]
- Dielschneider, R.F.; Henson, E.S.; Gibson, S.B. Lysosomes as Oxidative Targets for Cancer Therapy. Oxidative Med. Cell. Longev. 2017, 2017, 3749157. [Google Scholar] [CrossRef]
- Fan, Z.; Chang, Y.; Cui, C.; Sun, L.; Wang, D.H.; Pan, Z.; Zhang, M. Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy. Nat. Commun. 2018, 9, 2605. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Xu, B. Regulating the Rate of Molecular Self-Assembly for Targeting Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 5770–5775. [Google Scholar] [CrossRef]
- Yan, R.; Hu, Y.; Liu, F.; Wei, S.; Fang, D.; Shuhendler, A.J.; Liu, H.; Chen, H.-Y.; Ye, D. Activatable NIR Fluorescence/MRI Bimodal Probes for in Vivo Imaging by Enzyme-Mediated Fluorogenic Reaction and Self-Assembly. J. Am. Chem. Soc. 2019, 141, 10331–10341. [Google Scholar] [CrossRef]
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70. [Google Scholar] [CrossRef]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Wu, Y.; Li, X.; Zhou, Y.; Wang, Z.; Liang, H.; Ding, F.; Hong, S.; Steinmetz, N.F.; et al. Molecularly Stimuli-Responsive Self-Assembled Peptide Nanoparticles for Targeted Imaging and Therapy. ACS Nano 2023, 17, 8004–8025. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, N.; Fu, B.; Huang, F.; Liu, J. Self-assembling peptide-based nanodrug delivery systems. Biomater. Sci. 2019, 7, 4888–4911. [Google Scholar] [CrossRef] [PubMed]
- An, H.-W.; Hou, D.; Zheng, R.; Wang, M.-D.; Zeng, X.-Z.; Xiao, W.-Y.; Yan, T.-D.; Wang, J.-Q.; Zhao, C.-H.; Cheng, L.-M.; et al. A Near-Infrared Peptide Probe with Tumor-Specific Excretion-Retarded Effect for Image-Guided Surgery of Renal Cell Carcinoma. ACS Nano 2020, 14, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.C.; Bera, T.; Chatterjee, T.; Samanta, J.; Sengupta, A.; Bhattacharyya, M.; Guha, S. Supramolecular Dipeptide-Based Near-Infrared Fluorescent Nanotubes for Cellular Mitochondria Targeted Imaging and Early Apoptosis. Bioconjug. Chem. 2021, 32, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Z.; Signore, S.J.D.; Rodal, A.A.; Xu, B. Active Probes for Imaging Membrane Dynamics of Live Cells with High Spatial and Temporal Resolution over Extended Time Scales and Areas. J. Am. Chem. Soc. 2018, 140, 3505–3509. [Google Scholar] [CrossRef]
- Cai, Y.; Shi, Y.; Wang, H.; Wang, J.; Ding, D.; Wang, L.; Yang, Z. Environment-Sensitive Fluorescent Supramolecular Nanofibers for Imaging Applications. Anal. Chem. 2014, 86, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Huang, H.; Kang, X.; Yang, L.; Xi, Z.; Sun, H.; Pluth, M.D.; Yi, L. NBD-Based Synthetic Probes for Sensing Small Molecules and Proteins: Design, Sensing Mechanisms and Biological Applications. Chem. Soc. Rev. 2021, 50, 7436–7495. [Google Scholar] [CrossRef] [PubMed]
- Meglio, P.D.; Ianaro, A.; Ghosh, S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-κB activation. Arthritis Rheum. 2005, 52, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.-M.; Chen, K.; Liu, X.-J.; Xie, W.-R.; Wang, H. Cell-permeable Tat-NBD peptide attenuates rat pancreatitis and acinus cell inflammation response. World J. Gastroenterol. 2009, 15, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Farber-Katz, S.E.; Dippold, H.C.; Buschman, M.D.; Peterman, M.C.; Xing, M.; Noakes, C.J.; Tat, J.; Ng, M.M.; Rahajeng, J.; Cowan, D.M.; et al. DNA damage triggers golgi dispersal via DNA-PK and GOLPH3. Cell 2014, 156, 413. [Google Scholar] [CrossRef]
- Kirkham, S.; Hamley, I.W.; Smith, A.M.; Gouveia, R.M.; Connon, C.J.; Reza, M.; Ruokolainen, J. A self-assembling fluorescent dipeptide conjugate for cell labelling. Colloids Surf. B Biointerfaces 2016, 137, 104–108. [Google Scholar] [CrossRef]
- Cheng, J.; Fu, S.; Qin, Z.; Han, H.; Yang, X. Self-assembled natural small molecule diterpene acids with favorable anticancer activity and biosafety for synergistically enhanced antitumor chemotherapy. J. Mater. Chem. B 2021, 9, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cheetham, A.G.; Lock, L.L.; Cui, H. Cellular Uptake and Cytotoxicity of Drug–Peptide Conjugates Regulated by Conjugation Site. Bioconjug. Chem. 2013, 24, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lock, L.L.; Cheetham, A.G.; Cui, H. Enhanced Cellular Entry and Efficacy of Tat Conjugates by Rational Design of the Auxiliary Segment. Mol. Pharm. 2014, 11, 964–973. [Google Scholar] [CrossRef]
- Choi, S.W.; Hong, H.K.; Jeon, J.; Choi, J.Y.; Kim, M.; Kim, P.; Lee, B.C.; Woo, S.J. FITC-Labeled RGD Peptides as Novel Contrast Agents for Functional Fluorescent Angiographic Detection of Retinal and Choroidal Neovascularization. Cells 2023, 12, 1902. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S. Stimuli-Responsive Self-Assembly of a Naphthalene Diimide by Orthogonal Hydrogen Bonding and Its Coassembly with a Pyrene Derivative by a Pseudo-Intramolecular Charge-Transfer Interaction. Angew. Chem. Int. Ed. 2014, 53, 1092–1097. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Al Kobaisi, M.; Bhosale, S.V.; Bhargava, S.; Bhosale, S.V. Flower-like supramolecular self-assembly of phosphonic acid appended naphthalene diimide and melamine. Sci. Rep. 2015, 5, 14609. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, G.; Smulders, M.M.J.; Stefankiewicz, A.R. Steering the Self-Assembly Outcome of a Single NDI Monomer into Three Morphologically Distinct Supramolecular Assemblies, with Concomitant Change in Supramolecular Polymerization Mechanism. Adv. Sci. 2019, 6, 1900577. [Google Scholar] [CrossRef] [PubMed]
- Kolhe, N.B.; Devi, R.N.; Senanayak, S.P.; Jancy, B.; Narayan, K.S.; Asha, S.K. Structure engineering of naphthalene diimides for improved charge carrier mobility: Self-assembly by hydrogen bonding, good or bad? J. Mater. Chem. 2012, 22, 15235–15246. [Google Scholar] [CrossRef]
- Peng, Y.; Jin, X.; Zheng, Y.; Han, D.; Liu, K.; Jiang, L. Direct Imaging of Superwetting Behavior on Solid–Liquid–Vapor Triphase Interfaces. Adv. Mater. 2017, 29, 1703009. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors 2022, 12, 550. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Y.; Wu, Z.; Peng, Y.; Liu, Y.; Chen, Y.; Shao, L.; Zeng, Z.; Liu, Y. A lysosomes and mitochondria dual-targeting AIE-active NIR photosensitizer: Constructing amphiphilic structure for enhanced antitumor activity and two-photon imaging. Mater. Today Bio 2023, 21, 100721. [Google Scholar] [CrossRef]
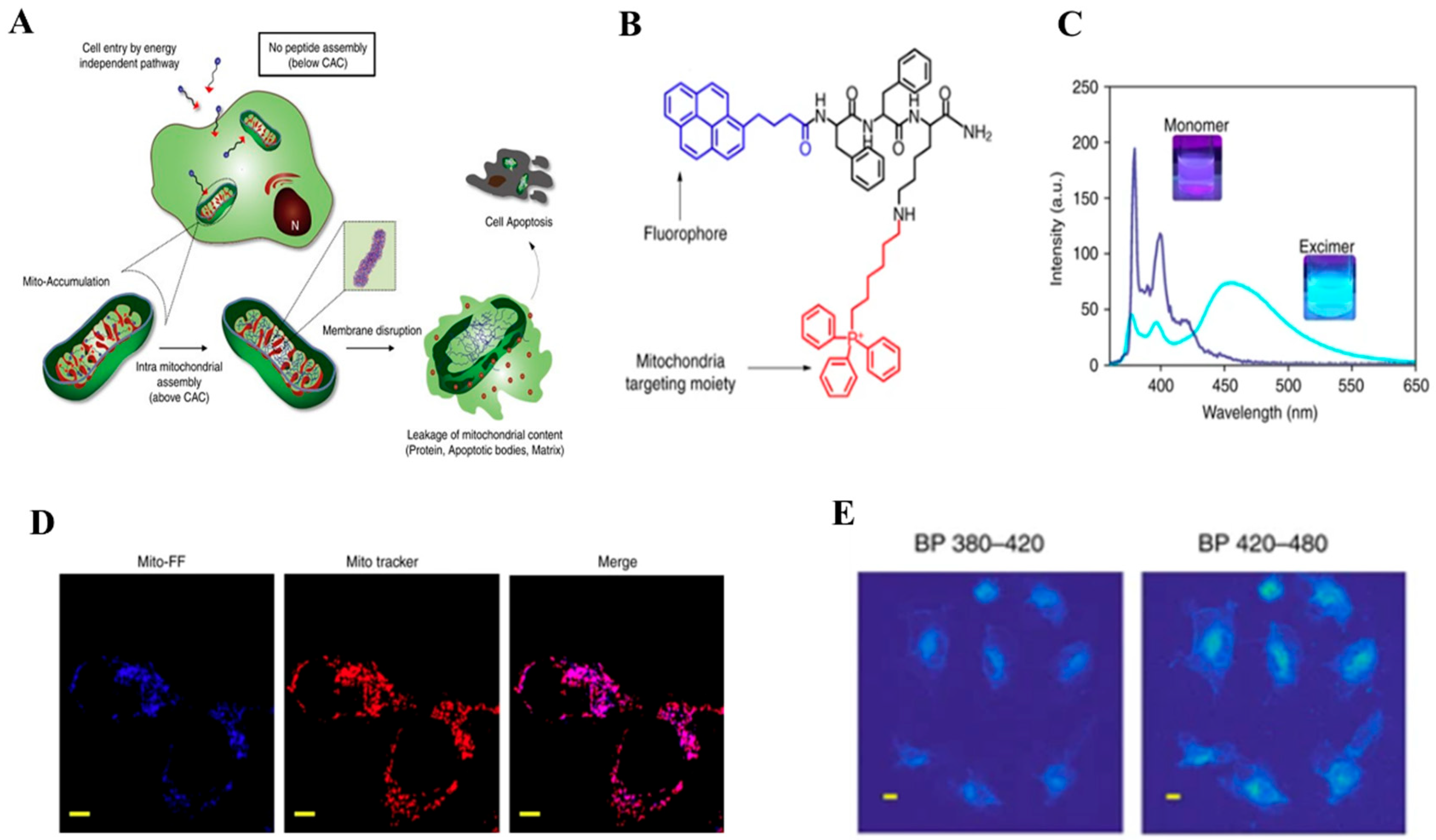
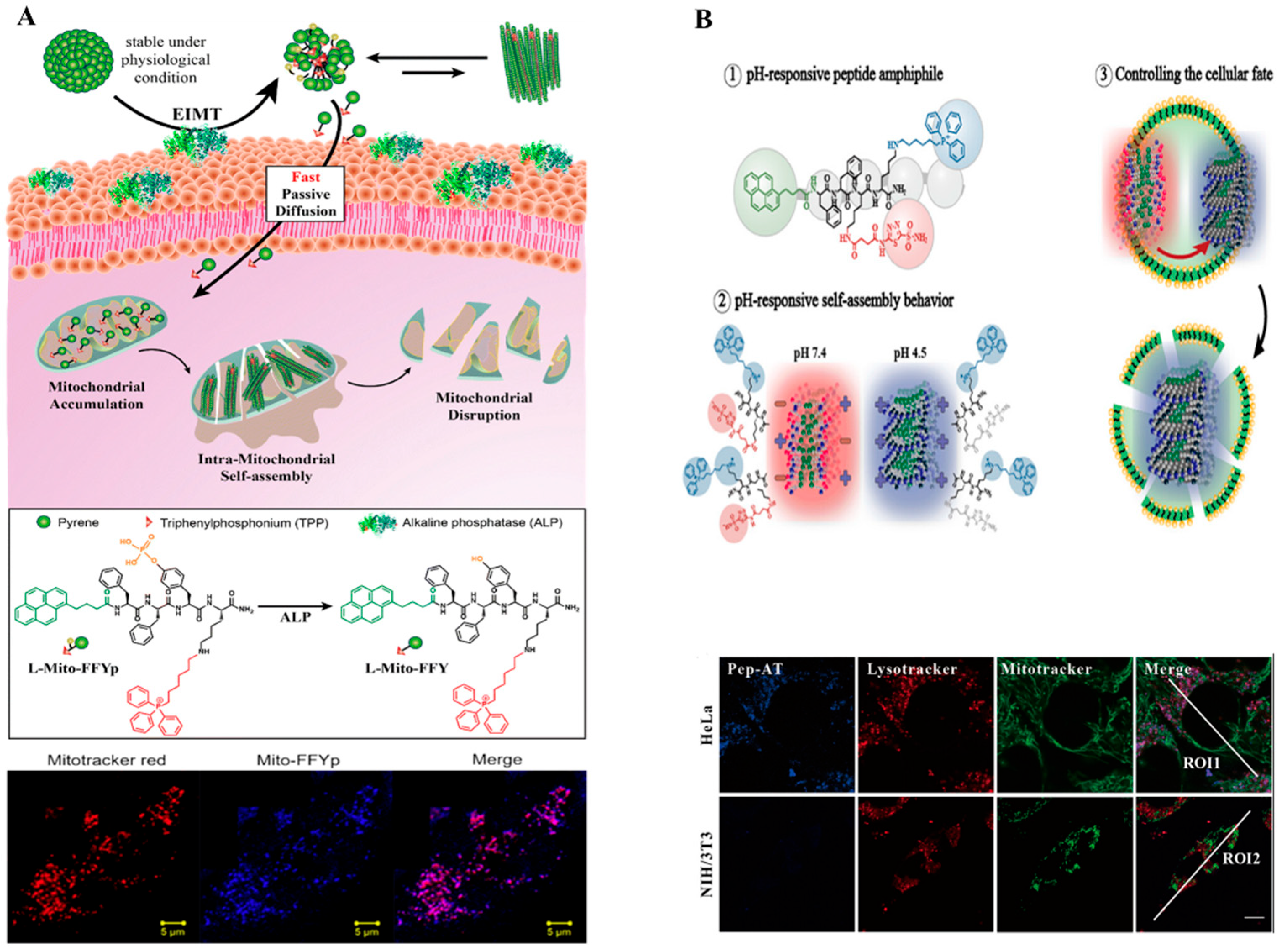
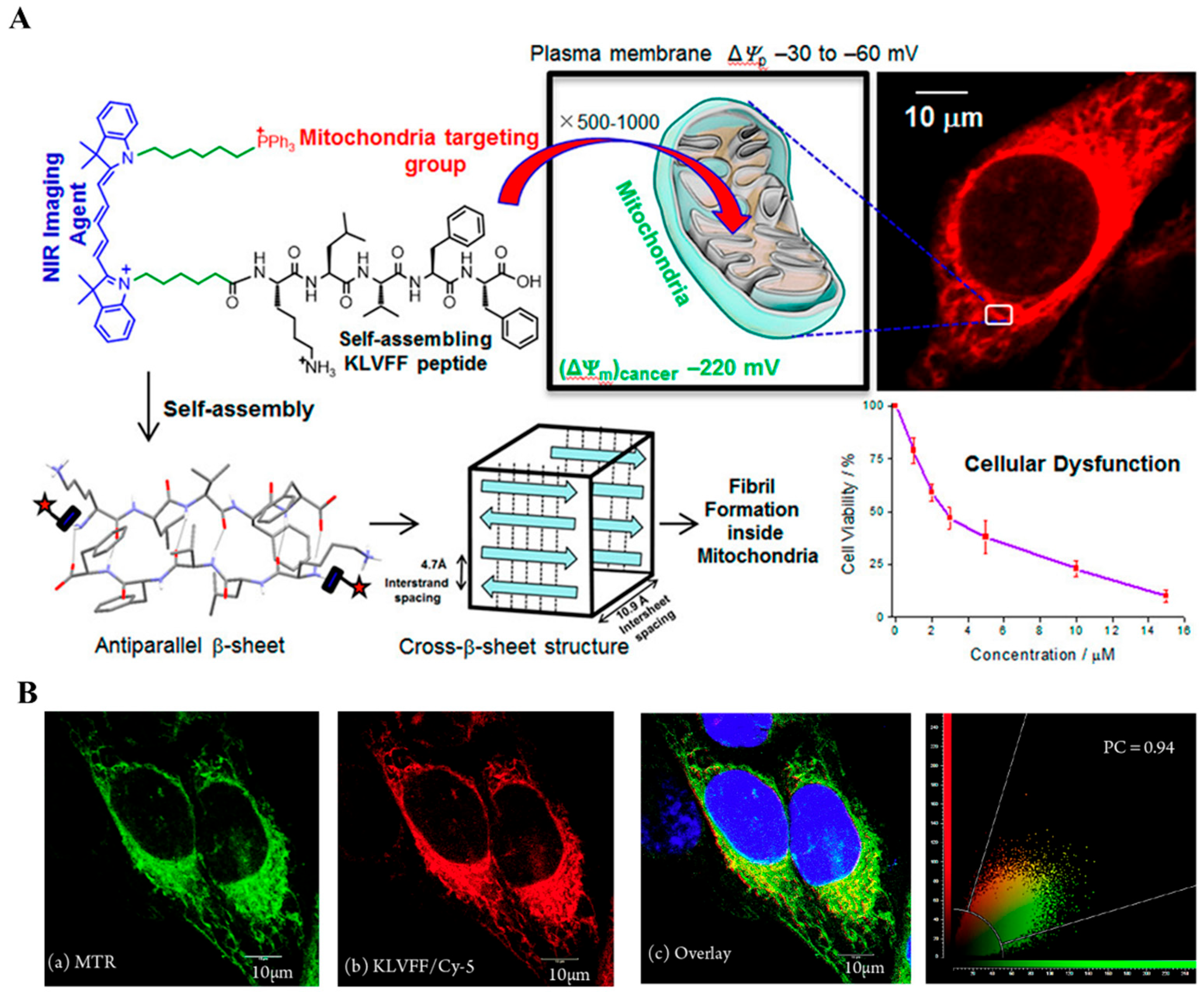
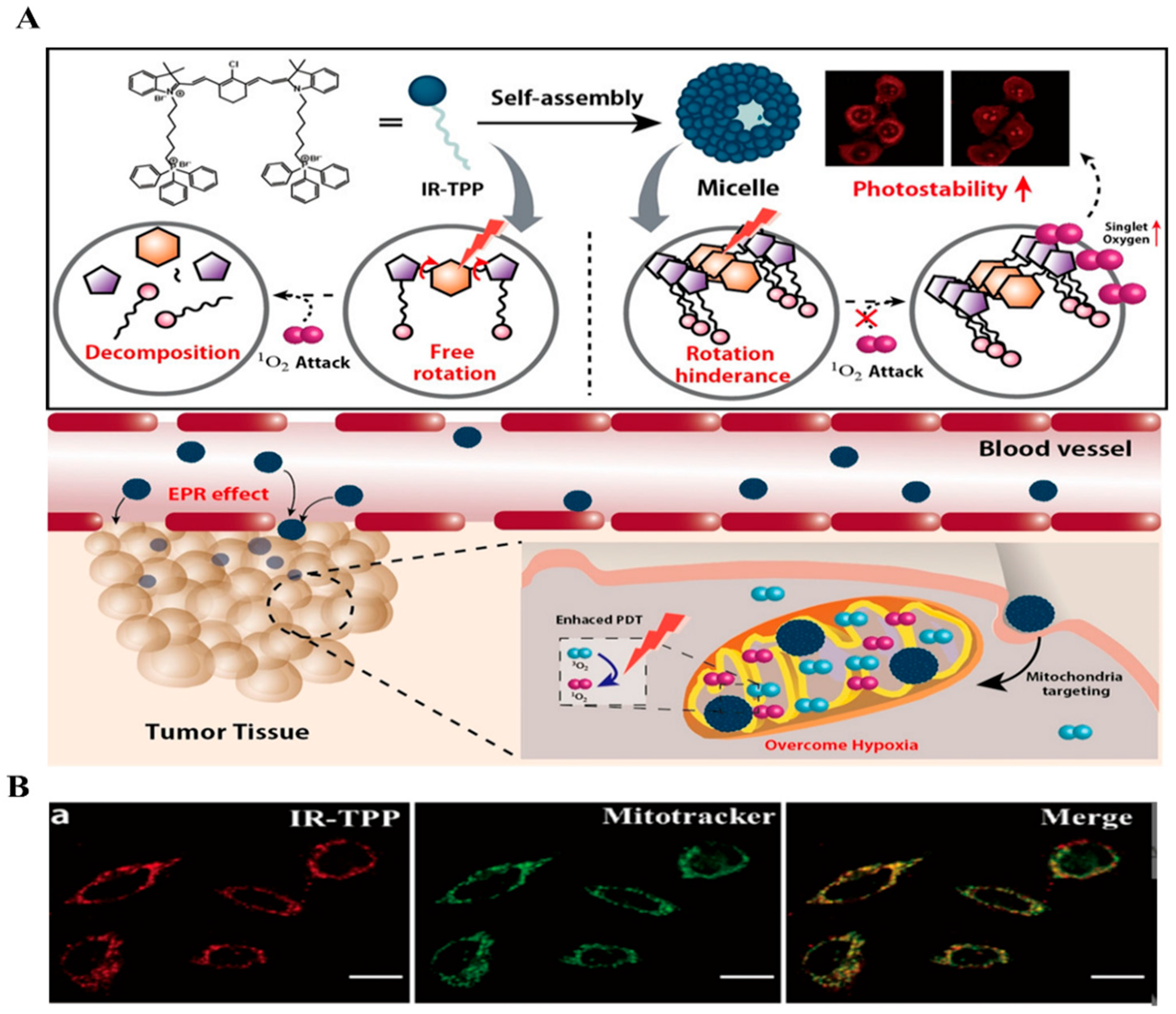

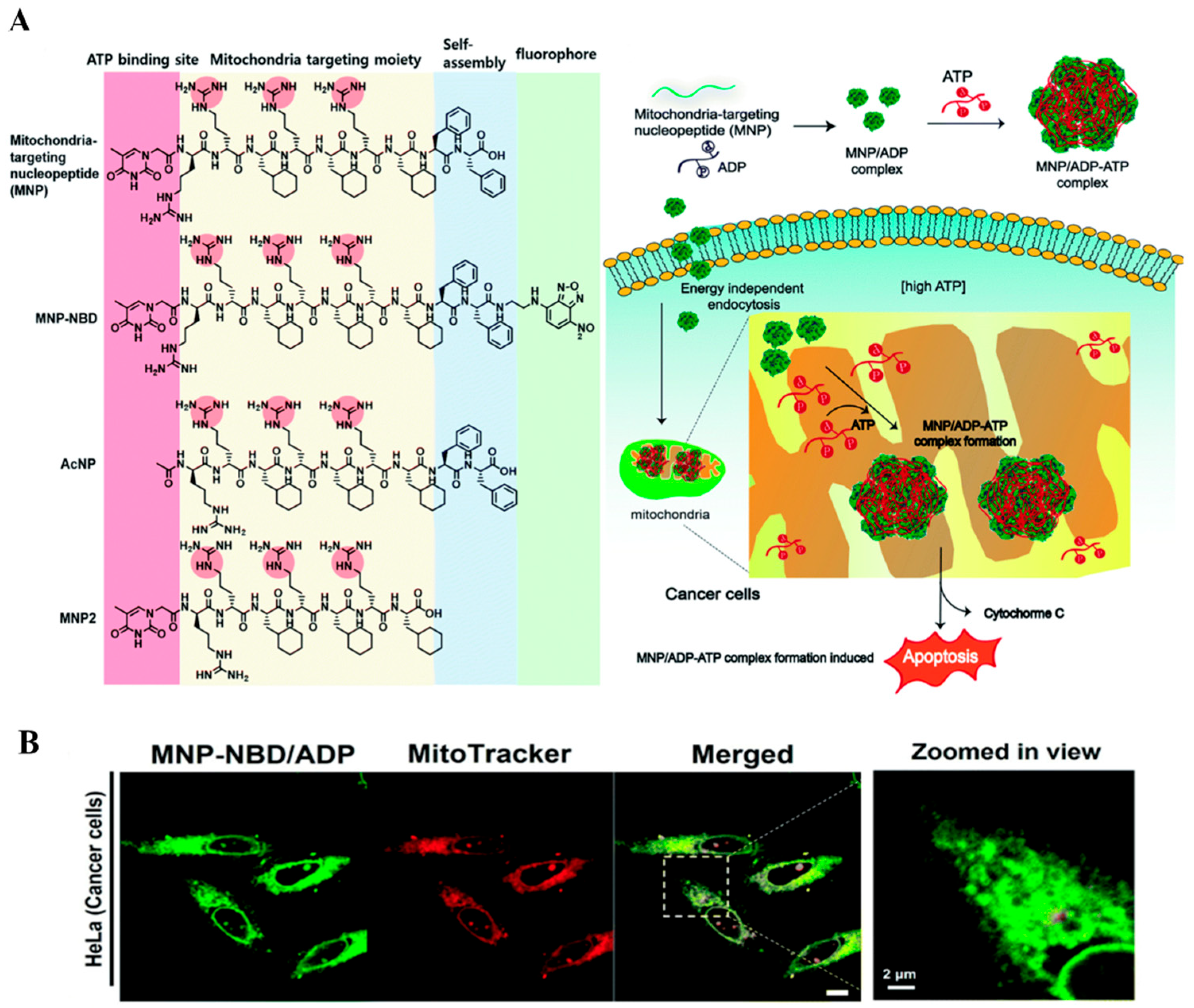
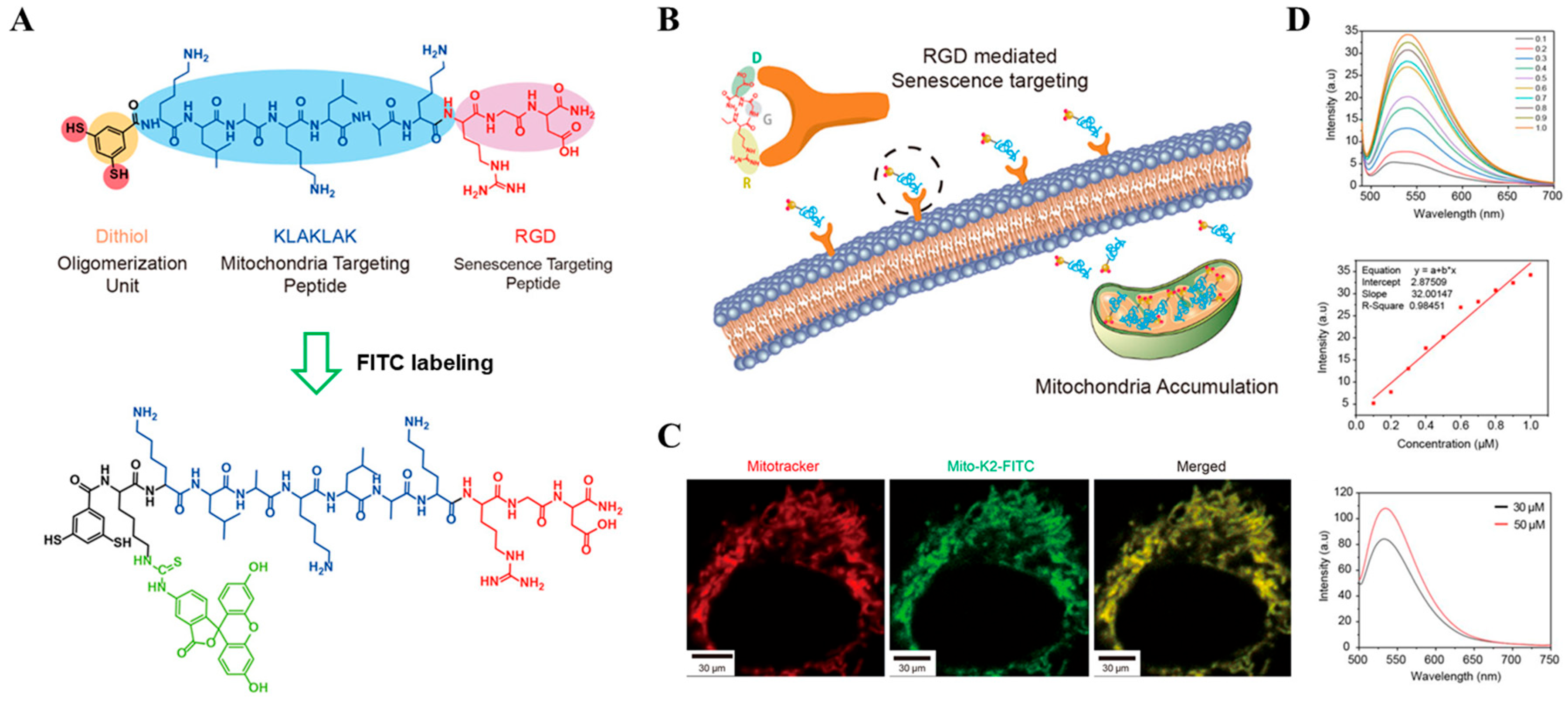

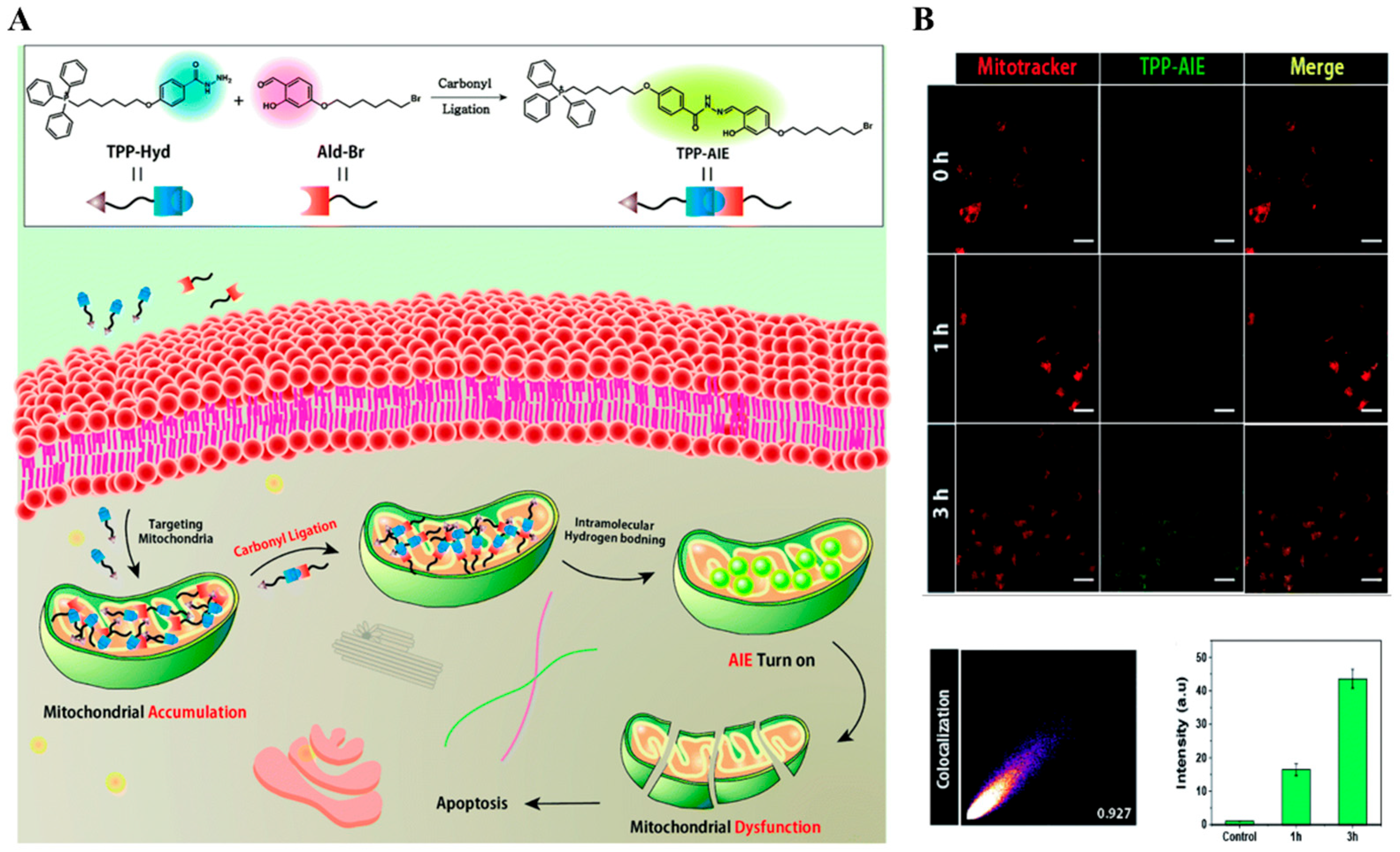
| Types of Monomer | λex and λemi | Molecular Design (Target-Organelle) | Shape of Structure | Functional Mechanism | Ref. |
|---|---|---|---|---|---|
| Pyrene-based | 343 nm 375–405 nm | Pyrene + FF + TPP (Mitochondrial) | Fiber | Mitochondrial membrane disruption | [48] |
| Pyrene + FF(D/L) + TPP (Mitochondrial) | Super fibril | Mitochondrial membrane disruption | [49] | ||
| Pyrene + FFYp + TPP (Mitochondrial) | Micelle to fiber | Mitochondrial membrane disruption | [50] | ||
| Pyrene + FF(SA) + TPP (Mitochondrial) | Micelle to fiber | Mitochondrial membrane disruption | [51] | ||
| Pyrene + FF + AZ (Lysosome) | Nanofiber | Lysosomal membrane disruption | [52] | ||
| NIR-based | 651 nm 672 nm | NIR + KLVFF + TPP (Mitochondrial) | Amyloid fibrils | Mitochondrial dysfunction | [53] |
| 785 nm 810 nm | NIR + TPP (Mitochondrial) | Micelle | ROS generation, PDT | [54] | |
| 745 nm 810 nm | NIR + FF + KGRR + Y1L (Lysosome) | Nanofiber | Lysosomal enlargement and damages | [55] | |
| NBD-based | 466 nm 539 nm | NBD + FFGKsuccG (Mitochondrial) | Nanofibers | Depolarization of mitochondria membrane, ROS generation, | [56] |
| NBD + Nap-ffk (Mitochondrial) | Micelle to fiber | Anticancer drug delivery | [57] | ||
| NBD + ff + pS1 (Golgi body) | Nanofiber | Reveals the dynamics of cancer cells golgi body | [58] | ||
| NBD + MNP (Mitochondrial) | Micelle | ROS generation, metabolic disorder | [59] | ||
| FITC-based | 498 nm 519 nm | FITC + dithiol + RGD (Mitochondrial) | α-helix | Mitochondrial membrane disruption | [60] |
| NDI-based | 395 nm 420 nm | NDI + Lyso + RGD (Lysosome) | Fiber | Lysosomal swelling and damage | [61] |
| AIE-based | variable | AIE + TPP (Mitochondrial) | Nanoaggregate | Mitochondrial dysfunction | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.S.; Kim, S.; Lim, C.; Lee, J.; Seu, M.-S.; Ryu, J.-H. Organelle Targeting Self-Assembled Fluorescent Probe for Anticancer Treatment. Chemosensors 2024, 12, 138. https://doi.org/10.3390/chemosensors12070138
Hasan MS, Kim S, Lim C, Lee J, Seu M-S, Ryu J-H. Organelle Targeting Self-Assembled Fluorescent Probe for Anticancer Treatment. Chemosensors. 2024; 12(7):138. https://doi.org/10.3390/chemosensors12070138
Chicago/Turabian StyleHasan, Md Sajid, Sangpil Kim, Chaelyeong Lim, Jaeeun Lee, Min-Seok Seu, and Ja-Hyoung Ryu. 2024. "Organelle Targeting Self-Assembled Fluorescent Probe for Anticancer Treatment" Chemosensors 12, no. 7: 138. https://doi.org/10.3390/chemosensors12070138
APA StyleHasan, M. S., Kim, S., Lim, C., Lee, J., Seu, M.-S., & Ryu, J.-H. (2024). Organelle Targeting Self-Assembled Fluorescent Probe for Anticancer Treatment. Chemosensors, 12(7), 138. https://doi.org/10.3390/chemosensors12070138








