Abstract
Gas-sensing technology is crucial for the detection of toxic and harmful gases to ensure environmental safety and human health. Gas sensors convert the changes in the conductivity of the sensing material resulting from the adsorption of gas molecules into measurable electrical signals. Rare earth orthoferrite-based perovskite oxides have emerged as promising candidates for gas-sensing technology owing to their exceptional structural, optical, and electrical properties, which enable the detection of various gases. In this article, we review the latest developments in orthoferrite-based gas sensors in terms of sensitivity, selectivity, stability, operating temperature, and response and recovery times. It begins with a discussion on the gas-sensing mechanism of orthoferrites, followed by a critical emphasis on their nanostructure, doping effects, and the formation of nanocomposites with other sensing materials. Additionally, the role of the tunable bandgap and different porous morphologies with a high surface area of the orthoferrites on their gas-sensing performance were explored. Finally, we identified the current challenges and future perspectives in the gas-sensing field, such as novel doping strategies and the fabrication of miniaturized gas sensors for room-temperature operation.
Keywords:
gas sensor; orthoferrite; nanoparticle; thin film; chemiresistive gas sensor; response; nanocomposite; nanofiber; doping 1. Introduction
Rapid urbanization and advancements in modern industrial products have contributed significantly to air pollution, resulting in the emission of volatile organic compounds (VOCs) from industrial processes, residences, public spaces, and transportation vehicles [1,2]. Additionally, the leakage of flammable and toxic gases further reduces air quality, posing a serious risk to human health [3]. According to the World Health Organization, VOCs are the most common atmospheric pollutants with melting points below room temperature and boiling points between 50 and 260 °C, highlighting the need for efficient monitoring systems [4]. Gas-sensing technologies have become crucial for air quality monitoring, toxic gas detection, and VOC identification [5,6,7,8]. To address these challenges, various gas sensors, including calorimetric, potentiometric, catalytic, chemiresistive, and conductometric-based sensors, have been developed [9]. Effective sensors with a low detection limit, excellent selectivity, long-term stability, high sensitivity, and quick response and recovery times are essential for gas-sensing applications.
Chemiresistive gas sensors involve an active sensing material whose conductivity is highly sensitive to environmental changes. Among chemiresistive sensors, metal oxide semiconductor (MOS) materials have gained prominence owing to their high selectivity, simple fabrication, low production cost, long-term stability, and potential for miniaturization [10]. The sensing mechanism of MOS gas sensors is based on the adsorption of gas molecules onto the surface of the material, which affects the concentration of charge carriers and alters their electrical resistance [11,12]. The performance of chemiresistive sensors can be evaluated using key metrics such as the detection limit, response and recovery times, sensitivity, and selectivity. These parameters are usually influenced by various factors such as the operating temperature, concentration of the target gas, and environmental conditions such as humidity. Among these factors, the operating temperature plays a critical role in determining the efficiency and reliability of the sensor because it affects its conductivity and surface reactions. However, the requirement for high operating temperatures limits their practical application owing to their energy efficiency, reduced stability, and risks in flammable environments [13]. Long-term exposure to high temperatures can also degrade the microstructure of sensing materials, thereby reducing their performance [14].
Perovskite oxides with an ABO3 structure demonstrate superior gas-sensing performance compared with individual metal oxide semiconductors, primarily because of their ability to generate oxygen vacancies, which are essential for gas detection [15,16]. These materials have significant potential in various optoelectronic applications owing to their unique physicochemical properties and tunable electronic conductivities [17]. Furthermore, the ability of perovskite structures to accommodate a variety of dopants with different ionic radii at A or B sites enables modifications to charge carrier concentrations and band gap widths without altering the crystal lattice, thus improving their gas-sensing performance [18,19,20].
Rare earth orthoferrites (i.e., RFeO3, where R indicates a rare earth element) have demonstrated potential applications in areas such as optoelectronics [21], solid oxide fuel cells [22], magneto-optic devices [23], magnetic refrigeration [24,25], spintronics [26], and gas sensors [27,28] owing to their unique optical, electrical, and magnetic properties [29]. The excellent chemical stability and catalytic properties of orthoferrites allow them to effectively adsorb gases and cause changes in resistance when exposed to specific gases [15,30]. Typically, perovskite oxides are synthesized through a mechanical process followed by high-temperature heat treatment. However, this approach often results in the desired perovskite structure with the formation of large aggregated crystal particles with insufficient porosity, which hinders their practical use in gas sensing [31]. A lower porosity limits the active surface area available for interactions with gas molecules, thereby diminishing the efficiency of the gas-sensing reaction [32]. To achieve a better gas-sensing performance, sensor materials with larger specific surface areas are crucial because they provide more active sites for adsorption and interaction with the target gas molecules. Various chemical methods have been adopted to overcome the low porosity of perovskites obtained via mechanical processes. Rare earth orthoferrites, synthesized using different chemical methods such as sol–gel [33], hydrothermal [34], and electrospinning [35], have demonstrated superior gas-sensing performance owing to their enhanced surface area and improved interactions with gas molecules. These techniques enable the fabrication of nanomaterials with precise control over the morphology, composition, and functionality of sensors. To enhance the gas-sensing capabilities of orthoferrites, various strategies, such as doping with different metals at R-sites, Fe-sites, or both sites, the development of composite nanomaterials, and employing light activation [36], have been explored. Light illumination on the sensing material surface can alter the electronic properties of the surface, thereby enhancing the sensing performance. In addition, innovative sensing structures, including nanoparticles with different morphologies [37], nanorods [27], nanotubes [38], nanofibers [39], and nanosheets/thin films [40], have been developed to alter the surface-to-volume ratio and improve gas adsorption and sensing performance. Compared with other gas sensors, the low operating temperatures, high stability, high selectivity, and potential for miniaturization make orthoferrites ideal for creating compact, reliable, and efficient gas sensors.
Perovskite oxides with an ABO3 structure have emerged as a promising candidate for gas-sensing applications due their structural flexibility and tunable physicochemical properties. Among these oxides, RFeO3 compounds exhibit unique structural features, flexible A-site and B-site composition, and unique gas-sensing characteristics compared to other perovskites. Despite the increased research interest on RFeO3-based gas sensors in the recent years, a comprehensive and dedicated review on these compounds is still lacking. Therefore, the aim of this review is to address the gap by providing the detailed overview of the recent developments of RFeO3-based gas sensors, emphasizing their potential for future environmental and industrial applications. This review highlights the recent experimental research on rare earth orthoferrite-based gas sensors featuring different morphologies, with a focus on the enhancement of selectivity, sensitivity, response and recovery time, and stability. This study explores the advantages of doped orthoferrites over their undoped counterparts in gas-sensing applications. It begins by providing an overview of the crystal structures and gas-sensing mechanisms of orthoferrites. This is followed by a discussion of the effect of nanostructures on the enhancement of gas-sensing properties, along with recent progress in orthoferrite-based gas-sensing materials with diverse morphologies, highlighting their practical applications in environmental monitoring. Finally, this review summarizes the findings, addresses current challenges, and explores future prospects, shedding light on the potential advancements and applications of orthoferrite-based gas-sensing technologies.
2. Crystal Structure
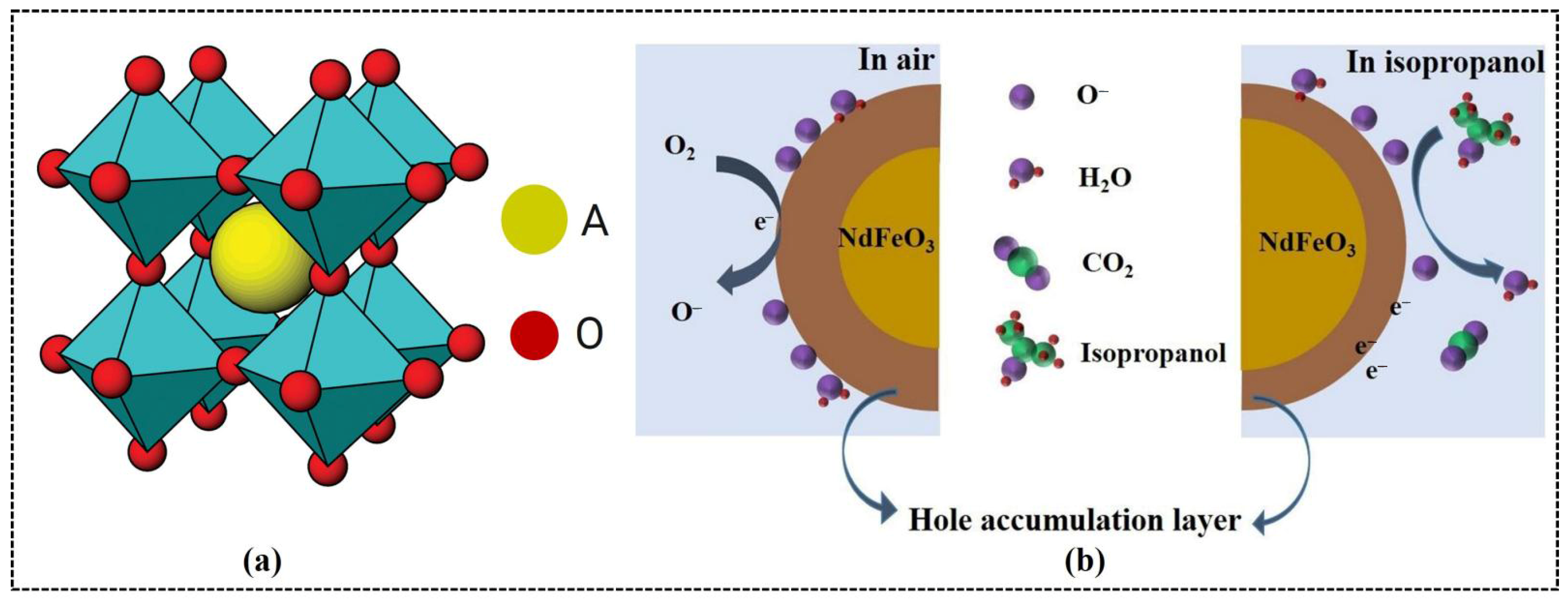
Perovskite metal oxides with the general formula ABO3 (Figure 1a) have attracted considerable attention owing to their unique structural, chemical, and electronic properties [41]. In ABO3, the A-site is typically occupied by rare earth metals (R) with a coordination number of 12, whereas the B-site is generally filled with transition metals with a coordination number of 6. Ideally, the perovskite structure has cubic symmetry at room temperature [42].
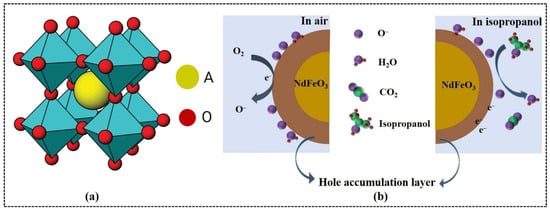
Figure 1.
(a) Ideal cubic perovskite structure for ABO3 (cyan, BO6 units). (b) Gas-sensing mechanism of NdFeO3 sensor. (a) Reproduced with permission [43]. Copyright 2012, American Chemical Society. (b) Reproduced with permission [34]. Copyright 2022, Elsevier B.V.
The perovskite oxides exhibit an extraordinary capability to incorporate 90% of the metallic elements into their lattices in a stable and effective manner [24,44,45]. This has resulted in the development of a wide range of functional materials with tunable physical properties for specific applications. The stabilities of the perovskite oxides were assessed using the Goldschmidt tolerance factor (t):
where rA, rB, and rO are the ionic radii of the A-site cations, B-site cations, and oxygen anions, respectively. For an ideal cubic structure, t = 1, whereas it is in the range of 0.71 to 0.89 for an orthorhombic structure due to a mismatch in the ionic radii of the A- and B-cations [46].
Perovskite oxides with rare earth metals (R) at the A-site have become the focus of electrochemical sensors, such as potentiometric and amperometric sensors, during the late 20th and early 21st centuries [47]. Later, these materials were explored for use in chemiresistive gas sensors, which exhibit a change in resistance owing to gas adsorption [8,48]. The strategy of doping at the A- and B-sites demonstrated significant changes in the gas-sensing performance, making perovskite oxides a highly flexible platform for advanced material design.
Among perovskite oxides, RFeO3 materials have been extensively explored for gas-sensing applications, particularly owing to their high stability, superior electrical properties, and catalytic behavior [18,19,20]. Orthoferrite-based gas sensors have demonstrated an ability to detect various toxic and harmful gases with efficient performance metrics, such as low detection limits, rapid response and recovery times, and stable operation under harsh environmental conditions [27]. The feasibility of substituting different cations at the R-site, Fe-site, or both sites enables the development of gas sensors with improved sensitivity, selectivity, and response/recovery times [49,50]. In this paper, we review orthoferrite-based chemiresistive sensors in different forms at the nanoscale and discuss the changes in gas-sensing behavior after doping at the R-site, Fe-site, or both sites.
3. Gas-Sensing Mechanism
The gas-sensing mechanism of perovskite oxides can be understood from the interaction between the gas molecules and sensor material in terms of a change in the resistance of the sensing material. Changes in the electrical properties of the material result from the adsorption and desorption of gas molecules on the surface of the sensor upon exposure to the target gas. These interactions provide a deep understanding of the charge distribution, carrier concentration, and localized electrical behavior of the sensing material, which eventually influences its sensing mechanism.
Rare earth orthoferrites exhibit a p-type semiconducting behavior, with holes as the predominant charge carriers [51]. These holes, generated by the ionization of R3+ cation vacancies in the crystal lattice, are crucial to the sensing mechanism. Orthoferrites absorb oxygen molecules onto their surfaces from an air environment (left picture of Figure 1b). The strong electron affinity of oxygen leads to interactions with free electrons on the surface of RFeO3 and generates chemisorbed oxygen species, such as O2−, O−, and O2− [52,53]. The detailed reactions are as follows:
O2(gas) → O2(ads)
O2(ads) + e− → O2−(ads) (T < 100 °C)
O2−(ads) + e− → 2O2−(ads) (100 °C < T < 300 °C)
O−(ads) + e− → O2−(ads) (T > 300 °C)
In this process, electrons are transferred from the surface of RFeO3 to the oxygen species, which increases the concentration of holes on the surface and the width of the hole accumulation layer (left picture of Figure 1b). This modification of the electronic properties of the surface alters the electrical resistance of the material. In a gas environment, the response of the RFeO3 sensor depends primarily on the interaction between the gas molecules and chemisorbed oxygen species. Such interactions modify the hole accumulation layer near the surface (Figure 1b) as well as the mobility of the charge carriers within the semiconductor, which directly affects the resistance [34].
The sensing process in RFeO3 relies on measuring the resistance change resulting from the adsorption and desorption of gas molecules on their surface. Adsorption alters resistance, which is measured and characterized by sensing parameters such as sensitivity, selectivity, response, and recovery time. The desorption phase is equally important, because it allows the sensor to return to its original electrical state by releasing gas molecules from its surface. This regeneration is crucial for repeated use and ensures consistent performance in subsequent detection cycles.
4. Gas-Sensing Parameters
4.1. Operating Temperature
The temperature at which the sensor delivers the highest response to the target gas is known as the optimal operating temperature [6]. At this temperature, the interaction between the sensor surface and target gas reaches an equilibrium state, ensuring the maximum sensitivity and efficiency of the sensor. Typical operating temperatures for orthoferrite-based gas sensors range from 110 to 350 °C. At room temperature, the reaction between the gas molecules and the sensor surface is too slow or may not happen. That is why heating the sensor is required to promote the surface reactions. Sensors with low operating temperatures are more important for practical applications than those with excessively high operating temperatures because they do not require additional heating elements, making them suitable for portable and low-power applications. Consequently, developing sensors that exhibit high efficiency at room temperature remains a challenge in advancing gas-sensing technology.
4.2. Response and Recovery Time
Response and recovery times are critical parameters for evaluating the performance of a gas sensor because they directly reflect the sensor’s ability to respond and detect changes in the presence of the target gas. The response time indicates how quickly the sensor can respond to the gas, resulting in a stable signal (usually defined as 90% of the full response) when transitioning from an air environment to the target gas environment [6]. By contrast, recovery time refers to the duration required for the sensor to stabilize back to 90% of its original value after the gas is removed and the environment is restored to air. Sensors with faster response and recovery times are more effective in providing early warnings to minimize the risks and potential damage. Response and recovery times of RFeO3-based gas sensors vary widely with a quick response time of under 10 s to several minutes, depending on gas type, concentration, and nanostructure.
4.3. Selectivity
The selectivity of a gas sensor is the ability of the sensor to identify and respond to a specific target gas while minimizing interference from other gases present in the environment. This parameter is evaluated by exposing the sensor to various gases under identical operating conditions. If the sensor demonstrates an excellent response to a particular gas compared to other gases, it is considered to have excellent selectivity for that gas.
4.4. Response
The response of a gas sensor is defined as the ratio of its resistance in air (Ra) to that in the target gas environment (Rg) [6]. For p-type semiconductor gas sensors, the response to reducing gases is often calculated as S = Rg/Ra, whereas, for oxidizing gases, the response is S = Ra/Rg. By contrast, the response can be expressed in percentage form: S = (Rg − Ra)/Rg × 100% for reducing gases, and S = (Ra − Rg)/Ra × 100% for oxidizing gases. Conversely, the definition of the gas response value is reversed for n-type semiconductor gas sensors.
4.5. Sensitivity
The sensitivity of a gas sensor, reflected by its response to an exposed gas, enables the detection of a specific gas by measuring the changes in its electrical properties. The sensitivity of a gas sensor can be extracted from the slope of the response–concentration fitting curve [15,54]. It indicates the strength of the gas sensor’s ability to detect the gas by measuring the rate of change in the sensor’s response with varying gas concentrations.
4.6. Limit of Detection
The limit of detection (LOD) of a gas sensor is the lowest concentration of gas that can be detected reliably. A gas sensor with a low LOD is essential for detecting toxic and harmful gases at an early stage before creating a panic. A sensor with higher sensitivity and faster response time may easily detect trace amounts of the target gases. The long-term use of a sensor under harsh conditions may degrade its sensitivity, leading to an increase in the LOD. The LOD of RFeO3-based gas sensors varies depending on the sensing material and the target gas, with several sensors achieving in the range of 1 ppb to 5 ppm.
4.7. Stability
The stability of a gas sensor is a key parameter that reflects its ability to provide consistent and reliable responses to a gas with a fixed concentration over an extended period, without significantly deviating from its initial value. Repeated measurements should be conducted over time to assess the sensor stability. Sensors with excellent stability maintain their performance even under different environmental conditions such as temperature and humidity.
5. Nanostructures
Recently, nanostructured materials have demonstrated remarkable gas-sensing capabilities compared to their bulk counterparts [55]. The dimensions of the nanostructures and their chemical properties play a vital role in determining the performance of the sensor in terms of sensitivity, selectivity, stability, and response/recovery times. The smaller size of the nanostructure provides a more active surface area for gas molecules to interact with the sensing material, thereby enhancing the performance. In addition, a higher surface-to-volume ratio of nanostructures can significantly improve their sensitivity towards targeted gas molecules.
Nanomaterials can be classified into different categories such as zero-dimensional nanoparticles, one-dimensional nanorods/nanotubes/nanofibers, two-dimensional nanosheets/thin films, and three-dimensional nanocrystals. These dimensional differences significantly influence the gas-sensing performance of nanomaterials. Orthoferrites have been widely explored for gas-sensing applications because of their high specific surface areas, porosities, and chemical reactivities. For example, the smaller particle size of a (La0.8Ca0.2)0.6Bi0.4FeO3 sensor demonstrated superior gas-sensing performance compared to a sensor with a larger particle size [56]. The unique channel-like structure of GdFeO3 nanorods favors the easy diffusion of gas molecules and enhances their sensitivity compared with GdFeO3 butterflies [27]. The strain effects dominated with the increase in the thickness of the LaFe0.8Co0.2O3 thin films, leading to a better response to CO at a lower thickness [57]. Additionally, external factors such as the operating temperature, humidity, and concentration of the target gas can influence the sensing capabilities. Nanostructured gas sensors based on orthoferrites have been fabricated using strategic synthesis methods [57,58] to alter the size, morphology, and crystallographic structure of the sensor, which can significantly affect its response. The exceptional advantages of these methods for controlling the size, shape, and arrangement of nanoparticles are helpful for optimizing the performance of gas sensors. In the following sections, we provide a comprehensive review of the latest research findings on rare earth orthoferrite-based gas sensors with various nanostructured morphologies. Table 1, Table 2, Table 3 and Table 4 summarize the important gas-sensing parameters of orthoferrites from the existing literature.

Table 1.
Summary of orthoferrite-based nanoparticle gas sensors reported in the literature.
5.1. Nanoparticles
Orthoferrites in the form of nanoparticles have been prepared through various suitable synthesis methods such as sol–gel [33,64,81], hydrothermal [34,37], citrate–nitrate auto-combustion [82], microwave thermal treatment [83], microwave-assisted aqueous solution [84], and solvothermal [62] methods through a series of steps. The first step is to mix the appropriate ratio of metal salts with basic precipitants to form a precipitate. Next, the precipitate is washed and dried to convert it into nanoparticles. Finally, heat treatment or surface modification techniques are used to refine the morphology and properties of the nanoparticles. The size of the nanoparticles and nanocrystals is influenced less by the choice of synthesis method and more by the preparation and control of the salt solution. However, the synthesis method significantly influences the morphology of the resulting nanoparticles [82].
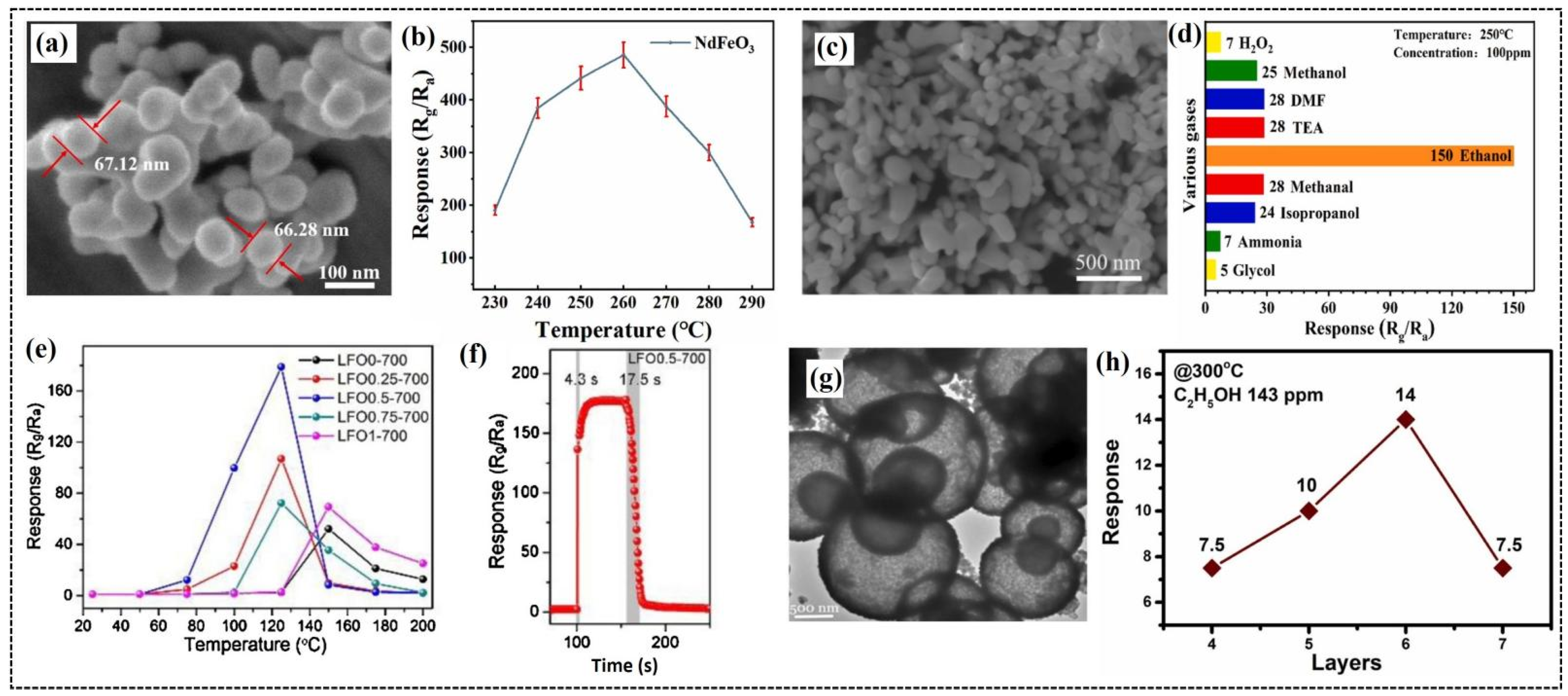
Guo et al. reported the excellent gas-sensing performance of NdFeO3 nanoparticles synthesized using a hydrothermal method, which resulted in a granular morphology with an average size of 65 nm [37] (Figure 2a). These nanoparticles demonstrated exceptional gas-sensing properties for n-butanol detection, with a high response of 441 to 100 ppm at an optimal operating temperature of 260 °C and a low detection limit of 0.024 ppm. The response of the sensor initially increased with an increasing temperature, reaching a high value at 260 °C, beyond which it started decreasing (Figure 2b). At lower temperatures, insufficient activation energy prevents effective interactions between the chemisorbed oxygen species and gas molecules. By contrast, the evaporation of adsorbed gas molecules intensifies at higher temperatures, reducing the number of adsorbed gas molecules and leading to a decrease in the response of the sensor. The sensor exhibited excellent selectivity for n-butanol over other gases, such as ethanol, isopropanol, triethylamine, and ammonia, along with rapid response and recovery times of 33 and 7 s, respectively. It also exhibited consistent repeatability across six cycles, stable performance over 15 days, and a response value of 84 at 50% relative humidity. The outstanding performance of the sensor was attributed to the large surface area of the nanoparticles, oxygen vacancies, and unique electronic characteristics of the perovskite structure, which was further supported by X-ray photoelectron spectroscopy (XPS) analysis, highlighting the potential of this system for efficient gas-sensing applications.
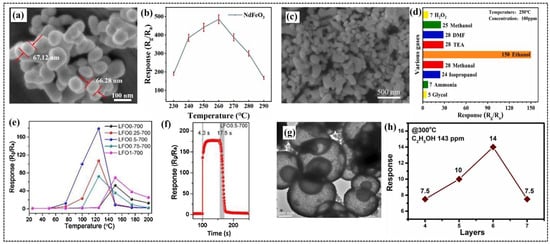
Figure 2.
(a) Scanning electron microscopy (SEM) image of NdFeO3 nanoparticles. (b) Responses to 100 ppm of n-butanol at different operating temperatures from 230 to 290 °C. (c) High-resolution SEM image of NdFeO3 nano-coral granules. (d) Response of NdFeO3 sensor to different test gases (concentration of 100 ppm) at 250 °C. (e) Sensing responses of LFO0-700, LFO0.25-700, LFO0.5-700, LFO0.75-700, and LFO1-700 were detected under the temperature range from 25 to 200 °C toward 100 ppm of formaldehyde. (f) Response/recovery curves of LFO0.5-700 sensor to 100 ppm of formaldehyde at the optimal operating temperature. (g) Transmission electron microscopy (TEM) image of LFO-HS. (h) Response of x-layer (x = 4, 5, 6, or 7) LFO-HS gas sensors to 143 ppm of C2H5OH at 300 °C. (a,b) Reproduced with permission [37]. Copyright 2024, Elsevier B.V. (c,d) Reproduced with permission [62]. Copyright 2021, Elsevier B.V. (e,f) Reproduced with permission [8]. Copyright 2020, Elsevier B.V. (g,h) Reproduced with permission [63]. Copyright 2021, Elsevier B.V.
Liu et al. fabricated a LaFeO3 nanopowder-based planar electrode gas sensor to reduce the operating temperature [36]. They demonstrated an enhancement in the performance of the sensor under illumination with multiwavelength light (370, 420, and 470 nm). Among the sensors with various annealing temperatures (500–900 °C), the sensor that was annealed at 700 °C exhibited superior performance at a relatively low operating temperature of 110 °C compared with previously reported sensors [33]. Light illumination studies revealed that, when the sensor was exposed to 30 ppm of ethanol and methanol, the sensor response reached 121.6 and 59.7, respectively, without light illumination. However, under illumination with 370 nm light, the response increased to 154.5 for ethanol and 109.3 for methanol. The UV–visible absorption spectra and energy band analysis further confirmed that the photons at 370 nm exceeded the band gap of LaFeO3, facilitating higher photon-energy-assisted electron transitions, thereby reducing the resistance and enhancing the sensitivity of the sensor.
In a separate study conducted by Chai et al., LnFeO3 (Ln = Nd, Dy, Er) nanoparticles were prepared using a microwave-assisted hydrothermal method, and the influence of rare earth elements on the gas-sensing performance was explored [34]. Nitrogen adsorption/desorption studies revealed that the specific surface area of NdFeO3 (8.142 m2/g) was smaller than those of DyFeO3 (9.679 m2/g) and ErFeO3 (9.800 m2/g). Gas sensors were fabricated on Al2O3 substrates using Pt/Au electrodes and were tested for isopropanol sensing. Among the sensors, the NdFeO3 gas sensor demonstrated the highest response at 275 °C for 2 ppm of isopropanol, which is attributed to its lowest Ln-O binding energy [51] and high oxygen defect concentration, which was confirmed by the XPS analysis that showed values of 56.4%, 54.1%, and 54.7% for NdFeO3, DyFeO3, and ErFeO3, respectively. A higher concentration of oxygen vacancies provides more active sites for gas adsorption, whereas the lowest binding energy facilitates the breaking of Ln-O bonds, allowing the generation of atomic vacancies in the crystal structure. These surface vacancies promote greater adsorption of oxygen species on the material surface, significantly enhancing the interactions with gas molecules and resulting in a high performance of the gas sensor. Cao et al. reported the influence of raw materials such as ethylene glycol (EG) and polyethylene glycol (PEG) and the incorporation of pre-calcination on the ethanol-sensing properties of LaFeO3−δ nanoparticles [67]. The sensor synthesized using EG as the raw material, combined with a pre-calcination step at 400 °C prior to the final calcination at 600 °C (EG400 + 600) exhibited a significantly enhanced sensing performance at 112 °C due to a higher Fe/La ratio at the surface.
Sheng et al. investigated the potential of NdFeO3 nano-coral granules (Figure 2c), prepared via a solvothermal method, for detecting VOCs [62]. The sensor exhibited a response value of 150 to 100 ppm of ethanol (Figure 2d) with a response time of 19 s and recovery time of 22 s at 250 °C. The high specific surface area of the nano-coral granules provides more active sites on the material surface and promotes oxygen adsorption. Additionally, the porous structure of the sensor facilitates the easy diffusion of gas molecules through the interior of the material and enhances its sensitivity towards gas sensing. Yang et al. prepared ErFeO3 nanoparticles through a hydrothermal method followed by a calcination and an acidification procedure [65]. The rough surface of the nanoparticles played an important role in enhancing the sensitivity of the gas sensor [85]. This sensor exhibited optimal performance at 270 °C, demonstrating high sensitivity and repeatability for isopropanol detection, with a rapid response time of 6 s and a recovery time of 25 s at 100 ppm. The sensor also demonstrated remarkable sensitivity to isopropanol, exhibiting a response of 2.31 even at a low concentration of 2 ppm.
Yang et al. reported the fabrication of a LaFeO3 gas sensor with a hierarchical porous nanostructure synthesized using F108 as the structuring agent, followed by annealing at 700 °C [8]. The fabricated sensors were designated as LFO0-700, LFO0.25-700, LFO0.5-700, LFO0.75-700, and LFO1-700, based on the quantity of F108 used during synthesis, ranging from 0.00 to 1.00 g, and explored this sensor for the trace detection of formaldehyde. The specific surface area of the nanostructures increased with increasing F108 content, reaching the highest value for LFO0.5-700 at 0.5 g of F108. This sensor exhibited the highest response of 178 (Figure 2e) for 100 ppm of formaldehyde, which is 3.5 times higher than LFO without F108 (LFO0-700), and at a relatively low operating temperature of 125 °C compared to 150 °C for other sensors. The sensor demonstrated faster response and recovery times of 4.3 and 17.5 s (Figure 2f), respectively, with reliable stability over 15 weeks. The superior performance of the sensor was attributed to the addition of F108, which improved its structural properties and sensing capabilities, thereby opening a feasible avenue for the fabrication of oxides with low operating temperatures and enhanced sensing performance.
As mentioned previously, the morphology of the sensing material significantly affects its gas-sensing properties. To explore this, Phan et al. conducted a comparative study of the gas-sensing properties of hollow core and porous shell LaFeO3 (LFO-HS) nanoparticles synthesized through a facile hydrothermal method and bulk LaFeO3 (LFO) prepared using a solid-state reaction [63]. Morphological studies revealed a unique hollow core structure surrounded by a porous shell for the LFO-HS nanoparticles (Figure 2g), while irregular clumps were observed for bulk LFO. Nitrogen adsorption–desorption studies revealed a higher surface area for LFO-HS (44.7 m2/g) compared to LFO (16.7 m2/g). The precursor solution prepared by dispersing 20 mg of LFO-HS and LFO in 1 mL of dimethylformamide was used to fabricate a six-layer gas sensor by drop coating onto a Pt electrode-patterned SiO2/Si substrate. The hollow core and porous shell structures were retained in the sensor film, promoting the sensor response to the target gas. The sensor with six-layer LFO-HS demonstrated significantly higher response (Figure 2h) to 143 ppm of ethanol at 300 °C compared to the six-layer LFO sensor, which is attributed to the larger active surface area of LFO-HS, which promotes efficient gas adsorption. The sensor also exhibited excellent selectivity for ethanol over acetone and ammonia. The sensor demonstrated an ability for the trace-level detection of ethanol, and its porous structure resulted in longer response and recovery times.
Zhang et al. reported the gas-sensing properties of LnFeO3 (Ln = La, Pr, Nd, or Sm) nanoparticles synthesized using the citrate sol–gel method [64]. All these compounds exhibited a p-type semiconducting behavior, with an optimal gas-sensing performance at 210 °C. The sensors demonstrated sensitivity to various volatile sulfur compounds (VSCs), with the highest response to dimethyl disulfide (DMDS) followed by methyl sulfide (DMS), hydrogen sulfide (H2S), and carbon disulfide (CS2), which was attributed to differences in adsorption energies. The increased response with respect to DMDS concentration is attributed to an increase in the reaction rate of DMDS with chemisorbed oxygen [86]. The cross-interference effects between the noise factors NO2, CO, and EtOH on the performance of the LnFeO3 gas sensors were studied using DMDS as the optimal representative gas. The response values of the LaFeO3, PrFeO3, NdFeO3, and SmFeO3 sensors varied by ±20%, ±10%, ±10%, and ±5%, respectively, in the presence of interfering gases. However, the overall response trends of the four sensors were unaffected by this interference.
5.1.1. A-Site Doping
Rare earth orthoferrites have attracted considerable attention for diverse applications [87,88] because of their stability, abundant oxygen vacancies, and tunable rare earth and iron sites. Doping RFeO3 compounds significantly affects their structural, optical, and electrical properties.
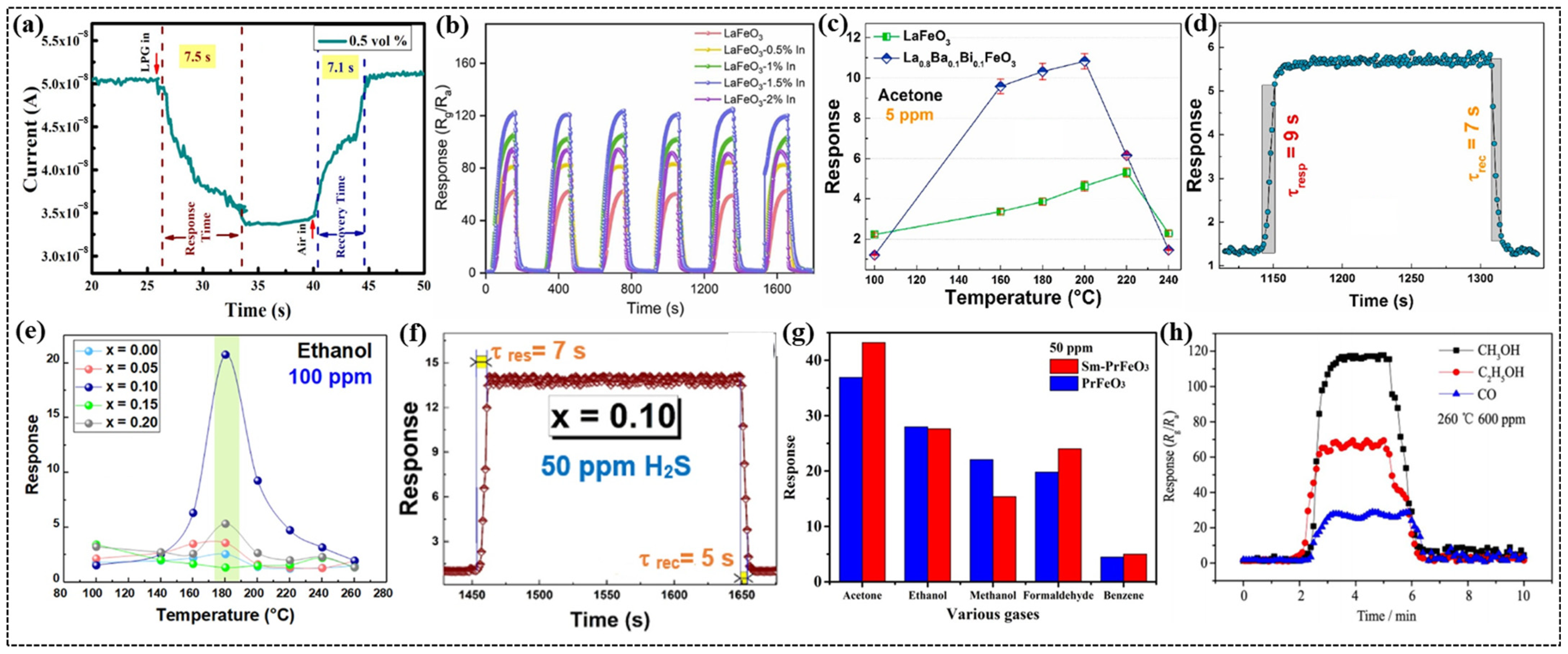
Liquified petroleum gas (LPG), which is highly flammable and widely used as a fuel for cooking, heating, and transportation, causes respiratory issues. Most LPG sensors operate at high temperatures, limiting the efficiency and lifespan of the sensing materials and highlighting the need for low-temperature-operating sensors [89]. Bharati et al. investigated the impact of bismuth doping on the room-temperature LPG-sensing properties of PrFeO3 nanomaterials [68]. The specific surface area of the nanomaterials increased significantly with Bi doping, ranging from 16.331 to 37.645 m2/g, while the band gap decreased from 2.27 to 1.95 eV upon doping. This study highlighted the enhanced sensing performance due to Bi doping, with the Pr0.8Bi0.2FeO3 composition achieving the highest response. This sensor demonstrated rapid response and recovery times of 15.3 and 22.4 s, respectively, for 0.5 vol% LPG because of its mesoporous structure that promotes gas adsorption. The increased sensitivity and reliable performance across multiple cycles were attributed to the higher specific surface area and creation of additional active sites for oxygen adsorption, which facilitated enhanced electron transfer during gas sensing. In another study, Bharati et al. demonstrated that the incorporation of Ca ions into PrFeO3 nanoparticles significantly improved LPG sensing performance [28]. The Pr0.8Ca0.2FeO3 sensor exhibited the highest response of 1.3 with quicker response and recovery times of 7.5 and 7.1 s (Figure 3a), respectively. The reproducibility, high sensitivity, and fast response and recovery times of the sensor underscore its potential for use in LPG sensing technologies.
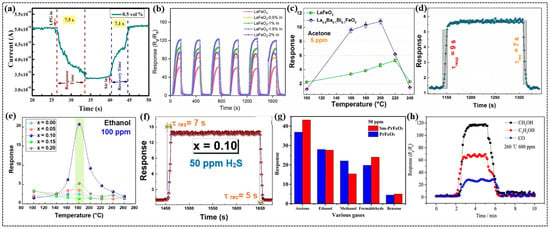
Figure 3.
(a) Transit sensing graph of Pr0.8Ca0.2FeO3 composition. (b) Reproductive cycles of sensor responses based on pure, 0.5%, 1%, 1.5%, and 2% In-doped LaFeO3 to 100 ppm of formaldehyde at 125 °C. (c) Response vs. temperature of the LaFeO3- and La0.8Ba0.1Bi0.1FeO3-based sensors towards acetone gas. (d) Response (τresp) and recovery (τrec) times of the La0.8Ba0.1Bi0.1FeO3 gas sensor to 1000 ppb of acetone gas. (e) Response vs. temperature of La1–2xBaxBixFeO3 (x = 0.00, 0.05, 0.10, 0.15, or 0.20)-based sensors. (f) Response and recovery time of the La0.8Ba0.1Bi0.1FeO3 gas sensor to 50 ppm H2S. (g) Response to various test gases (concentration of 50 ppm). (h) Response and recover characteristics of Gd0.9Ca0.1FeO3 for different gases at 260 °C. (a) Reproduced with permission [28]. Copyright 2024, American Chemical Society. (b) Reproduced with permission [49]. Copyright 2022, Elsevier B.V. (c,d) Reproduced with permission [69]. Copyright 2024, Elsevier B.V. (e,f) Reproduced with permission [70]. Copyright 2021, Elsevier B.V. (g) Reproduced with permission [71]. Copyright 2020, Elsevier B.V. (h) Reproduced with permission [73]. Copyright 2017, Elsevier B.V.
Xiao et al. developed a highly sensitive and selective formaldehyde (HCHO) gas sensor based on an In-doped LaFeO3 porous structure with an ultralow detection limit of 1 ppb [49]. Gas-sensing studies revealed that indium doping significantly enhanced sensor performance, where 1.5% In-doped LaFeO3 exhibited the highest response of 122 (Figure 3b) for 100 ppm formaldehyde at 125 °C, nearly double the response of pure LaFeO3. This sensor demonstrated fast response and recovery times of 36 and 40 s, respectively, which were ascribed to the increased surface area and porous morphology introduced by In doping. The incorporation of In ions generated a higher concentration of oxygen vacancies, providing more active sites for oxygen adsorption, thereby achieving an ultralow detection limit. Density functional theory calculations further revealed that the chemisorption of HCHO on the In-doped LaFeO3 surface was stronger than that on pristine LaFeO3, enabling stronger interactions and resulting in an improved response.
Using the auto-combustion method, Benali et al. synthesized La0.8Ba0.1Bi0.1FeO3 nanoparticles and explored their potential for sensing parts per billion quantities of acetone gas [69]. This synthesis method produced crystalline nanoparticles with smaller crystallite sizes than those of LaFeO3. Positron annihilation lifetime spectroscopy (PALS) revealed a high concentration of vacancy defects in the material. The sensor response to acetone increased with the operating temperature, peaking at 200 °C and then decreasing (Figure 3c), and this trend is attributed to the interplay between the temperature-dependent activation energy of gas molecules and the surface reaction. This sensor exhibited a fast response time of 9 s and a recovery time of 7 s (Figure 3d) to 1000 ppb of acetone because of the increased rate of gas–molecule interactions.
Benali et al. investigated the effect of particle size on the gas-sensing properties of (La0.8Ca0.2)0.6Bi0.4FeO3 (LCBFO) compounds synthesized using various methods [56]. The sol–gel (SG) method resulted in LCBFO-SG nanoparticles with a smaller crystallite size (32.469 nm) than LCBFO-SS nanoparticles (65.566 nm) prepared using a solid-state (SS) reaction method. The lower activation energy 0.369 eV of LCBFO-SG compared to 0.427 eV of LCBFO-SS, indicates easier carrier transport in the SG compound. Both sensors demonstrated different optimal operating temperatures for different gases such as 220 °C for ethanol, 200 °C for acetone, and 160 °C for H2S. The rapid response and recovery times and high sensitivity of the LCBFO-SG sensor were attributed to its smaller particle size. The LCBFO-SG sensor exhibited higher response for H2S at 160 °C while showing a similar response to ethanol and acetone at 200–220 °C, indicating its potential use in multi-gas detection applications.
Using the auto-combustion method and glycine as fuel, Benali et al. synthesized La1–2xBaxBixFeO3 (0 ≤ x ≤ 0.20) nanoparticles and studied their gas-sensing performance [70]. X-ray diffraction (XRD) analysis revealed the single-phase formation for the compounds with x ≤ 0.10, while secondary phases appeared for x = 0.15 and 0.20. Atomic force microscopy studies revealed that the compound with x = 0.10 exhibited the most homogeneous surface roughness, facilitating the generation of more adsorption sites to enhance the sensitivity of the sensor. The La0.8Ba0.1Bi0.1FeO3 sensor demonstrated the highest response of 21 to 100 ppm of ethanol (Figure 3e) at a significantly lower operating temperature of 180 °C [80,90,91]. The sensor exhibited rapid response and recovery times of approximately 10 and 6 s, respectively, for 50 ppm of ethanol. Furthermore, it showed the highest response to H2S gas at 200 °C and very short response and recovery times of 7 and 5 s (Figure 3f), respectively, for 50 ppm of H2S.
Pei et al. employed a hydrothermal method to prepare Sm-doped PrFeO3 nanoparticles with a coral-like morphology [71]. UV–Vis spectroscopy revealed that Sm doping reduced the band gap and enhanced the conductivity of the sensor. The Sm-PrFeO3 sensor demonstrated the highest response to acetone at 270 °C, which was 10 °C lower than the operating temperature of the undoped PrFeO3 sensor (Figure 3g). This sensor exhibited exceptional selectivity for acetone over other gases such as ethanol, methanol, formaldehyde, and benzene, with a response of 44.94 at 50 ppm. The sensor also showed significantly faster response and recovery times of 15 and 16 s, respectively, which are attributed to the cage-like morphology and lower bandgap value achieved via doping. The system exhibited a low concentration detection as low as 5 ppm, stability over five cycles, and retained its response under a 71% relative humidity, making it suitable for acetone detection.
Panpan et al. examined the effect of Ca doping on the CO2 gas-sensing properties of Yb1−xCaxFeO3 (x = 0, 0.1, 0.2, 0.3) nanoparticles [72]. The sensors were fabricated by mixing Yb1−xCaxFeO3 powders with deionized water to form a paste, which was then packed into an alumina ceramic tube with two Au electrodes on both sides. The Yb0.8Ca0.2FeO3 sensor demonstrated the highest response to 5000 ppm of CO2, at 260 °C. In another study, Zhang et al. reported a high sensitivity of a Yb0.8Ca0.2FeO3 sensor towards 0.5 ppm of acetone with response and recovery times of 29 and 55 s, respectively [74]. Xiaofeng et al. explored nanocrystalline Gd1−xCaxFeO3 sensors for methanol gas detection [73]. Among the sensors studied, the Gd0.9Ca0.1FeO3 sensor exhibited the best response to methanol compared to other gases, such as ethanol, CO, and formaldehyde (Figure 3h). The sensor demonstrated responses of 117.7, 72.7, and 31.9 to 600 ppm of methanol, ethanol, and CO gases, respectively, at 260 °C.
5.1.2. B-Site Doping
The exceptional thermal and chemical stability of orthoferrites, combined with their adaptability to incorporate various metals at Fe-sites, introduces structural point defects, such as vacancies and variations in oxygen stoichiometry, which influence the electronic structure of the active sites and significantly improve their gas-sensing properties [41].
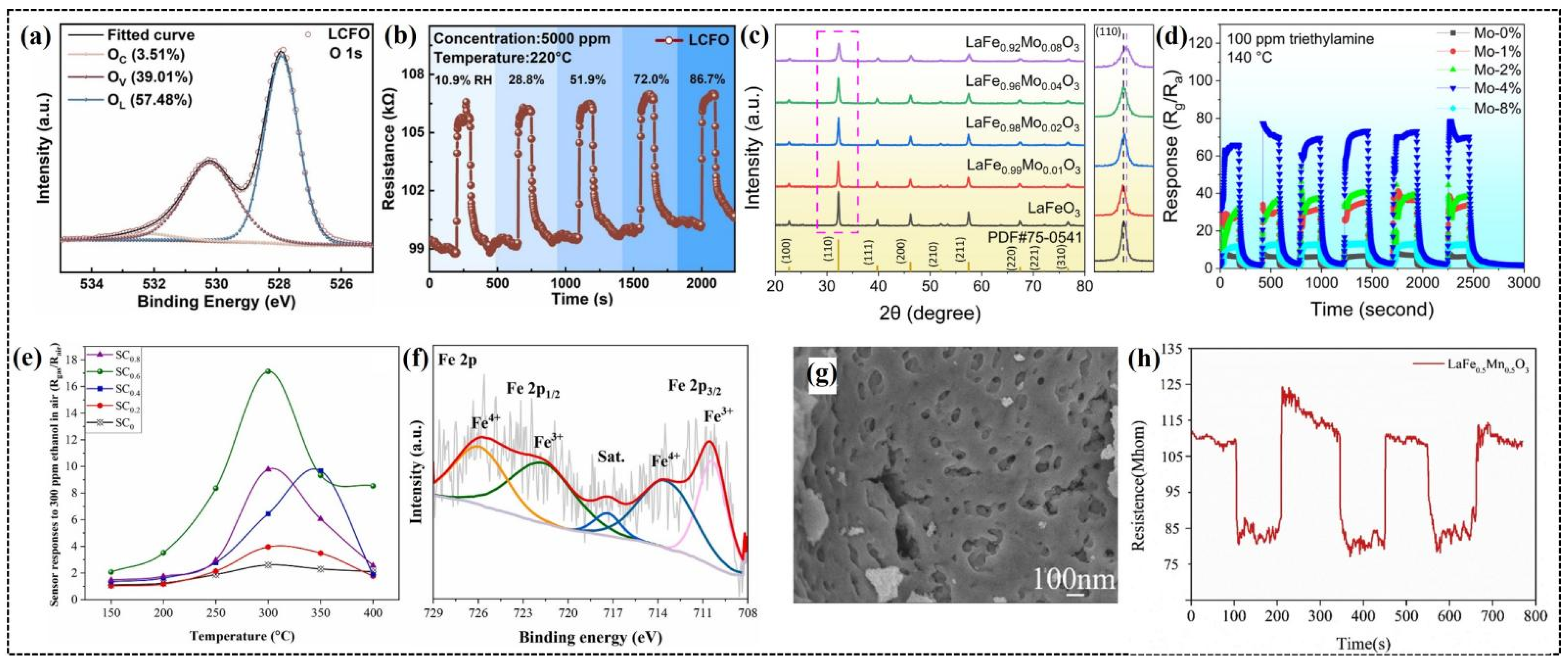
Duan et al. reported the enhanced gas-sensing performance of Co-doped LaFeO3 nanoparticles [15]. The incorporation of Co ions in LaCo0.1Fe0.9O3 (LCFO) reduced the agglomeration of nanoparticles compared to undoped LaFeO3 (LFO), providing more active sites for gas adsorption onto the surface of the sensing material. Co doping significantly increased the proportion of oxygen vacancies in LCFO (39.01%) (Figure 4a) compared to LFO (32.71%), which enhanced gas sensing by facilitating oxygen molecule adsorption and accelerating oxidation–reduction reactions. The LCFO gas sensor demonstrated superior CO2 sensing capabilities, including a wide detection range, fast response and recovery times (6 and 42 s, respectively), and excellent humidity resistance with minimal changes in the response (Figure 4b). Density functional theory (DFT) calculations further revealed that Co doping shifted the Fe d-band center, increasing the adsorption energy of CO2 on Fe sites (from −2.75 to −4.21 eV) and introducing strong adsorption at Co sites (−3.83 eV). The enhanced CO2 sensing mechanism of LCFO compared to that of LFO is attributed to the combination of higher oxygen vacancy concentrations, as confirmed by XPS analysis, and stronger CO2 adsorption.
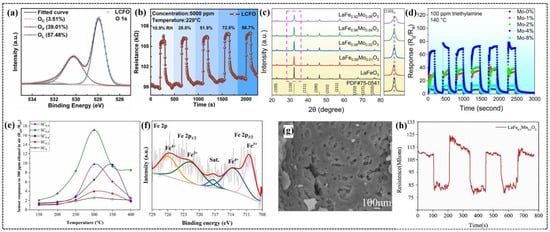
Figure 4.
(a) O 1s XPS spectrums of LaCo0.1Fe0.9O3. (b) Real-time response and recovery curves of the LaCo0.1Fe0.9O3 sensor toward 5000 ppm of CO2 under different RHs. (c) XRD patterns of LaFe1−δMoδO3 materials. (d) Cyclic stability of LaFe1−δMoδO3 sensors to 100 ppm of TEA gas at 140 °C. (e) Response of LaFe1−xCuxO3 (x = 0.0–0.8) gas sensors (SC0–SC0.8) to 300 ppm of ethanol in air at various operating temperatures. (f) XPS analysis of LaFe0.4Cu0.6O3 nano-perovskite (SC0.6), high-resolution XPS scan spectra over iron (Fe 2p). (g) SEM image of LaFe0.5Mn0.5O3 powders. (h) Three-cycle response–recovery test of a LaFe0.5Mn0.5O3 sensor to 50 ppm at 190 °C. (a,b) Reproduced with permission [15]. Copyright 2023, Elsevier B.V. (c,d) Reproduced with permission [50]. Copyright, MDPI. (e,f) Reproduced with permission [75]. Copyright 2024, Elsevier B.V. (g,h) Reproduced with permission [78]. Copyright 2019, Elsevier B.V.
Shen et al. synthesized LaFeO3 nanoparticles with different Mo doping ratios to improve the triethylamine (TEA) gas-sensing performance [50]. XRD confirmed the lattice distortion due to Mo doping, which introduced defects and increased the number of active sites for gas–molecule interactions (Figure 4c). Morphological studies revealed a porous nanosheet structure with a large surface area that promoted efficient gas diffusion. Among the samples, 4% Mo-doped LaFeO3 demonstrated the best performance (Figure 4d), with a response approximately 11 times higher than that of undoped LaFeO3 at a relatively low operating temperature of 140 °C. In addition, the sensor exhibited fast response and recovery times of 12 and 59 s, respectively, with excellent selectivity, stability, and humidity resistance. The superior performance of the sensor was ascribed to a higher oxygen vacancy concentration of 4% Mo-doped LaFeO3 compared to other samples, promoting the adsorption of gas molecules on the surface of the sensor [92], thereby improving the gas-sensing performance.
Derakhshi et al. synthesized Cu-doped LaFe1−xCuxO3 nanoparticles to improve VOC detection [75]. The introduction of Cu significantly improved ethanol sensing performance and reduced crystallite size from 20.5 nm for LaFeO3 to 12.7 nm for LaFe0.4Cu0.6O3. The higher specific surface area of the LaFe0.4Cu0.6O3 sensor led to excellent VOC detection, with responses of 17, 18, and 16 for 300 ppm of ethanol, acetone, and acetaldehyde, respectively, at 300 °C. This sensor demonstrated a 6.5-fold greater response (Figure 4e) to ethanol than pure LaFeO3, primarily due to the mixed valences (Fe4+/Fe3+ and Cu2+/Cu+) and oxygen vacancies (Figure 4f), which generated more active sites for gas adsorption.
Bharati et al. developed a room-temperature carbon dioxide (CO2) sensor using Co-doped PrFeO3 nanoparticles with crystallite sizes ranging from 19 to 23 nm [76]. Morphological studies showed greater agglomeration of the nanoparticles at higher Co contents, likely due to the magnetic interactions between the constituent ions. The PrFe0.9Co0.1O3 sensor exhibited good selectivity for 500 ppm of CO2 at room temperature, with fast response and recovery times of 17.2 and 18.4 s, respectively. Li et al. systematically investigated the effect of Mn doping on the gas-sensing properties of LaFe1−xMnxO3 (x = 0, 0.3, 0.4, 0.5 and 0.6) nanoparticles [78]. A gradual decrease in the lattice parameters and average grain size was evident with an increase in the Mn content, primarily due to the smaller ionic radius of Mn3+ compared to that of Fe3+. SEM analysis revealed that the nanoparticles with x = 0, 0.3, and 0.4 composition appeared compact with smooth surfaces, while a porous morphology emerged at x = 0.5 (Figure 4g), which disappeared at x = 0.6. The LaFe0.5Mn0.5O3 sensor exhibited the highest response to 50 ppm of ethanol at a reduced operating temperature of 190 °C compared to 210 °C for undoped LaFeO3. This enhancement is ascribed to the porous morphology and larger specific surface area of the sensor. Surprisingly, the LaFe0.5Mn0.5O3 sensor exhibited n-type semiconductor behavior (Figure 4h), which is opposite to that of p-type LaFeO3, because of the increased electron concentration resulting from the replacement of Fe3+ ions with Mn3+ ions, leading to the formation of Mn4+ ions [93,94]. The sensor exhibited good selectivity towards ethanol with stable performance over a 40-day testing period and a faster recovery time of 5 s compared with 31 s for the LaFeO3 sensor.
Benali et al. synthesized La0.8Ca0.1Pb0.1Fe0.95Mg0.05O3 (LCPFMO) nanoparticles and examined their gas-sensing properties at different frequencies and temperatures [80]. The LCPFMO sensor demonstrated enhanced ethanol sensitivity with increasing frequency (100 Hz to 1 MHz) and ethanol concentration (250–1000 ppm). This sensor exhibited the highest sensitivity at a frequency of 1 MHz to 1000 ppm ethanol at 225 °C.
5.2. Nanorod/Nanotube/Nanofibers
In the domain of gas sensing, one-dimensional (1D) nanostructures, such as nanorods, nanotubes, and nanofibers, have attracted significant attention over nanoparticles owing to several key advantages. They provide more active sites than nanoparticles to facilitate a greater interaction between the gas molecules and the sensing material, leading to an enhancement in sensitivity [95]. In addition, the unique channel-like structure of nanotubes and nanorods allows for the better diffusion of gas molecules [96]. This structure not only increases the interaction of the target gas molecules with the sensor material but also the detection speed. The high specific surface area of 1D nanostructures further enhances the sensitivity of the sensors [97]. The 1D nanostructure stands out for its excellent electron transport properties, which are crucial for converting the interactions between the gas molecules and the sensor material into detectable electrical signals. The unique structural and electrical properties of 1D nanostructures make them suitable candidates for gas-sensing applications, which is a key area of ongoing research.

Table 2.
Summary of orthoferrite-based nanorod/nanotube/nanofiber gas sensors reported in the literature.
Table 2.
Summary of orthoferrite-based nanorod/nanotube/nanofiber gas sensors reported in the literature.
| Material | Detection Gas | O.T. (°C) | Conc. (ppm) | Response | tres/trec (s) | LOD | Reference |
|---|---|---|---|---|---|---|---|
| NdFeO3 | Trietheylamine | 190 | 100 | 18.9 | 72/35 | [6] | |
| GdFeO3 | N-Propanol | 140 | 100 | 58.113 | 28/28 | 1 ppm | [27] |
| YFeO3 | Ethanol | 350 | 100 | 18 | 19/9 | [35] | |
| LaFeO3 | Ethanol | 160 | 100 | 9.4 | 2/4 | [38] | |
| ErFeO3 | Ethylene glycol | 230 | 100 | 15.8 | 61/39 | 35 ppb | [39] |
| SmFeO3 | Ethylene glycol | 200 | 100 | 14.3 | 47/56 | [98] | |
| Sm0.95Ho0.05FeO3 | Glycol | 200 | 100 | 26.12 | 50/32 | [99] | |
| SmFeO3 | Ethylene glycol | 240 | 100 | 18.19 | 41/47 | [100] | |
| LaFeO3 | Acetone | 120 | 40 | 46.4 | 14/49 | 135 ppb | [101] |
| LaFeO3 | Oxygen | 650 | 5% O2 | 8.3 | 15/18 | [102] | |
| LaFeO3 | Hydrogen sulfide | 150–300 | 0.5–4 | 20–90% | 90–240/300–500 | 500 ppb | [103] |
| PrFeO3 | Acetone | 180 | 200 | 141.3 | 4/4 | [104] | |
| La0.75Ba0.25FeO3 | Ethanol | 210 | 500 | 136.1 | 42/40 | [90] | |
| SmFeO3 | Acetone | 140 | 100 | 9.98 | 17/16 | [105] | |
| Ag-LaFeO3 | Formaldehyde | 230 | 5 | 4.8 | 2/4 | 5 ppm | [106] |
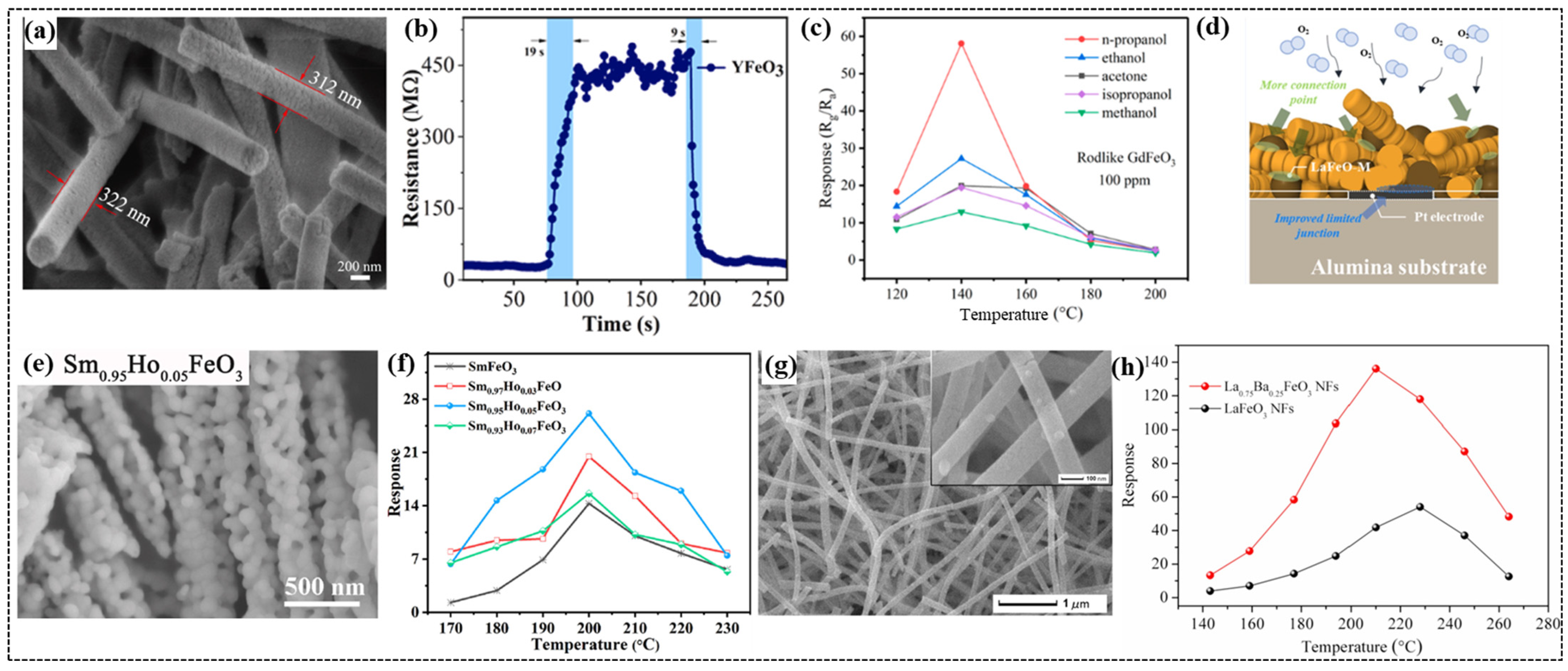
Guo et al. employed electrospinning to synthesize NdFeO3 nanorods with an average diameter of 275 nm and a specific surface area of 10.298 m2/g [6]. The gas sensor based on the NdFeO3 nanorods demonstrated a high response value of 18.9 to 100 ppm of triethylamine (TEA) at 190 °C. This sensor exhibited excellent selectivity for TEA over other gases because of the higher adsorption capacity and stronger reducibility of TEA molecules on the surface of the NdFeO3 nanorods. Liu et al. synthesized one-dimensional YFeO3 nanorods using electrospinning and evaluated their applications towards ethanol sensing [35]. The YFeO3 nanorods with a porous structure (Figure 5a) exhibited optimum performance at 350 °C, and this inflection point was attributed to the balance between the molecular activation energy and thermal effects on adsorption. This sensor showed excellent selectivity for ethanol at a low detection limit of 1 ppm with a response value of 2 compared to other gases such as NH3, H2O2, and formaldehyde. This sensor demonstrated fast response and recovery times of 19 and 9 s (Figure 5b), respectively, for 100 ppm of ethanol and maintained a stable response over a 30-day period. It exhibited good resistance to humidity by retaining a response value up to 60% relative humidity. Beyond this humidity level, the water molecular film formed on the surface of the sensor altered its response because of the partial blocking of target gas interactions with the sensing material. These findings suggest that the YFeO3 nanorod-based sensor is reliable and efficient for ethanol detection under various environmental conditions.
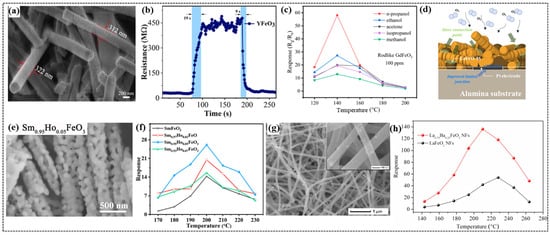
Figure 5.
(a) SEM images of YFeO3. (b) Response–recovery curve of YFeO3 to 100 ppm of ethanol at 350 °C. (c) Relationship between temperature and response to 100 ppm of different gases of the sensors based on GdFeO3 rods. (d) Schematic of the sensing mechanism of the LaFeO3 oxygen sensor with a mixed morphology (LaFeO-M) of powders and fibers. (e) SEM image Sm0.95Ho0.05FeO3 nanofibers. (f) Response of the Sm1−xHoxFeO3 (0 ≤ x ≤ 0.07) sensors to 100 ppm of glycol at different operating temperatures (170–230 °C). (g) FE-SEM image of La0.75Ba0.25FeO3 nanofibers. (h) Operating temperature dependence of the sensing response to 500 ppm of ethanol gas for the LaFeO3 and La0.75Ba0.25FeO3 nanofiber-based sensors. (a,b) Reproduced with permission [35]. Copyright 2023, Elsevier B.V. (c) Reproduced with permission [27]. Copyright, MDPI. (d) Reproduced with permission [102]. Copyright 2020, Elsevier B.V. (e,f) Reproduced with permission [99]. Copyright 2021, Elsevier B.V. (g,h) Reproduced with permission [90]. Copyright 2018, Elsevier B.V.
Lin et al. synthesized GdFeO3 nanostructures with rod and butterfly morphologies using an ecofriendly coprecipitation method [27]. By regulating the alcohol–water ratio of the solution, the amount of polyvinylpyrrolidone (PVP), and the standing time (12 h for butterflies and 36 h for rods), the desired morphologies were achieved. After annealing the precursors at 800 °C for 3 h, structural analysis confirmed the orthorhombic structure for both morphologies. SEM images displayed the rods with a diameter of 109.3 nm and a length of 1.536 μm, while butterflies with a length of 6.208 μm and a thickness of 359.9 nm were obtained. The GdFeO3 rod-based sensor exhibited a significantly higher response than the butterfly-based sensor for all the tested gases (Figure 5c), which was attributed to the porous structure of the rods, providing more active sites to interact with gases. The rod-based sensor demonstrated better selectivity for n-propanol, with a response of more than twice that for the other gases. This sensor demonstrated the highest response value of 58.113 at 140 °C with response and recovery times of 28 s each due to its rough surface. Furthermore, the stability and repeatability over a period of seven days highlight the potential use of GdFeO3 sensors for n-propanol detection. Wei et al. successfully fabricated an ethylene glycol sensor based on ErFeO3 nanofibers using uniaxial electrospinning [39]. The ErFeO3 nanofiber sensor demonstrated the highest response of 15.8 to 100 ppm ethylene glycol at 230 °C. The sensor demonstrated outstanding selectivity for ethylene glycol with a low detection limit of 35 ppm over other gases and excellent stability over 14 days.
Mun et al. investigated the influence of morphology on the gas-sensing properties of an LaFeO3 sensor [102]. They employed the sol–gel method to prepare nanopowders (LaFeO-P), and electrospinning was used to produce nanofibers (LaFeO-F). A mixed structure (LaFeO-M) combining nanopowders and nanofibers in a 1:1 ratio was also prepared. Gas sensors were fabricated on alumina substrates with prepatterned plating electrodes using screen printing. SEM images revealed that nanopowders (P) had irregular shapes with a rough surface, while nanofibers (F) exhibited a uniform structure with lengths of 2–3 μm. By contrast, the mixed structure (M) exhibited a morphology with nanorods bridging the narrow contact points between the interlinked nanofibers. The LaFeO-M sensor demonstrated the highest response of 13.1 to oxygen with 5% partial pressure, which was 2.64 times that of LaFeO-P at an optimal temperature of 650 °C, likely due to the higher concentration of oxygen vacancies, as confirmed by XPS analysis. This sensor exhibited fast response and recovery times of 10 and 19 s, respectively, compared to those of LaFeO-P (16 and 23 s, respectively) and LaFeO-P (15 and 18 s, respectively). The superior performance of the LaFeO-M sensor is attributed to the formation of ionosorbed oxygen species (Figure 5d) at elevated temperatures as well as the mixed morphology, which provides a balance between the dense microstructure of the nanopowders and the narrow contact between the interlinked nanofibers.
Han et al. reported the superior gas-sensing properties of rough SmFeO3 nanofibers, which exhibited a high response value of 18.19 to 100 ppm of ethylene glycol at 240 °C [100]. This sensor demonstrated a low detection limit of 5 ppm and reliable stability over a test period of 35 days, with a minimal decrease in response to moisture. The gas-sensing mechanism of the sensor was explained based on the hole accumulation layer (HAL). In air, the thickness of the HAL increases when oxygen molecules are absorbed onto the sensor surface, whereas the thickness of the HAL decreases when the sensor interacts with ethylene glycol because of a reduction in the hole concentration. This change in resistance enhanced the detection of ethylene glycol. In a subsequent study, Han et al. employed a doping strategy to improve the gas-sensing performance of SmFeO3 nanofibers [99]. They fabricated a Ho-doped SmFeO3 nanofiber-based sensor and explored the effects of Ho doping on its structural, morphological, and gas-sensing properties. The SEM results showed the highly porous nature of the SmFe0.95Ho0.05FeO3 nanofibers (Figure 5e) with interconnected nanoparticles. The SmFe0.95Ho0.05FeO3 nanofiber sensor exhibited a significantly improved response of 26.12 to 100 ppm of glycol at 200 °C (Figure 5f). It also exhibited an outstanding response of 10.20 to 5 ppm of glycol, surpassing the performance of other glycol sensors reported in the literature [100]. The enhanced gas-sensing properties of the Ho-doped SmFeO3 nanofiber-based sensor were attributed to the reduced crystallite size, which provided a larger surface area for gas adsorption and increased porosity, which facilitated faster gas diffusion into the sensing layer.
Zhang et al. fabricated LaFeO3 nanotubes using electrospinning for rapid ethanol detection [38]. The synthesis process involved the application of a high voltage to a solution containing lanthanum nitrate hexahydrate, ferric nitrate nonahydrate, citric acid, and polyvinylpyrrolidone (PVP) to deposit the nanofibers onto a collector. The resulting LaFeO3/PVP composite nanofibers were heated at 600 °C to obtain LaFeO3 nanotubes. SEM and TEM revealed the hollow structure of the nanotubes, with an outer diameter of 50 nm and an uneven inner diameter ranging from 15 to 30 nm. The LaFeO3 nanotube gas sensor demonstrated a high response of 9.4 to 100 ppm of ethanol at 160 °C, with a fast response and recovery times of 2 and 4 s, respectively. The stable response of the sensor over a month indicated the potential for using LaFeO3 nanotubes in the development of high-performance ethanol gas sensors. Xiang et al. explored the effect of Ba-doping on the gas-sensing properties of LaFeO3 nanofibers [90] (Figure 5g). All doped nanofiber sensors demonstrated superior gas-sensing performance compared to their undoped counterparts. Among the sensors, the La0.75Ba0.25FeO3 sensor exhibited the highest response of 136.1 to 500 ppm of ethanol gas at 210 °C (Figure 5h). The enhancement in the gas-sensing performance is attributed to the change in the electronic structure and defect chemistry of the LaFeO3 lattice owing to the substitution of Ba2+ for La3+.
5.3. Thin Film/Nanosheet
In recent studies, altering the concentration of defect vacancies has been shown to be an effective method to enhance gas-sensing properties [107,108,109]. Various techniques, such as heat treatment, cation and anion doping, solution treatment, and interfacial effects [110] have been employed to modulate oxygen vacancy defects.

Table 3.
Summary of orthoferrite-based nanosheet/thin film gas sensors reported in the literature.
Table 3.
Summary of orthoferrite-based nanosheet/thin film gas sensors reported in the literature.
| Material | Detection Gas | O.T. (°C) | Conc. (ppm) | Response | tres/trec (s) | LOD | Reference |
|---|---|---|---|---|---|---|---|
| LaFe0.99P0.01O3−δ | Acetone | 180 | 100 | 30 | 50 ppb | [40] | |
| La0.8Ca0.1Pb0.1FeO3 | Ethanol | 250 | 5 | 2.04 | 200/580 | [111] | |
| LaFeO3 | Formaldehyde | 120 | 1 | 1.35 | 50 ppb | [112] | |
| LaFeO3 | SO2 | 120 | 3 | 7.6% | 15/14 | [55] | |
| LaFe0.8Co0.2O3 | CO | 225 | 500 | 86% | 100/500 | [57] | |
| La0.8Pb0.1Ca0.1Fe0.8Co0.2O3 | Ozone | 170 | 0.4 | 3.9 | 2.1/- | [113] | |
| PrFeO3 | CO2 | 180 | 5000 | 3.846 | 441/98 | [87] | |
| LaFeO3 | Ethanol | 128 | 200 | 55 | 13/11 | [114] | |
| LaFeO3 | Acetone | 260 | 0.5 | 2.068 | 62/107 | [115] |
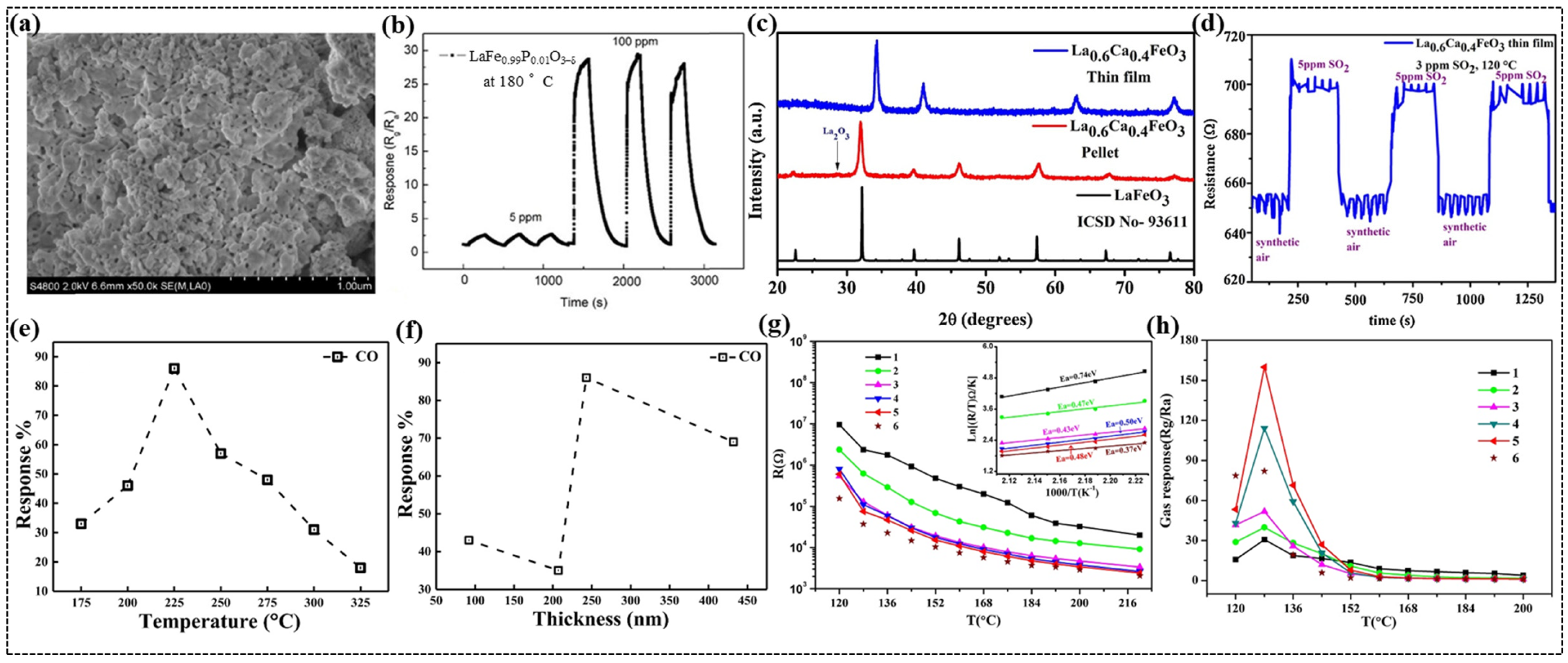
Qin et al. synthesized phosphorous-doped LaFe1−xPxO3 (x = 0.005, 0.01, 0.03, 0.05) (LPFO) nanosheets using a sol–gel method [40]. SEM analysis revealed a porous structure with loosely stacked nanoparticles for pristine LaFeO3, whereas an improved structural arrangement of the nanoparticles was observed when LaFeO3 doping was used. At a specific doping concentration of P (x = 0.01), a well-defined stacked, porous, nanometer-thin layer was observed (Figure 6a), which was disrupted by a further increase in the P content. This indicates the influence of optimized doping levels on the morphology and functional properties of the material. In addition, TEM imaging revealed regular circular pores with diameters of approximately 15 nm, confirming the mesoporous structure of the compound with x = 0.01. Among all the sensors, the LPFO sensor with x = 0.01 demonstrated the highest response and lowest operating temperature of 180 °C (Figure 6b). The superior performance of the x = 0.01 sensor is ascribed to the increase in oxygen vacancies due to P-doping, along with the mesoporous structure and larger specific surface area of the nanoparticles compared with the undoped LaFeO3 sensor.
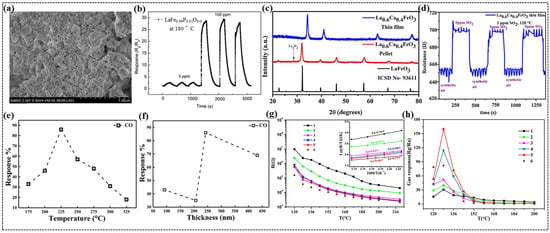
Figure 6.
(a) SEM image of LaFe0.99P0.01O3−δ nanosheet. (b) Continuity measurement curves of LF0.99P0.01O3−δ sensors. (c) Powder-XRD patterns of La0.6Ca0.4FeO3 nanoparticles in the form of pellet and thin film. (d) Sensing response of La0.6Ca0.4FeO3 thin film towards SO2 gas. (e) Variation in response % with temperature for 500 ppm of CO in 243 nm of LaFe0.8Co0.2O3 (LFCO) thin film. (f) Variation in CO gas response with film thickness in LaFe0.8Co0.2O3. (g) Temperature dependence of resistance from 120 °C to 220 °C in the air for LaFeO3 sensors with the different number of sensing layers. The inset shows the corresponding ln[R/T] vs. 1000/T curves from 184 °C to 220 °C. (h) Working temperature dependence of the gas response to 200 ppm of ethanol for LaFeO3 sensors with the different number of layers. (a,b) Reproduced with permission [40]. Copyright 2021, Elsevier B.V. (c,d) Reproduced with permission [55]. Copyright 2020, Elsevier B.V. (e,f) Reproduced with permission [57]. Copyright 2021, Elsevier B.V. (g,h) Reproduced with permission [114]. Copyright 2018, Elsevier B.V.
Smiy et al. fabricated a La0.8Ca0.1Pb0.1FeO3 thin-film sensor using a drop-coating method to investigate the effect of double doping on gas-sensing properties [111]. At 250 °C, the sensor exhibited the highest response to ethanol, which was attributed to the creation of favorable defect sites due to doping. Aranthady et al. synthesized Ca-doped LaFeO3 chemiresistive gas sensors in the form of pellets and thin films using the sol–gel method and DC magnetron sputtering, respectively, to detect low-concentration SO2 gases [55]. XRD studies confirmed similar structures for both the pellets and thin films. However, a slight shift in peak positions towards higher angles and an increase in peak intensity in the case of thin films compared to the pellets (Figure 6c) were observed owing to residual stress as well as the preferred orientation of the crystallites. SEM imaging revealed the agglomeration of nanoparticles with irregular morphologies, providing more active sites for surface reactions. Among all the sensors, La0.6Ca0.4FeO3 pellet sensor demonstrated excellent sensing performance towards 5 ppm of SO2 at 160 °C. Surprisingly, at a lower temperature of 120 °C, the La0.6Ca0.4FeO3 thin film sensor exhibited significantly enhanced sensitivity to 3 ppm of SO2 (Figure 6d) compared to the pellet sensor. This enhancement was ascribed to the larger surface area and stable microstructure of the thin film, which facilitated easier charge movement across the surface.
In another study, Bhowmick et al. explored the impact of thickness on CO sensing properties of LaFe0.8Co0.2O3 thin films synthesized using a modified sol–gel method [57]. XRD analysis confirmed the structural stability of the thin films with varying thickness in the range of 92–432 nm. SEM images further provided insights into morphology and revealed the arrangement of spherical nanoparticles with porous microstructure. Notably, the degree of porosity increased with film thickness up to 243 nm, beyond which the strain effects restricted the development of porosity, impacting the gas diffusion and sensing performance. Among all the thin films, 243 nm thin film demonstrated the highest response to CO, peaking approximately 87% at 225 °C (Figure 6e), which is attributed to the balance between gas diffusion and chemisorption processes. The thinner films lacked sufficient adsorption sites for chemisorption process, while excessively thick films limited gas diffusion due to increased resistance. The superior sensing performance of 243 nm thin film (Figure 6f) is attributed to the highly porous structure and low activation energy for adsorption, which improved the gas–solid interactions.
Chen et al. reported the gas-sensing properties of PrFeO3 and NdFeO3 thick-film sensors fabricated through the sol–gel route [87]. The maximum response of the PrFeO3 sensor was 8.44 to 1000 ppm of CO2 under relative humidity of 58% at 160 °C, while the NdFeO3 sensor exhibited the highest response of 2.36 to 3000 ppm of CO2 with relative humidity of 72% at 200 °C. Nieto et al. investigated the effect of Ca doping on the gas-sensing properties of Sm1−xCaxFe0.7Co0.3O3 thin films synthesized using a modified sol–gel method [116]. A remarkable improvement in the gas-sensing performance was achieved through the incorporation of Ca2+ ions into the films. The thin films with calcium content x ≥ 0.2, demonstrated the highest response to 200 ppm of CO at 300 °C and for 500 ppm C3H8, at similar temperature conditions.
Cao et al. fabricated LaFeO3 thick film sensors by coating LaFeO3 nanoparticles on the ceramic tube, which resulted in the sensors with thickness ranging from 40 to 234 μm [114]. A reduction in the electrical resistance was evident with the increase in the number of layers, suggesting that an enhanced cross-sectional area is available for the easy flow of the charge carriers (Figure 6g). However, the reduction in the resistance is more pronounced in the case of two- and three-layer films with a lower activation energy Ea of 0.47 eV and 0.43 eV, respectively (inset of Figure 6g). This smaller activation energy further contributes to improve the conductivity of the charge carriers. The optimal operating temperature for all the sensors is 128 °C to 200 ppm of ethanol, while the five-layer film demonstrated the highest response (Figure 6h), striking the balance between surface reaction efficiency and gas diffusion. The three-layer sensor exhibited fast response and recovery times, which is ascribed to lower Ea facilitating quicker interaction between ethanol molecules and adsorbed oxygen species.
5.4. Nanocomposites
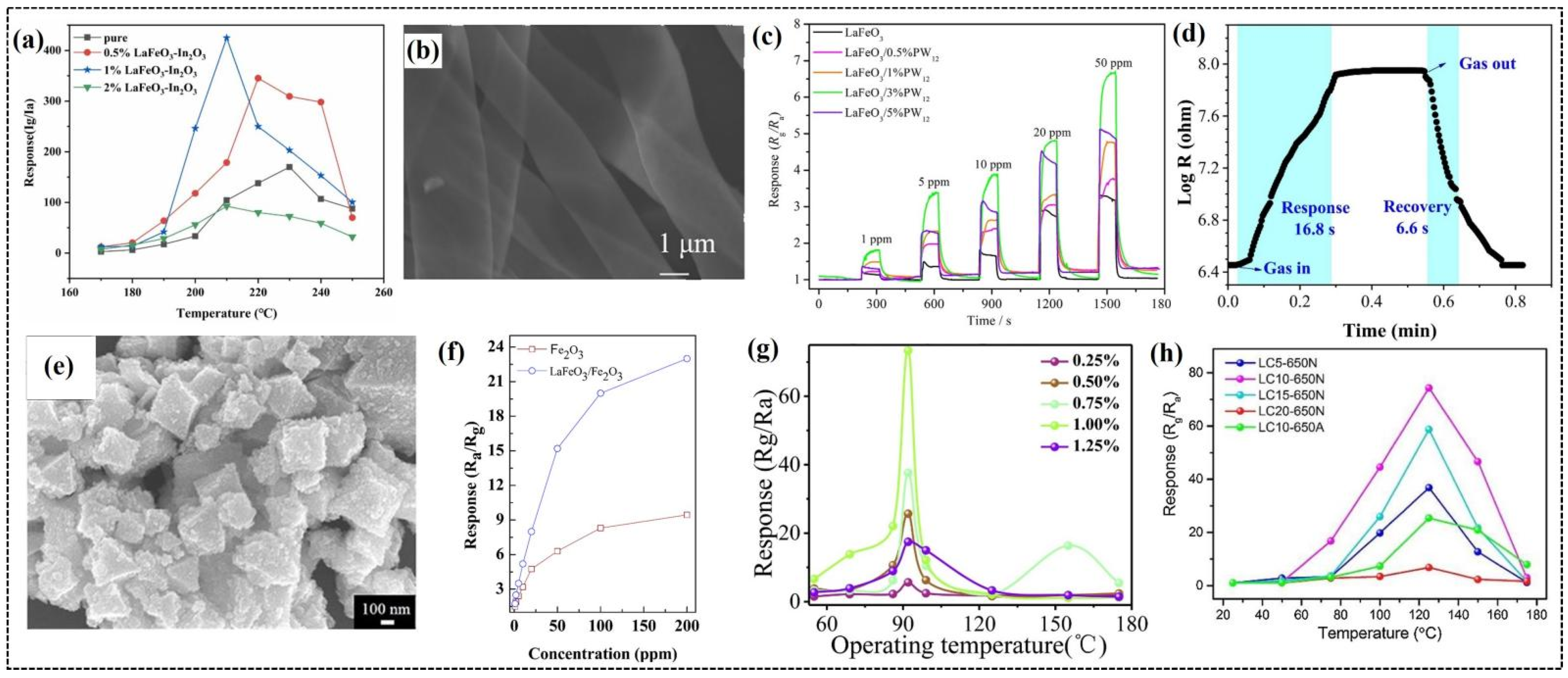
Meng et al. demonstrated the superior gas-sensing performance of LaFeO3-In2O3 nanocomposites synthesized using the hydrothermal method [117]. Individual nanoparticles of LaFeO3 and In2O3 were prepared separately, and LaFeO3-In2O3 composites were produced by mixing them with desired ratios. Gas-sensing tests demonstrated an excellent sensitivity of 1% LaFeO3-In2O3 composite with a remarkable response value of 425 to 100 ppm of 2-butanone at an operating temperature of 210 °C, nearly three times higher than that of pure In2O3 (Figure 7a). This sensor exhibited an impressive detection limit of 10 ppb for 2-butanone, with long-term sustainability and reduced response–recovery times compared to the In2O3 sensor. This enhanced gas-sensing performance is ascribed to the p-n heterojunction created by the combination of n-type In2O3 and p-type LaFeO3, facilitated charge transfer, and increased oxygen adsorption, improving the response to 2-butanone. In addition to that, the unique rod-like morphology provides a large surface area and more active sites for gas adsorption. The inherent catalytic nature of LaFeO3 promoted the redox reaction between 2-butanone and adsorbed oxygen, further contributing to enhanced sensing performance.
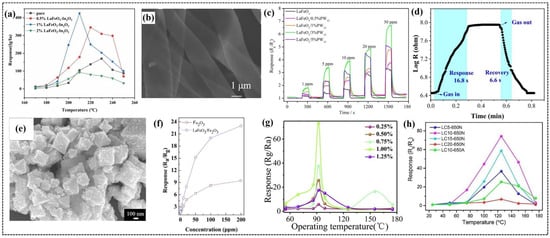
Figure 7.
(a) Response values of In2O3, 0.5% LaFeO3-In2O3, 1% LaFeO3-In2O3, and 2% LaFeO3-In2O3 sensors to 100 ppm of 2-butanone at different temperatures. (b) SEM images of LaFeO3/3%PW12 nanoribbons. (c) Response curves of LaFeO3/PW12 nanocomposite to different concentrations of acetone at 250 °C. (d) Dynamic response curve of LaFeO3/2.5wt%SnO2 QDs to 100 ppm of HCOOH. (e) Typical FESEM images of LaFeO3/α-Fe2O3 composite. (f) Responses of the pure α-Fe2O3 and LaFeO3/α-Fe2O3 composites to different concentrations of acetone at 230 °C. (g) Response curves with different amounts (0.25–1.25%) of doping of nitrogen-doped carbon quantum dot(NCQD)/Ag-LaFeO3 NALPN for 5 ppm of methanol. (h) Response values of LC5-650N, LC10-650N, LC15-650N, LC20-650N, and LC10-650A sensors to 50 ppm of formaldehyde over a temperature range of 25 °C to 175 °C. (a) Reproduced with permission [117]. Copyright 2023, Elsevier B.V. (b,c) Reproduced with permission [118]. Copyright 2024, Elsevier B.V. (d) Reproduced with permission [119]. Copyright 2022, Elsevier B.V. (e,f) Reproduced with permission [120]. Copyright 2018, American Chemical Society. (g) Reproduced with permission [121]. Copyright 2019, Elsevier B.V. (h) Reproduced with permission [122]. Copyright 2021, Elsevier B.V.
Wang et al. employed the electrospinning technique to synthesize one-dimensional LaFeO3 nanoribbon-based composite materials decorated with different mass fractions of phosphotungstic acid (PW12) and investigated their gas-sensing properties [118]. Among the fabricated sensors, the LaFeO3/3%PW12 sensor (Figure 7b) demonstrated the highest response of 3.35 (Figure 7c) to 5 ppm of acetone at 250 °C, which is nearly 2.6 times the response value and 90 °C lower than the operating temperature of pure LaFeO3 nanoribbons. In the composite, PW12 acted as an electron acceptor and facilitated the efficient charge carrier migration between the two components, thereby enhancing the sensor’s sensitivity and selectivity towards acetone gas. The sensor exhibited maximum resistance to relative humidity in the range of 33–94% and maintained its stability by retaining the response for 30 days with minimal fluctuations.
Xia et al. reported the development of a novel method to prepare SnO2 quantum dot (QD)-sensitized LaFeO3 composites for detecting conductometric formic acid (HCOOH) gas [119]. Initially, they synthesized SnO2 QDs and dispersed them in LaFeO3 sol–gel precursors, followed by a calcination, which resulted in the composite with porous morphology. The smaller size of SnO2 QDs improved the porosity of LaFeO3, promoting effective gas diffusion, leading to an excellent selectivity and sensitivity towards HCOOH at a lower operating temperature. The sensor fabricated using the composite with 2.5 wt% SnO2 QDs (LSO-2) demonstrated a high response value of 31.5 to 100 ppm of HCOOH at 210 °C, and a low detection limit of 1 ppm. This sensor exhibited greater stability by retaining consistent performance for 6 days, and fast response and recovery times of 16.8 and 6.6 s (Figure 7d), respectively, due to its porous structure. The difference in the work function and band gap width created a built-in electric field at the SnO2 QDs/LaFeO3 interface, which enhanced the gas-sensing performance. The region of SnO2 QDs were electron depleted with an abundance of captured electrons, due to their smaller size. During the gas-sensing process, these electrons were released when the sensor was exposed to target gas, and some of them were expected to combine with the free holes of LaFeO3, further enhancing the sensor’s resistance and response.

Table 4.
Summary of orthoferrite-based nanocomposite gas sensors reported in the literature.
Table 4.
Summary of orthoferrite-based nanocomposite gas sensors reported in the literature.
| Material | Detection Gas | O.T. (°C) | Conc. (ppm) | Response | tres/trec (s) | LOD | Reference |
|---|---|---|---|---|---|---|---|
| LaFeO3/3%PW12 | Acetone | 250 | 5 | 3.35 | 52.64 ppb | [118] | |
| 1% LaFeO3/In2O3 | 2-Butanone | 210 | 100 | 425 | 103/21 | 10 ppb | [117] |
| LaFeO3/2.5wt% SnO2 QD | Formic acid | 210 | 100 | 31.5 | 16.8/6.6 | 1 ppm | [119] |
| p-SmFeO3/p-YFeO3 | Ethanol | 120 | 30 | 163.593 | 37/129 | 380 ppb | [123] |
| LaFeO3/C | Formaldehyde | 125 | 50 | 74.3 | 500 ppb | [122] | |
| Au/LaFeO3 | Ethanol | 200 | 100 | 44 | 24/8 | [124] | |
| Ag/LaFeO3 | Ethanol | 190 | 100 | 155 | 30/5 | [125] | |
| SmFeO3/ZnO | Acetone | 350 | 10 | 45 | 10/250 | 100 ppb | [126] |
| α-Fe2O3/LaFeO3 | Acetone | 350 | 100 | 48.3 | 16.5/2 | 166.2 ppm | [127] |
| Ag/LaFeO3 NCQD | Methanol | 92 | 5 | 73 | 50.9/54.3 | [121] | |
| α-Fe2O3/LaFeO3 | Acetone | 230 | 100 | 20 | 3/5 | 1 ppm | [120] |
| Co-Fe2O3/SmFeO3 | Methanol | 155 | 5 | 19.7 | 47/19 | 40 ppb | [128] |
| 3wt% Pd/SmFeO3 | Acetone | 240 | 500 ppb | 7.21 | 8/15 | [129] | |
| 2wt% Pd/LaFeO3 | Acetone | 200 | 1 | 1.9 | 4/2 | [130] |
Zhang et al. explored the advantage of metal–organic framework (MOF)-templated LaFeO3/α-Fe2O3 porous nano-octahedrons for detecting acetone gas [120]. They used MIL-53(Fe) as a template to synthesize nano-octahedrons through a self-sacrificial template method. This method starts with the formation of MIL-53(Fe) nano-octahedrons, followed by the growth of La-Fe hydroxides on the MIL-53(Fe) surface. Subsequent pyrolysis transformed the as-prepared precursor into LaFeO3/α-Fe2O3 porous nano-octahedrons. The porous morphology is confirmed by FESEM (Figure 7e) and TEM analysis, which provides a large surface area for gas adsorption and the efficient diffusion of gas molecules. Gas-sensing studies revealed that the sensor based on LaFeO3 decorated with α-Fe2O3 nano-octahedrons exhibited a significantly higher sensitivity to acetone compared to pure α-Fe2O3 sensors. This sensor’s response is three times higher than that of pure α-Fe2O3 for 100 ppm of acetone at 230 °C (Figure 7f), which is attributed to the porous nanostructure and the formation of p-n heterojunction between LaFeO3 and α-Fe2O3. The sensor demonstrated excellent selectivity towards acetone over other tested gases due to the formation of hydrogen bonds between the surface hydroxyl groups of the sensing material and the carbonyl group of acetone, resulting in an enhanced acetone adsorption capacity. This sensor displayed long-term stability and superior reversibility along with rapid response and recovery times of 1 and 3 s, respectively.
Rong et al. developed a highly sensitive and selective methanol gas sensor based on a nitrogen-doped carbon quantum dot(NCQD)/Ag-LaFeO3 (NALPN) heterojunction prepared via a microwave-assisted synthesis route [121]. They fabricated sensors with different weight ratios of NCQDs to Ag-LaFeO3 and tested them for gas-sensing applications. Among the sensors, the 1% NALPN sensor demonstrated excellent sensitivity with a response of 73 to 5 ppm of methanol at 92 °C (Figure 7g). This enhanced sensitivity is ascribed to the formation of p-n heterojunction between p-type Ag-LaFeO3 and n-type NCQDs, which facilitates the adsorption of oxygen molecules and improves the charge separation, resulting in a larger change in the resistance upon exposure to methanol. The presence of carboxyl functional groups (-COOH) on the surface of NCQDs along with the cavities on the surface of Ag-LaFeO3 further enhanced the selectivity towards methanol compared to other tested gases. The sensor exhibited long-term stability over 30 days and fast response and recovery times of 19 and 54.3 s, respectively.
Ma et al. reported the synthesis of porous LaFeO3/C nanocomposites for the first time using a facile foaming sol–gel technique and explored their applicability in sensing formaldehyde [122]. They controlled the amount of carbon in the composite with the desired microstructure by adjusting the content of polyvinylpyrrolidone (PVP) during the synthesis process. Morphology studies displayed the loose porous structure of the nanocomposite annealed in the N2 atmosphere, compared to pristine LaFeO3 annealed in air. The porous structure provides more active sites on the surface of the sensing material to interact with formaldehyde gas molecules. This eventually led to enhancing the sensing ability of the sensor and reducing the response times. This was evident from the fast response and recovery times (11/19 s) demonstrated by LaFeO3 with a 10% PVP sensor (LC10-650N) (Figure 7h). The sensitivity of this sensor is three times higher than that of pristine LaFeO3, with a response of 74.3 to 50 ppm of formaldehyde at 125 °C, which was ascribed to the increased conductivity resulting from the strong interaction between LaFeO3 and carbon. The sensor displayed excellent selectivity to formaldehyde over other tested gases, likely due to catalytic activity of the sensing material to aldehyde group.
This comprehensive review of RFeO3-based gas sensors identified several materials and composites as potential candidates for real-world applications. Among the materials reported in Table 1, Table 2, Table 3 and Table 4, NdFeO3 nanoparticles stand out for their high sensitivity to n-butanol, highlighting their potential for VOC detection. Doped PrFeO3 compounds, such as Pr0.8Ca0.2FeO3 and PrFe0.9Co0.1O3, have demonstrated excellent sensing capabilities at room temperature for LPG and CO2 detection, respectively. Furthermore, orthoferrites with smaller amounts of Pd doping (e.g., 3 wt% Pd/SmFeO3, 2 wt% Pd/LaFeO3) have shown excellent selectivity and rapid response–recovery times at low concentrations (sub-ppm levels) and operating temperatures below 250 °C, making them highly attractive for integration into miniaturized or wearable applications. Additionally, LaFeO3-based nanocomposites incorporating materials such as SnO2 QDs, ZnO, or α-Fe2O3 significantly exhibited improved sensing characteristics due to better charge transport and gas diffusion. Moreover, one-dimensional nanostructures such as nanotubes and nanofibers, particularly GdFeO3 and SmFeO3 nanostructures, offer improved response times due to their high surface-to-volume ratios and interconnected porosity, which promote faster gas adsorption/desorption kinetics.
6. Conclusions and Future Perspectives
This article provides a comprehensive review on the latest advancements of rare earth orthoferrite-based gas sensors with a critical emphasis on their nanostructure, doping effects and heterostructures. Different synthesis methods were employed to modify the microstructure of orthoferrites such as the specific surface area and porosity in order to improve the gas–solid interactions. Doping at the R-site and Fe-site of orthoferrites suggested the alteration of the crystal structure, defect concentration, and electronic properties. This led to enhancing the specific surface area of the sensing material and providing activation energy to improve gas-sensing performance, including selectivity, sensitivity, lower operating temperatures and fast response/recovery times. Moreover, the formation of heterojunction with other sensing materials improved the surface reactivity and modulated the gas-sensing capabilities of orthoferrite-based gas sensors.
The advancement of nanotechnology has significantly enhanced the gas-sensing capabilities of orthoferrites and established them as a promising candidate for next generation sensing technologies. The research related to the development of orthoferrite-based gas sensors suggested that the sensitivity is no longer a constraint for their practical applications. However, there are prominent hurdles in achieving high selectivity, room temperature operation, long-term stability, and better resistance to humidity. The higher operating temperatures not only consume excessive power but also create safety issues. To address these challenges, future research should be focused on probing the underlying gas-sensing mechanism of orthoferrite compounds. With the help of advanced characterization techniques along with computational modeling, it would be possible to obtain a deep understanding of the role of crystal structure, oxygen vacancies on the interactions between the target gas, and the surface of the sensing materials. This will help the research community to select suitable dopants to develop sensing materials with enhanced sensing capabilities. Though various dopants were explored, the undiscovered dopants with different combinations could yield improved gas-sensing properties of orthoferrites. Additionally, exploring the combination of orthoferrites with other sensing materials such as metal oxides, noble metals, and polymers can further improve the gas-sensing performance. By controlling the porosity and exploring unique nanostructure morphologies with an increased surface area, the sensitivity of orthoferrite gas sensors could be further improved. One of the key challenges of orthoferrite gas sensors that need to be addressed is their long-term stability under harsh environmental conditions. To overcome these limitations, future research should be focused on designing novel sensing materials with a high surface area, controlled morphology, and engineered defects to improve the reactions between the target gas and the sensing material. Furthermore, the development of gas sensors using advanced fabrication techniques and integrating the flexible substrates and wireless communication systems will play a crucial role in the next generation of smart gas sensors. Finally, the design and fabrication of gas sensors suitable for miniaturization and able to support low-power operation at room temperature, with better selectivity and sensitivity, will propel the field of gas sensors to reach new heights of advancement and practical applications.
Author Contributions
Conceptualization, G.K.; validation, G.K.; data curation, G.K.; writing—original draft preparation, G.K.; writing—review and editing, S.H.; supervision, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-0024456 and No. RS-2024-00445819).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, L.; Zeng, W. Room-Temperature Gas Sensing of ZnO-Based Gas Sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Gao, S.; Wang, C.; Li, X.; Yuan, R.; Zhang, Q.; Zhao, J.; Chu, H. Amorphous CoSnO3 for Conductometric Triethylamine Gas Sensing. Sens. Actuators B Chem. 2024, 401, 135086. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Niu, Y.; Su, H.; Qi, Y. Simple Thermocatalytic Oxidation Degradation of VOCs. Catal. Lett. 2022, 152, 1801–1818. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Hsieh, J.-C.; Chen, C.-A.; Chen, W.-L.; Meng, H.-F.; Lu, C.-J.; Horng, S.-F.; Zan, H.-W. Ultrasensitive Detection of Hydrogen Sulfide Gas Based on Perovskite Vertical Channel Chemo-Sensor. Sens. Actuators B Chem. 2021, 326, 128988. [Google Scholar] [CrossRef]
- Guo, J.; Ma, S.; Fan, G.; Ni, P.; Ma, N.; Wang, Y.; Zhu, J.; Wang, H. P-Type NdFeO3 Nanorods Synthesized by Electrospinning Are Used in High Performance TEA Sensors. Ceram. Int. 2025, 51, 865–873. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, S.; Yang, G.; Gao, L.; Wu, Y.; Zhang, X. Oxygen Vacancies Assisted LaFeO3 Derived from Metal Organic Frameworks Endows a Practical HCHO Sensor with Excellent Sensing Characteristics. J. Ind. Eng. Chem. 2023, 126, 501–509. [Google Scholar] [CrossRef]
- Yang, K.; Ma, J.; Qiao, X.; Cui, Y.; Jia, L.; Wang, H. Hierarchical Porous LaFeO3 Nanostructure for Efficient Trace Detection of Formaldehyde. Sens. Actuators B Chem. 2020, 313, 128022. [Google Scholar] [CrossRef]
- Srinivasan, P.; Ezhilan, M.; Kulandaisamy, A.J.; Babu, K.J.; Rayappan, J.B.B. Room Temperature Chemiresistive Gas Sensors: Challenges and Strategies—A Mini Review. J. Mater. Sci. Mater. Electron. 2019, 30, 15825–15847. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A Review on Chemiresistive Room Temperature Gas Sensors Based on Metal Oxide Nanostructures, Graphene and 2D Transition Metal Dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Akbar, S.; Mulla, I.S.; Suryavanshi, S.S.; Bhandari, N.L.; Chaskar, M.G. Gas Sensors and Factors Influencing Sensing Mechanism with a Special Focus on MOS Sensors. J. Mater. Sci. 2023, 58, 559–582. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of Mesoporous Semiconductor Metal Oxides for Gas Sensing: Recent Advances and Emerging Challenges. Adv. Sci. 2023, 10, 2204810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in Designs and Mechanisms of Semiconducting Metal Oxide Nanostructures for High-Precision Gas Sensors Operated at Room Temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, Y.; Liu, B.; Duan, Z.; Zhang, Y.; Yuan, Z.; Tai, H. Enhancing the Carbon Dioxide Sensing Performance of LaFeO3 by Co Doping. Sens. Actuators B Chem. 2024, 402, 135136. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.; Xiang, W. Oxygen Vacancy Induced Performance Enhancement of Toluene Catalytic Oxidation Using LaFeO3 Perovskite Oxides. Chem. Eng. J. 2020, 387, 124101. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, I.-D. Recent Advances in ABO3 Perovskites: Their Gas-Sensing Performance as Resistive-Type Gas Sensors. J. Korean Ceram. Soc. 2020, 57, 24–39. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, Characterization and n-Propanol Sensing Properties of Perovskite-Type ZnSnO3 Nanospheres Based Gas Sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, M.; Li, T.; Zhang, Y.; Zou, H. Super-Fast Response Humidity Sensor Based on La0.7Sr0.3MnO3 Nanocrystals Prepared by PVP-Assisted Sol-Gel Method. Sens. Actuators B Chem. 2018, 258, 527–534. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.-G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Kotnana, G.; Jammalamadaka, S.N. Band Gap Tuning and Orbital Mediated Electron–Phonon Coupling in HoFe1−xCrxO3 (0 ≤ x ≤ 1). J. Appl. Phys. 2015, 118, 124101. [Google Scholar] [CrossRef]
- Gateshki, M.; Suescun, L.; Kolesnik, S.; Mais, J.; Świerczek, K.; Short, S.; Dabrowski, B. Structural, Magnetic and Electronic Properties of LaNi0.5Fe0.5O3 in the Temperature Range 5–1000 K. J. Solid State Chem. 2008, 181, 1833–1839. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Shang, J.; Shen, H.; Li, L.; Wang, F.; Shen, X.; Tian, T.; Kalashnikova, A.M.; Wu, A.; et al. Recent Advances of Rare Earth Orthoferrite RFeO3 Magneto-Optical Single Crystals. J. Cryst. Growth 2025, 649, 127939. [Google Scholar] [CrossRef]
- Kotnana, G.; Babu, P.D.; Jammalamadaka, S.N. Metamagnetic Transitions and Magnetocaloric Properties of HoCr1-xFexO3 (x = 0.25 and 0.75) Compounds. J. Supercond. Nov. Magn. 2022, 35, 2057–2067. [Google Scholar] [CrossRef]
- Kotnana, G.; Babu, P.D.; Jammalamadaka, S.N. Magnetic and Magnetocaloric Properties of HoCr0.75Fe0.25O3 Compound. AIP Adv. 2017, 8, 056407. [Google Scholar] [CrossRef]
- Dubrovin, R.M.; Roginskii, E.M.; Chernyshev, V.A.; Novikova, N.N.; Elistratova, M.A.; Eliseyev, I.A.; Smirnov, A.N.; Brulev, A.I.; Boldyrev, K.N.; Davydov, V.Y.; et al. Lattice Dynamics and Mixing of Polar Phonons in the Rare-Earth Orthoferrite TbFeO3. Phys. Rev. B 2024, 110, 134310. [Google Scholar] [CrossRef]
- Lin, J.; Liu, N.; Zhang, T.; Liang, H.; Zhang, G.; Wang, X. Gas-Sensing Performance of Gadolinium Ferrates with Rod and Butterfly Morphologies. Chemosensors 2023, 11, 355. [Google Scholar] [CrossRef]
- Bharati, K.; Gupta, M.; Rajkumari; Tiwari, P.R.; Singh, R.P.; Bhardwaj, B.; Singh, K.A.; Yadav, B.C.; Tripathi, S.; Kumar, S. LPG Sensing Study of Calcium-Doped Praseodymium Orthoferrite Nanomaterial. Anal. Chem. 2024, 96, 19491–19503. [Google Scholar] [CrossRef]
- White, R.L. Review of Recent Work on the Magnetic and Spectroscopic Properties of the Rare-Earth Orthoferrites. J. Appl. Phys. 1969, 40, 1061–1069. [Google Scholar] [CrossRef]
- Tang, L.; Chen, Z.; Zuo, F.; Hua, B.; Zhou, H.; Li, M.; Li, J.; Sun, Y. Enhancing Perovskite Electrocatalysis through Synergistic Functionalization of B-Site Cation for Efficient Water Splitting. Chem. Eng. J. 2020, 401, 126082. [Google Scholar] [CrossRef]
- Chatla, E.; Vankudothu, N.; Shravan Kumar Reddy, S.; Shanmukharao Smatham, S.; Sreenath Reddy, M.; Gopal Reddy, C.; Yadagiri Reddy, P. Impact of Tb Substitution on Structural, Electrical and Magnetic Properties of Ho1−xTbxFeO3 Orthoferrite. Appl. Phys. A 2023, 130, 10. [Google Scholar] [CrossRef]
- Balamurugan, C.; Song, S.-J.; Lee, D.-W. Porous Nanostructured GdFeO3 Perovskite Oxides and Their Gas Response Performance to NOx. Sens. Actuators B Chem. 2018, 272, 400–414. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, C.; Qin, H.; Hu, J. High-Performance Acetone Gas Sensor Based on Ferrite–DyFeO3. J. Mater. Sci. 2020, 55, 16300–16310. [Google Scholar] [CrossRef]
- Chai, H.; Li, Y.; Luo, Y.; Debliquy, M.; Zhang, C. Investigation on Isopropanol Sensing Properties of LnFeO3(Ln = Nd, Dy, Er) Perovskite Materials Synthesized by Microwave-Assisted Hydrothermal Method. Appl. Surf. Sci. 2022, 601, 154292. [Google Scholar] [CrossRef]
- Liu, J.M.; Ma, S.Y.; Liu, W.W.; Xu, C.Y.; Wei, J.S.; Jiang, H.T.; Liu, M.M.; Ma, N.N. A Novel Ethanol Sensor with High Response Based on One-Dimensional YFeO3 Nanorods. Ceram. Int. 2023, 49, 33082–33088. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, D.; Liu, W.; Miao, T.; Chen, J.; Cheng, B.; Qin, H.; Hu, J. Study on Gas Sensing Characteristics of LaFeO3 Sensor under Multi-Wavelength Light Illumination. Mater. Lett. 2023, 344, 134350. [Google Scholar] [CrossRef]
- Guo, J.; Ma, S.; Ma, N.; Liu, J.; Wei, J.; Xu, C.; Fan, G.; Ni, P. Hydrothermal Synthesis of NdFeO3 Nanoparticles and Their High Gas-Sensitive Properties. Ceram. Int. 2024, 50, 43654–43664. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Z.; Zou, H.; Ma, M. Fabrication of Electrospun LaFeO3 Nanotubes via Annealing Technique for Fast Ethanol Detection. Mater. Lett. 2018, 215, 58–61. [Google Scholar] [CrossRef]
- Wei, J.S.; Ma, S.Y.; Cai, Y.H.; Xu, C.Y.; Liu, J.M.; Jiang, H.T. A High-Performance Ethylene Glycol Sensor Based on Fibrous ErFeO3 Prepared by Electrostatic Spinning. Ceram. Int. 2023, 49, 32611–32618. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Shen, Y.; Zhang, R.; Meng, F. Phosphorus-Doped Porous Perovskite LaFe1-xPxO3-δ Nanosheets with Rich Surface Oxygen Vacancies for Ppb Level Acetone Sensing at Low Temperature. Chem. Eng. J. 2022, 431, 134280. [Google Scholar] [CrossRef]
- Warshi, M.K.; Mishra, V.; Sagdeo, A.; Mishra, V.; Kumar, R.; Sagdeo, P.R. Structural, Optical and Electronic Properties of RFeO3. Ceram. Int. 2018, 44, 8344–8349. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Review of Perovskite-Structure Related Cathode Materials for Solid Oxide Fuel Cells. Ceram. Int. 2020, 46, 5521–5535. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Kotnana, G.; Sahu, D.P.; Jammalamadaka, S.N. Inverse and Enhanced Magnetocaloric Properties of HoCrO3. J. Alloys Compd. 2017, 709, 410–414. [Google Scholar] [CrossRef]
- Kotnana, G.; Reddy, V.R.; Jammalamadaka, S.N. Magnetic and Hyperfine Interactions in HoFe1−xCrxO3 (0≤x≤1) Compounds. J. Magn. Magn. Mater. 2017, 429, 353–358. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric Oxides as Low-Cost and Highly-Efficient Oxygen Reduction/Evolution Catalysts for Low-Temperature Electrochemical Devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Conejo-Dávila, A.S.; Estrada-Monje, A.; Maldonado-Orozco, M.C.; Reyes-López, S.Y.; Zaragoza-Contreras, E.A. Electrochemical Perovskite-Based Sensors for the Detection of Relevant Biomarkers for Human Kidney Health. Chemosensors 2023, 11, 507. [Google Scholar] [CrossRef]
- Zhang, G.; Song, X.-Z.; Wang, X.-F.; Liu, N.; Li, X.; Wei, Z.; Qian, G.; Wang, Z.; Yu, S.; Tan, Z. LnFeO3 (Ln = La, Nd, Sm) Derived from Bimetallic Organic Frameworks for Gas Sensor. J. Alloys Compd. 2022, 902, 163803. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, X.; Ma, Z.; Yang, K.; Gao, X.; Wang, H.; Jia, L. Formaldehyde Gas Sensor with 1 Ppb Detection Limit Based on In-Doped LaFeO3 Porous Structure. Sens. Actuators B Chem. 2022, 371, 132558. [Google Scholar] [CrossRef]
- Shen, C.; Liang, H.; Zhao, Z.; Guo, S.; Chen, Y.; Tan, Z.; Song, X.-Z.; Wang, X. Mo-Doped LaFeO3 Gas Sensors with Enhanced Sensing Performance for Triethylamine Gas. Sensors 2024, 24, 4851. [Google Scholar] [CrossRef]
- Siemons, M.; Leifert, A.; Simon, U. Preparation and Gas Sensing Characteristics of Nanoparticulate P-Type Semiconducting LnFeO3 and LnCrO3 Materials. Adv. Funct. Mater. 2007, 17, 2189–2197. [Google Scholar] [CrossRef]
- Park, C.O.; Akbar, S.A. Ceramics for Chemical Sensing. J. Mater. Sci. 2003, 38, 4611–4637. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Jiang, Y.; Duan, Z.; Yuan, Z.; Liu, B.; Huang, Q.; Zhao, Q.; Yang, Y.; Tai, H. Synergistic Effect of Charge Transfer and Interlayer Swelling in V2CTx/SnS2 Driving Ultrafast and Highly Sensitive NO2 Detection at Room Temperature. Sens. Actuators B Chem. 2024, 411, 135788. [Google Scholar] [CrossRef]
- Aranthady, C.; Jangid, T.; Gupta, K.; Mishra, A.K.; Kaushik, S.D.; Siruguri, V.; Rao, G.M.; Shanbhag, G.V.; Sundaram, N.G. Selective SO2 Detection at Low Concentration by Ca Substituted LaFeO3 Chemiresistive Gas Sensor: A Comparative Study of LaFeO3 Pellet vs Thin Film. Sens. Actuators B Chem. 2021, 329, 129211. [Google Scholar] [CrossRef]
- Benali, A.; Benali, E.M.; Melo, B.M.G.; Tozri, A.; Bejar, M.; Dhahri, E.; Graça, M.-P.F.; Valente, M.A.; Peng, L.; Wu, J.; et al. Significant Improvement of Dielectric, Magnetic, and Gas-Sensing Properties of the (La0.8Ca0.2)0.6Bi0.4FeO3 Nanomaterial: Particles Size Effects. J. Mater. Sci. Mater. Electron. 2023, 34, 45. [Google Scholar] [CrossRef]
- Bhowmick, T.; Nag, S.; Majumder, S.B. Investigations on Lanthanum Iron Cobalt Oxide Thin Film as Selective Carbon Monoxide Sensor. J. Alloys Compd. 2021, 884, 161161. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Sackmann, A.; Weimar, U.; Bârsan, N. A Highly Selective Sensor to Acetylene and Ethylene Based on LaFeO3. Sens. Actuators B Chem. 2020, 303, 127204. [Google Scholar] [CrossRef]
- Ni, P.; Ma, S.; Ma, N.; Xu, C.; Fan, G.; Guo, J.; Wei, J.; Liu, J. Porous Hollow Sphere Structure PrFeO3 as an Efficient Sensing Material for N-Butanol Detection. J. Alloys Compd. 2024, 1002, 175392. [Google Scholar] [CrossRef]
- Liu, H.; Miao, T.; Liu, W.; Chen, J.; Cheng, B.; Qin, H.; Hu, J. Highly Sensitive Acetone Gas Sensor Based on YFeO3 Planar Electrode under Multi-Wavelength Light Illumination. Mater. Lett. 2023, 333, 133596. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Y.; Liu, W.; Chen, J.; Hu, J. Gas Response of SmFeO3 Planar Electrode Sensor to Volatile Organic Compounds Gases under Light Illumination. Mater. Lett. 2022, 326, 133009. [Google Scholar] [CrossRef]
- Sheng, H.; Ma, S.; Han, T.; Yun, P.; Yang, T.; Ren, J. A Highly Sensitivity and Anti-Humidity Gas Sensor for Ethanol Detection with NdFeO3 Nano-Coral Granules. Vacuum 2022, 195, 110642. [Google Scholar] [CrossRef]
- Nga Phan, T.T.; My Dinh, T.T.; Duc Nguyen, M.; Li, D.; Nhan Phan, C.; Kien Pham, T.; Tu Nguyen, C.; Huyen Pham, T. Hierarchically Structured LaFeO3 with Hollow Core and Porous Shell as Efficient Sensing Material for Ethanol Detection. Sens. Actuators B Chem. 2022, 354, 131195. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Jiang, C.; Guo, H.; Qu, F.; Shimakawa, Y.; Yang, M. Integrated Sensing Array of the Perovskite-Type LnFeO3 (Ln = La, Pr, Nd, Sm) to Discriminate Detection of Volatile Sulfur Compounds. J. Hazard. Mater. 2021, 413, 125380. [Google Scholar] [CrossRef]
- Yang, T.T.; Ma, S.Y.; Cao, P.F.; Xu, X.L.; Wang, L.; Pei, S.T.; Han, T.; Xu, X.H.; Yun, P.D.; Sheng, H. Synthesis and Characterization of ErFeO3 Nanoparticles by a Hydrothermal Method for Isopropanol Sensing Properties. Vacuum 2021, 185, 110005. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Gas Sensing and Electrochemical Properties of Rare Earthferrite, LnFeO3 (Ln = Nd, Sm). Ceram. Int. 2020, 46, 26682–26688. [Google Scholar] [CrossRef]
- Cao, E.; Yang, Y.; Cui, T.; Zhang, Y.; Hao, W.; Sun, L.; Peng, H.; Deng, X. Effect of Synthesis Route on Electrical and Ethanol Sensing Characteristics for LaFeO3-δ Nanoparticles by Citric Sol-Gel Method. Appl. Surf. Sci. 2017, 393, 134–143. [Google Scholar] [CrossRef]
- Bharati, K.; Tiwari, P.R.; Singh, R.P.; Singh, A.; Yadav, B.C.; Singh, M.P.; Kumar, S. Synthesis of Bismuth-Doped Praseodymium Ortho Ferrite Nanomaterials for LPG Sensing. Appl. Nanosci. 2024, 14, 277–289. [Google Scholar] [CrossRef]
- Benali, E.M.; Saher, L.; Benali, A.; Bejar, M.; Dhahri, E.; Wu, J.; Peng, L.; Gordo, P.M.; Pina, J.; Costa, B.F.O. Synthesis, Characterization, and Sensitivity Tests of La0.8Ba0.1Bi0.1FeO3 Nanoparticles towards a Few Parts-per-Billion of Acetone Gas. Heliyon 2024, 10, e26778. [Google Scholar] [CrossRef]
- Benali, E.M.; Benali, A.; Bejar, M.; Dhahri, E.; Khomchenko, V.A.; Peng, L.; Wu, J.; Costa, B.F.O. Structural, Morphological and Excellent Gas Sensing Properties of La1–2xBaxBixFeO3 (0.00 ≤ x ≤ 0.20) Nanoparticles. J. Alloys Compd. 2021, 883, 160856. [Google Scholar] [CrossRef]
- Pei, S.; Ma, S.; Xu, X.; Xu, X.; Almamoun, O. Modulated PrFeO3 by Doping Sm3+ for Enhanced Acetone Sensing Properties. J. Alloys Compd. 2021, 856, 158274. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, H.; Zhang, H.; Lü, W.; Hu, J. CO2 Gas Sensors Based on Yb1−xCaxFeO3 Nanocrystalline Powders. J. Rare Earths 2017, 35, 602–609. [Google Scholar] [CrossRef]
- Xiaofeng, W.; Ma, W.; Sun, K.; Hu, J.; Qin, H. Nanocrystalline Gd1–xCaxFeO3 Sensors for Detection of Methanol Gas. J. Rare Earths 2017, 35, 690–696. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, H.; Lv, W.; Zhang, H.; Hu, J. Gas Sensors Based on Ytterbium Ferrites Nanocrystalline Powders for Detecting Acetone with Low Concentrations. Sens. Actuators B Chem. 2017, 246, 9–19. [Google Scholar] [CrossRef]
- Derakhshi, Z.; Baghshahi, S.; Khodadadi, A.A.; Tamizifar, M. Cu-Doped LaFe1-xCuxO3 Perovskites Nano-Crystallites for Enhanced VOCs Detection. Ceram. Int. 2024, 50, 23175–23187. [Google Scholar] [CrossRef]
- Bharati, K.; Tiwari, P.R.; Singh, R.P.; Bala; Singh, A.; Yadav, B.C.; Kumar, S. Cobalt-Doped Praseodymium Ortho Ferrite as a Promising Nanomaterial for Carbon Dioxide Gas Sensing. J. Mater. Chem. C 2023, 11, 15581–15590. [Google Scholar] [CrossRef]
- Lee, K.; Hajra, S.; Sahu, M.; Mishra, Y.K.; Kim, H.J. Co+3 Substituted Gadolinium Nano-Orthoferrites for Environmental Monitoring: Synthesis, Device Fabrication, and Detailed Gas Sensing Performance. J. Ind. Eng. Chem. 2022, 106, 512–519. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Wang, A.; Wu, S.; Zhang, L. N-Type LaFe1-xMnxO3 Prepared by Sol-Gel Method for Gas Sensing. J. Alloys Compd. 2020, 816, 152647. [Google Scholar] [CrossRef]
- Smiy, S.; Saoudi, H.; Benali, A.; Bejar, M.; Dhahri, E.; Fiorido, T.; Bendahan, M.; Aguir, K. Correlation between Structural, Magnetic and Gas Sensor Properties of La0.885Pb0.005Ca0.11Fe1-xCoxO2.95(0.00 ≤ x ≤ 0.15) Compounds. Mater. Res. Bull. 2020, 130, 110922. [Google Scholar] [CrossRef]
- Benali, A.; Bejar, M.; Dhahri, E.; Graça, M.P.F.; Valente, M.A.; Radwan, A. High Ethanol Gas Sensing Property and Modulation of Magnetic and AC-Conduction Mechanism in 5% Mg-Doped La0.8Ca0.1Pb0.1FeO3 Compound. J. Mater. Sci. Mater. Electron. 2019, 30, 12389–12398. [Google Scholar] [CrossRef]
- Jaouali, I.; Hamrouni, H.; Moussa, N.; Nsib, M.F.; Centeno, M.A.; Bonavita, A.; Neri, G.; Leonardi, S.G. LaFeO3 Ceramics as Selective Oxygen Sensors at Mild Temperature. Ceram. Int. 2018, 44, 4183–4189. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, R.K.; Singh, P. Fabrication of Lanthanum Ferrite Based Liquefied Petroleum Gas Sensor. Sens. Actuators B Chem. 2016, 229, 25–30. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Optical, Magnetic and Gas Sensing Properties of LaFeO3 Nanoparticles Synthesized by Different Chemical Methods. J. Electron. Mater. 2019, 48, 6503–6511. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Reyes-Gómez, J.; Flores-Álvarez, J.M.; Guillén-Bonilla, H.; Olvera, M.d.l.L.; Rodríguez Betancourtt, V.M.; Verde-Gómez, Y.; Guillén-Cervantes, A.; Santoyo-Salazar, J. Synthesis, Characterization and Sensitivity Tests of Perovskite-Type LaFeO3 Nanoparticles in CO and Propane Atmospheres. Ceram. Int. 2016, 42, 18821–18827. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chen, Q.; Deng, X.Y.; Jiao, H.Y.; Wang, P.Y.; Gengzang, D.J. Synthesis and Characterization of In-Doped LaFeO3 Hollow Nanofibers with Enhanced Formaldehyde Sensing Properties. Mater. Lett. 2019, 236, 229–232. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A Fast Response & Recovery H2S Gas Sensor Based on α-Fe2O3 Nanoparticles with Ppb Level Detection Limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Qin, H.; Zhang, H.; Zhang, Z.; Zhou, G.; Gao, C.; Hu, J. CO2 Sensing Properties and Mechanism of PrFeO3 and NdFeO3 Thick Film Sensor. J. Rare Earths 2019, 37, 80–87. [Google Scholar] [CrossRef]
- Bhat, M.A.; Rana, P.; Mir, F.A.; Pathak, D. A Brief Study on Exploration of Ni Doped PrFeO3 Perovskite as Multifunctional Material. J. Mater. Sci. Mater. Electron. 2023, 34, 269. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Zhang, X.; Gong, L.; Zhang, Y.; Zhang, K. Preparation and Characterization of Ba-Doped LaFeO3 Nanofibers by Electrospinning and Their Ethanol Sensing Properties. Mater. Chem. Phys. 2018, 213, 122–129. [Google Scholar] [CrossRef]
- Benali, A.; Azizi, S.; Bejar, M.; Dhahri, E.; Graça, M.F.P. Structural, Electrical and Ethanol Sensing Properties of Double-Doping LaFeO3 Perovskite Oxides. Ceram. Int. 2014, 40, 14367–14373. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Shikha, P.; Komal; Kang, T.S.; Randhawa, B.S. Mn Doping Induced Physico-Chemical Changes in LaCe Ferrite Nanofabricated by Ionic Liquid Assisted Hydrothermal Route. J. Alloys Compd. 2017, 701, 788–796. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-Type LaFe1−xMnxO3 (X= 0, 0.3, 0.5, 0.7, 1.0) Oxygen Carriers for Chemical-Looping Steam Methane Reforming: Oxidation Activity and Resistance to Carbon Formation. Korean J. Chem. Eng. 2017, 34, 1651–1660. [Google Scholar] [CrossRef]
- Sun, K.-M.; Song, X.-Z.; Wang, X.-F.; Li, X.; Tan, Z. Annealing Temperature-Dependent Porous ZnFe2O4 Olives Derived from Bimetallic Organic Frameworks for High-Performance Ethanol Gas Sensing. Mater. Chem. Phys. 2020, 241, 122379. [Google Scholar] [CrossRef]
- Zhu, H.; Gu, X.; Zuo, D.; Wang, Z.; Wang, N.; Yao, K. Microemulsion-Based Synthesis of Porous Zinc Ferrite Nanorods and Its Application in a Room-Temperature Ethanol Sensor. Nanotechnology 2008, 19, 405503. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Mandal, K.; Pati, S.P.; Das, D. High Performance Gas Sensing Based on Nano Rods of Nickel Ferrite Fabricated by a Facile Solvothermal Route. Mater. Res. Express 2018, 5, 065056. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.; Xu, X.; Cao, P.; Liu, W.; Xu, X.; Pei, S. Electrospinning Synthesis, Crystal Structure, and Ethylene Glycol Sensing Properties of Orthorhombic SmBO3 (B=Fe, Co) Perovskites. J. Alloys Compd. 2021, 876, 160211. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.; Yun, P.; Sheng, H.; Xu, X.; Cao, P.; Pei, S.; Alhadi, A. Synthesis and Characterization of Ho-Doped SmFeO3 Nanofibers with Enhanced Glycol Sensing Properties. Vacuum 2021, 191, 110378. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.Y.; Xu, X.L.; Xu, X.H.; Pei, S.T.; Tie, Y.; Cao, P.F.; Liu, W.W.; Wang, B.J.; Zhang, R.; et al. Rough SmFeO3 Nanofibers as an Optimization Ethylene Glycol Gas Sensor Prepared by Electrospinning. Mater. Lett. 2020, 268, 127575. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.C.; Mhlongo, G.H. LaBO3 (B= Fe, Co) Nanofibers and Their Structural, Luminescence and Gas Sensing Characteristics. Phys. B Condens. Matter 2020, 578, 411883. [Google Scholar] [CrossRef]
- Mun, T.; Koo, J.Y.; Lee, J.; Kim, S.J.; Umarji, G.; Amalnerkar, D.; Lee, W. Resistive-Type Lanthanum Ferrite Oxygen Sensor Based on Nanoparticle-Assimilated Nanofiber Architecture. Sens. Actuators B Chem. 2020, 324, 128712. [Google Scholar] [CrossRef]
- Queraltó, A.; Graf, D.; Frohnhoven, R.; Fischer, T.; Vanrompay, H.; Bals, S.; Bartasyte, A.; Mathur, S. LaFeO3 Nanofibers for High Detection of Sulfur-Containing Gases. ACS Sustain. Chem. Eng. 2019, 7, 6023–6032. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 Hollow Nanofibers as a Highly Efficient Gas Sensor for Acetone Detection. Sens. Actuators B Chem. 2018, 255, 2546–2554. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, Y.; Jiang, B.; He, J.; Zheng, X.; Liang, Q. Effect of Lanthanides on Acetone Sensing Properties of LnFeO3 Nanofibers (Ln = La, Nd, and Sm). IEEE Sens. J. 2017, 17, 2404–2410. [Google Scholar] [CrossRef]
- Wei, W.; Guo, S.; Chen, C.; Sun, L.; Chen, Y.; Guo, W.; Ruan, S. High Sensitive and Fast Formaldehyde Gas Sensor Based on Ag-Doped LaFeO3 Nanofibers. J. Alloys Compd. 2017, 695, 1122–1127. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Q.; Huang, Y.; Xu, K.; Chu, P.K.; Ma, F. Oxygen Vacancy Enhanced Gas-Sensing Performance of CeO2/Graphene Heterostructure at Room Temperature. Anal. Chem. 2018, 90, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Li, C.; Han, D.; Wang, Z. Manipulating the Defect Structure (VO) of In2O3 Nanoparticles for Enhancement of Formaldehyde Detection. ACS Appl. Mater. Interfaces 2018, 10, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Xu, Y.; Yang, K.; Ma, J.; Li, C.; Wang, H.; Jia, L. Mo Doped BiVO4 Gas Sensor with High Sensitivity and Selectivity towards H2S. Chem. Eng. J. 2020, 395, 125144. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The Role of Oxygen Vacancies of ABO3 Perovskite Oxides in the Oxygen Reduction Reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Smiy, S.; Bejar, M.; Dhahri, E.; Fiorido, T.; Aguir, K.; Bendahan, M. Preparation of Double-Doping LaFeO3 Thin Film for Ethanol Sensing Application. J. Mol. Struct. 2022, 1267, 133543. [Google Scholar] [CrossRef]
- Hu, J.; Chen, X.; Zhang, Y. Batch Fabrication of Formaldehyde Sensors Based on LaFeO3 Thin Film with Ppb-Level Detection Limit. Sens. Actuators B Chem. 2021, 349, 130738. [Google Scholar] [CrossRef]
- Smiy, S.; Bejar, M.; Dhahri, E.; Fiorido, T.; Bendahan, M.; Aguir, K. Ozone Detection Based on Nanostructured La0.8Pb0.1Ca0.1Fe0.8Co0.2O3 Thin Films. J. Alloys Compd. 2020, 829, 154596. [Google Scholar] [CrossRef]
- Cao, E.; Chu, Z.; Wang, H.; Hao, W.; Sun, L.; Zhang, Y. Effect of Film Thickness on the Electrical and Ethanol Sensing Characteristics of LaFeO3 Nanoparticle-Based Thick Film Sensors. Ceram. Int. 2018, 44, 7180–7185. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Wang, X.; Li, L.; Hu, J. Acetone Sensing Properties and Mechanism of Nano-LaFeO3 Thick-Films. Sens. Actuators B Chem. 2016, 235, 56–66. [Google Scholar] [CrossRef]
- Nieto, A.; Hernández, T.; Rivera De la Rosa, J.; Garza Méndez, F.J.; Olvera, M.d.l.L.; Serrano, T.; Gómez, I.; Kharisov, B. Investigation of Sm1−xCaxFe0.7Co0.3O3 Films (x = 0.0–0.3) Prepared by Modified Sol–Gel Spin-Coating Method for Carbon Monoxide and Propane Gas Sensing. J. Mater. Sci. Mater. Electron. 2018, 29, 15587–15596. [Google Scholar] [CrossRef]
- Meng, F.; Qin, L.; Gao, H.; Zhu, H.; Yuan, Z. Perovskite-Structured LaFeO3 Modified In2O3 Gas Sensor with High Selectivity and Ultra-Low Detection Limit for 2-Butanone. J. Alloys Compd. 2024, 970, 172464. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Li, F.; Yang, Y.; Yu, H.; Dong, X. Perovskite-Structured LaFeO3 Gas Sensor Modified by Polyoxometalate Electron Acceptor with High Sensitivity and Low Detection Limit for Acetone Gas☆. J. Rare Earths 2024. [Google Scholar] [CrossRef]
- Xia, Z.; Zheng, C.; Hu, J.; Yuan, Q.; Zhang, C.; Zhang, J.; He, L.; Gao, H.; Jin, L.; Chu, X.; et al. Synthesis of SnO2 Quantum Dot Sensitized LaFeO3 for Conductometric Formic Acid Gas Sensors. Sens. Actuators B Chem. 2023, 379, 133198. [Google Scholar] [CrossRef]
- Zhang, N.; Ruan, S.; Yin, Y.; Li, F.; Wen, S.; Chen, Y. Self-Sacrificial Template-Driven LaFeO3/α-Fe2O3 Porous Nano-Octahedrons for Acetone Sensing. ACS Appl. Nano Mater. 2018, 1, 4671–4681. [Google Scholar] [CrossRef]
- Rong, Q.; Zhang, Y.; Li, K.; Wang, H.; Hu, J.; Zhu, Z.; Zhang, J.; Liu, Q. Ag-LaFeO3/NCQDs p-n heterojunctions for superior methanol gas sensing performance. Mater. Res. Bull. 2019, 115, 55–64. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. C-Doped LaFeO3 Porous Nanostructures for Highly Selective Detection of Formaldehyde. Sens. Actuators B Chem. 2021, 347, 130550. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, D.; Miao, T.; Liu, W.; Chen, J.; Cheng, B.; Qin, H.; Hu, J. Highly Sensitive P-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds. Chemosensors 2023, 11, 187. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Wu, Z.; Xiong, X.; Li, J.; Zhou, J.; Gao, X. Excellent Ethanol Sensor Based on LaFeO3 Modified with Gold Nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 27587–27595. [Google Scholar] [CrossRef]
- Li, F.; Lv, Q.; Gao, L.; Zhong, X.; Zhou, J.; Gao, X. Ultra-Sensitive Ag-LaFeO3 for Selective Detection of Ethanol. J. Mater. Sci. 2021, 56, 15061–15068. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Acetone Sensing Behavior of P-SmFeO3/n-ZnO Nanocomposite Synthesized by Thermal Treatment Method. Sens. Actuators B Chem. 2020, 304, 127252. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.; Zou, H.; Zhang, Y.; Hu, J.; Wang, H.; Zi, B.; Zhang, J.; Zhu, Z.; Duan, L.; et al. Microwave-Assisted Synthesis of Porous and Hollow α-Fe2O3/LaFeO3 Nanostructures for Acetone Gas Sensing as Well as Photocatalytic Degradation of Methylene Blue. Nanotechnology 2020, 31, 215601. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Rong, Q.; Zhu, Z.; Liu, Q.; Zhang, J. High Selectivity Methanol Sensor Based on Co-Fe2O3/SmFeO3 p-n Heterojunction Composites. J. Alloys Compd. 2018, 765, 193–200. [Google Scholar] [CrossRef]
- Li, L.; Qin, H.; Zhang, L.; Hu, J. Ultrasensitive Sensing Performances to Sub-Ppb Level Acetone for Pd-Functionalized SmFeO3 Packed Powder Sensors. RSC Adv. 2016, 6, 60967–60974. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Pei, J.; Chen, Y.; Li, L.; Xie, J.; Hu, J. Sensing Performances to Low Concentration Acetone for Palladium Doped LaFeO3 Sensors. J. Rare Earths 2016, 34, 704–710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).