The Use of Drosophila to Understand Psychostimulant Responses
Abstract
:1. Introduction
1.1. Drosophila as a Model Organism to Study Addiction
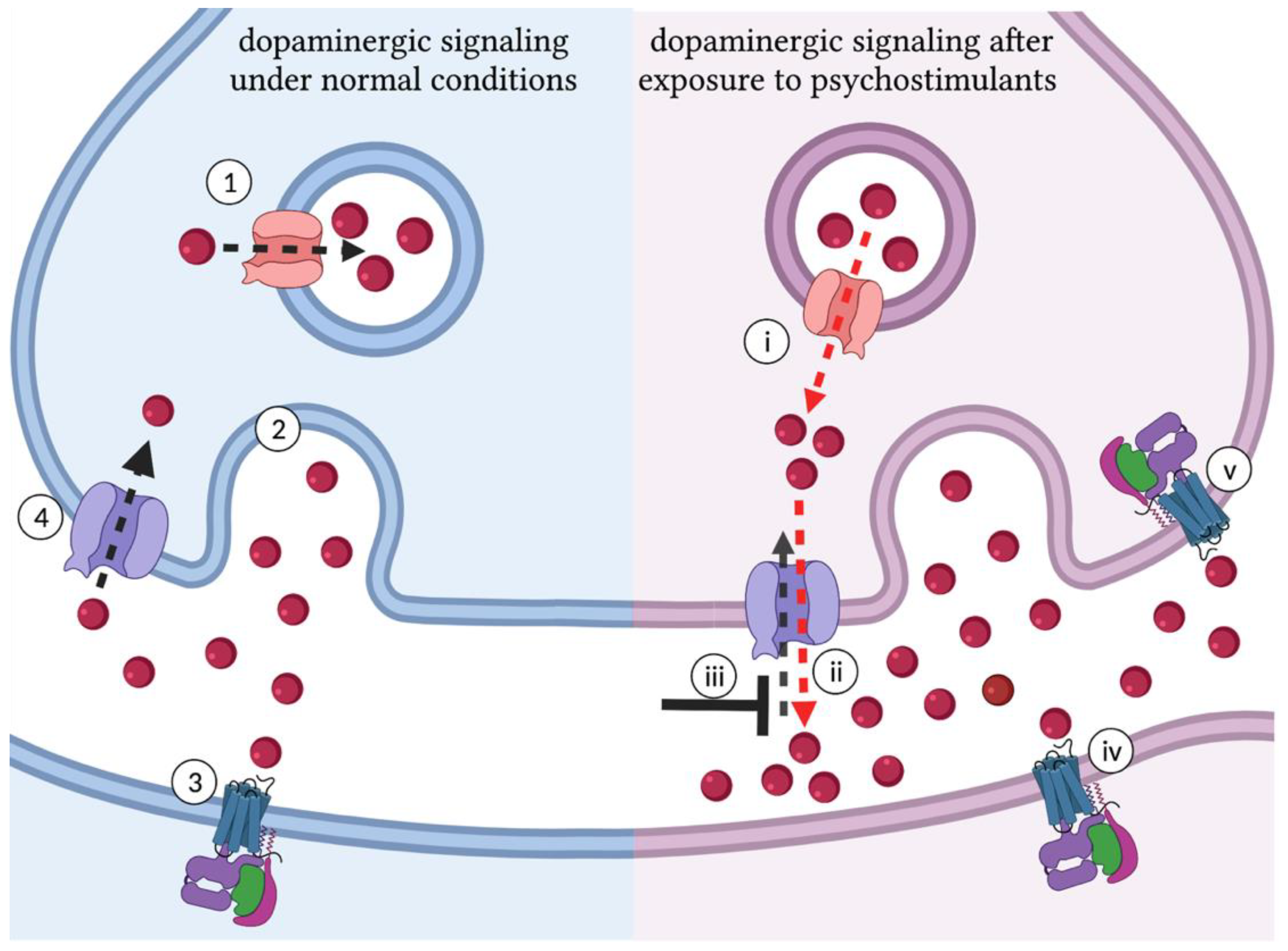
1.2. Dopamine Is Central to the Molecular Mechanisms of Psychostimulant Response
1.3. Behavioral Responses to Psychostimulants
2. Measuring Behavioral Responses to Psychostimulants in Drosophila
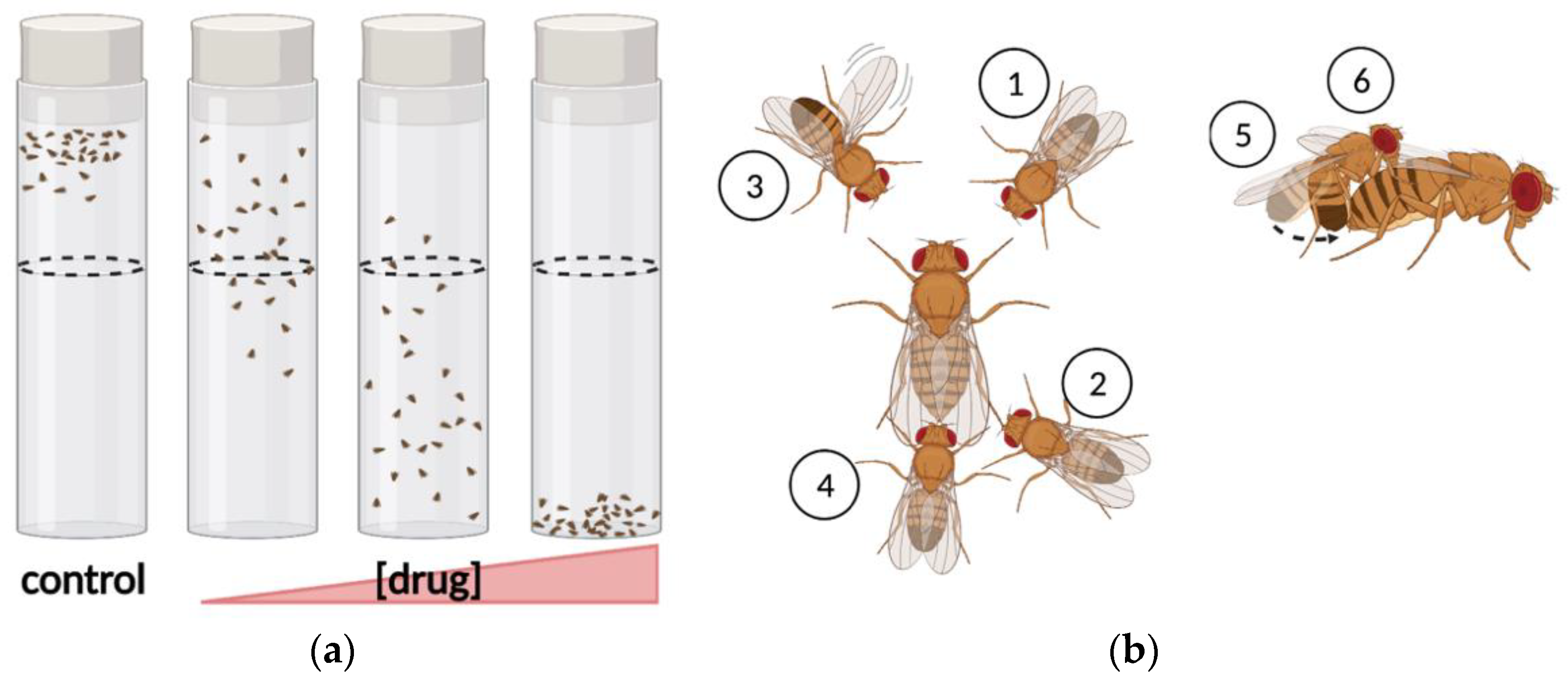
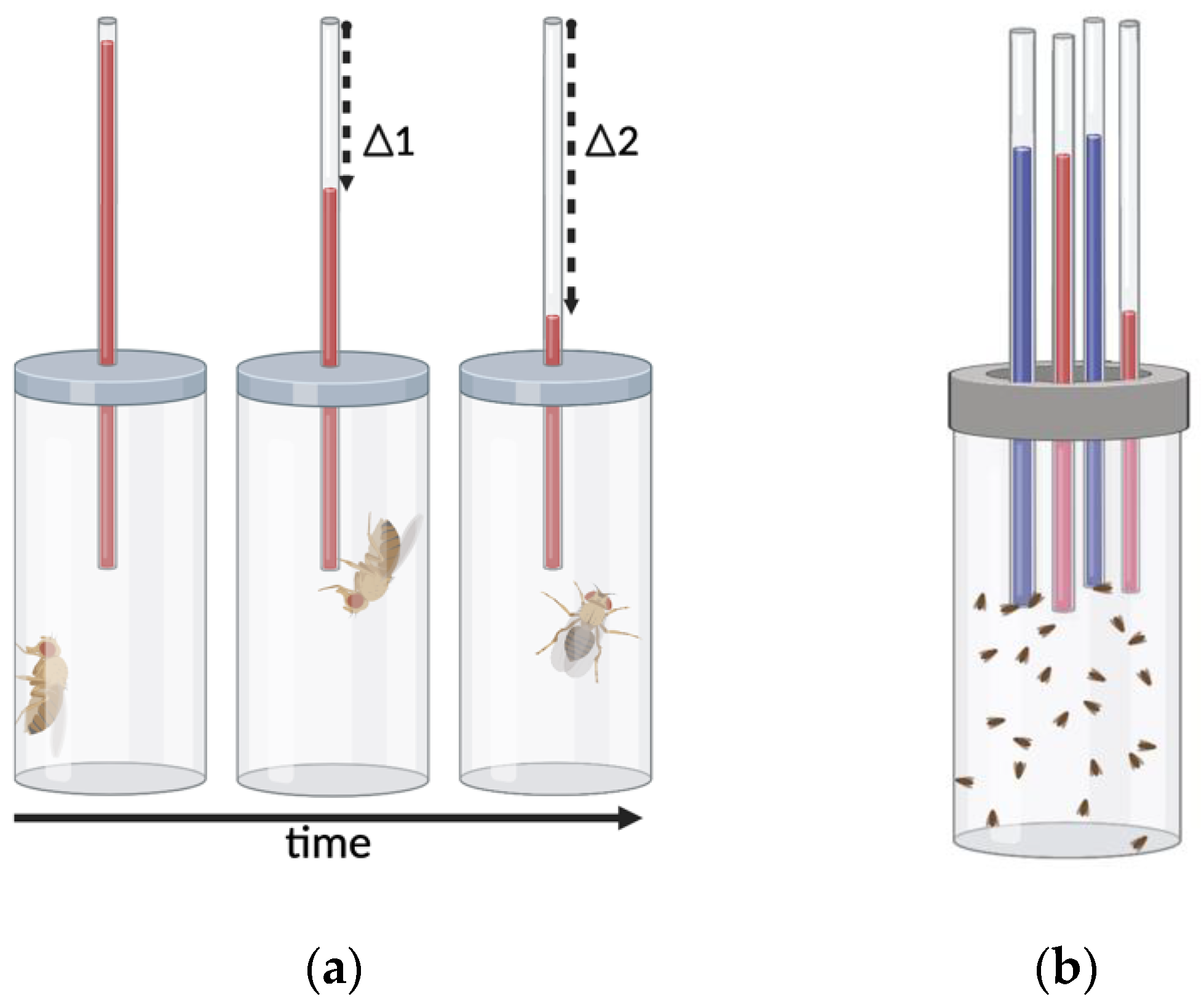
2.1. Assays of Motor-Activity
2.2. Assays of Motor-Impairment
2.3. Assays of Consumption and Preference
2.4. Attention-like Processes
3. Studying the Therapeutic Use of Psychostimulants with Drosophila
3.1. Attention Deficit Hyperactivity Disorder (ADHD)
3.2. Autism Spectrum Disorder (ASD)
4. Studying Psychostimulant Abuse with Drosophila
4.1. Using Drosophila to Study the Mechanism of Action of Psychostimulant Drugs
4.2. Using Drosophila to Identify Novel Genes Involved in Response to Psychostimulant Drugs
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dela Peña, I.; Gevorkiana, R.; Shi, W.X. Psychostimulants Affect Dopamine Transmission through Both Dopamine Transporter-Dependent and Independent Mechanisms. Eur. J. Pharmacol. 2015, 764, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Karch, S.B. Cocaine: History, Use, Abuse. J. R. Soc. Med. 1999, 92, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, N. Amphetamine-Type Stimulants: The Early History of Their Medical and Non-Medical Uses. Int. Rev. Neurobiol. 2015, 120, 9–25. [Google Scholar] [CrossRef]
- Guttmann, E.; Sargant, W. Observations on Benzedrine. BMJ 1937, 1, 1013–1015. [Google Scholar] [CrossRef] [Green Version]
- UNODC, I. World Drug Report; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative Efficacy and Tolerability of Medications for Attention-Deficit Hyperactivity Disorder in Children, Adolescents, and Adults: A Systematic Review and Network Meta-Analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Barateau, L.; Lopez, R.; Dauvilliers, Y. Treatment Options for Narcolepsy. CNS Drugs 2016, 30, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Devilbiss, D.M. Psychostimulants as Cognitive Enhancers: The Prefrontal Cortex, Catecholamines, and Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2011, 69, e101–e111. [Google Scholar] [CrossRef] [Green Version]
- Nickels, K.C.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Voigt, R.G.; Barbaresi, W.J. Stimulant Medication Treatment of Target Behaviors in Children with Autism: A Population-Based Study. J. Dev. Behav. Pediatr. 2008, 29, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, S.; Castelnau, P.; Morcillo, C.; Roux, S.; Bonnet-Brilhault, F. Psychostimulants for ADHD-like Symptoms in Individuals with Autism Spectrum Disorders. Expert Rev. Neurother. 2012, 12, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Ducci, F.; Goldman, D. The Genetic Basis of Addictive Disorders. Psychiatr. Clin. N. Am. 2012, 35, 495–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, N.A.; Yamamoto, K.M.; Froehlich, T.E. Genetic Influence on Efficacy of Pharmacotherapy for Pediatric Attention-Deficit/Hyperactivity Disorder: Overview and Current Status of Research. CNS Drugs 2020, 34, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Hewitt, J.K.; Young, S.E.; Corley, R.P.; Crowley, T.J.; Stallings, M.C. Genetic and Environmental Influences on Substance Initiation, Use, and Problem Use in Adolescents. Arch. Gen. Psychiatry 2003, 60, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendler, K.S.; Aggen, S.H.; Tambs, K.; Reichborn-Kjennerud, T. Illicit Psychoactive Substance Use, Heavy Use, Abuse, and Dependence in a US Population-Based Sample of Male Twins Kenneth. Psychol. Med. 2006, 36, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Goldman, D.; Oroszi, G.; Ducci, F. The Genetics of Addictions: Uncovering the Genes. Nat. Rev. Genet. 2005, 6, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Gardner, C.; Jacobson, K.C.; Neale, M.C.; Prescott, C.A. Genetic and Environmental Influences on Illicit Drug Use and Tobacco Use across Birth Cohorts. Psychol. Med. 2005, 35, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Roden, D.M.; George, A.L. The Genetic Basis of Variability in Drug Responses. Nat. Rev. Drug Discov. 2002, 1, 37–44. [Google Scholar] [CrossRef]
- del Valle Rodríguez, A.; Didiano, D.; Desplan, C. Power Tools for Gene Expression and Clonal Analysis in Drosophila. Nat. Methods 2011, 9, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, B.D.; Jenett, A.; Hammonds, A.S.; Ngo, T.-T.B.; Misra, S.; Murphy, C.; Scully, A.; Carlson, J.W.; Wan, K.H.; Laverty, T.R.; et al. Tools for Neuroanatomy and Neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 9715–9720. [Google Scholar] [CrossRef] [Green Version]
- Beumer, K.J.; Carroll, D. Targeted Genome Engineering Techniques in Drosophila. Methods 2014, 68, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Sivanantharajah, L.; Zhang, B. Current Techniques for High-Resolution Mapping of Behavioral Circuits in Drosophila. J. Comp. Physiol. A 2015, 201, 895–909. [Google Scholar] [CrossRef]
- Pfeiffer, B.D.; Ngo, T.T.B.; Hibbard, K.L.; Murphy, C.; Jenett, A.; Truman, J.W.; Rubin, G.M. Refinement of Tools for Targeted Gene Expression in Drosophila. Genet. 2010, 186, 735–755. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Ho, M.C.W.; Liu, Q.; Horiuchi, W.; Lin, C.C.; Task, D.; Luan, H.; White, B.H.; Potter, C.J.; Wu, M.N. A Genetic Toolkit for Dissecting Dopamine Circuit Function in Drosophila. Cell Rep. 2018, 23, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A Systematic Analysis of Human Disease-Associated Gene Sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Pandey, U.B.; Nichols, C.D. Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [Green Version]
- Kaun, K.R.; Azanchi, R.; Maung, Z.; Hirsh, J.; Heberlein, U. A Drosophila Model for Alcohol Reward. Nat. Neurosci. 2011, 14, 612–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.M.; Heberlein, U. Genetic Control of Acute Ethanol-Induced Behaviors in Drosophila. Alcohol. Clin. Exp. Res. 2000, 24, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Lathen, D.R.; Merrill, C.B.; Rothenfluh, A. Flying Together: Drosophila as a Tool to Understand the Genetics of Human Alcoholism. Int. J. Mol. Sci. 2020, 21, 6649. [Google Scholar] [CrossRef] [PubMed]
- Chvilicek, M.M.; Titos, I.; Rothenfluh, A. The Neurotransmitters Involved in Drosophila Alcohol-Induced Behaviors. Front. Behav. Neurosci. 2020, 14, 607700. [Google Scholar] [CrossRef] [PubMed]
- Deadwyler, S.A. Electrophysiological Correlates of Abused Drugs: Relation to Natural Rewards. Ann. N. Y. Acad. Sci. 2010, 1187, 140–147. [Google Scholar] [CrossRef]
- Hyman, S.E.; Malenka, R.C.; Nestler, E.J. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef] [Green Version]
- Baik, J.H. Dopamine Signaling in Reward-Related Behaviors. Front. Neural Circuits 2013, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.K.; Batchelor, H.M.; Liu, B.; Khanna, A.; Morales, M.; Schoenbaum, G. Dopamine Neurons Respond to Errors in the Prediction of Sensory Features of Expected Rewards. Neuron 2017, 95, 1395–1405.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Chiara, G.; Bassareo, V. Reward System and Addiction: What Dopamine Does and Doesn’t Do. Curr. Opin. Pharmacol. 2007, 7, 69–76. [Google Scholar] [CrossRef]
- Schultz, W. Predictive Reward Signal of Dopamine Neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.D.; Eiden, L.E. Functional Identification and Molecular Cloning of a Human Brain Vesicle Monoamine Transporter. J. Neurochem. 1993, 61, 2314–2317. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Reith, M.E.A. Structure and Function of the Dopamine Transporter. Eur. J. Pharmacol. 2000, 405, 329–339. [Google Scholar] [CrossRef]
- Imperato, A. Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and Psychostimulant Recognition by the Dopamine Transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Riddle, E.L.; Fleckenstein, A.E.; Hanson, G.R. Role of Monoamine Transporters in Mediating Psychostimulant Effects. AAPS J. 2005, 7, 847–851. [Google Scholar] [CrossRef]
- Sulzer, D.; Chen, T.K.; Lau, Y.Y.; Kristensen, H.; Rayport, S.; Ewing, A. Amphetamine Redistributes Dopamine from Synaptic Vesicles to the Cytosol and Promotes Reverse Transport. J. Neurosci. 1995, 15, 4102–4108. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of Neurotransmitter Release by Amphetamines: A Review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef]
- Venton, B.J.; Seipel, A.T.; Phillips, P.E.M.; Wetsel, W.C.; Gitler, D.; Greengard, P.; Augustine, G.J.; Wightman, R.M. Cocaine Increases Dopamine Release by Mobilization of a Synapsin-Dependent Reserve Pool. J. Neurosci. 2006, 26, 3206–3209. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gu, H.H.; Zhan, C.G. Mechanism for Cocaine Blocking the Transport of Dopamine: Insights from Molecular Modeling and Dynamics Simulations. J. Phys. Chem. B 2009, 113, 15057–15066. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Baler, R.; Telang, F. Imaging Dopamine’s Role in Drug Abuse and Addiction. Neuropharmacology 2009, 56, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Drevets, W.C.; Gautier, C.; Price, J.C.; Kupfer, D.J.; Kinahan, P.E.; Grace, A.A.; Price, J.L.; Mathis, C.A. Amphetamine-Induced Dopamine Release in Human Ventral Striatum Correlates with Euphoria. Biol. Psychiatry 2001, 49, 81–96. [Google Scholar] [CrossRef]
- Ellinwood, E.H.; Balster, R.L. Rating the Behavioral Effects of Amphetamine. Eur. J. Pharmacol. 1974, 28, 35–41. [Google Scholar] [CrossRef]
- McClung, C.; Hirsh, J. Stereotypic Behavioral Responses to Free-Base Cocaine and the Development of Behavioral Sensitization in Drosophila. Curr. Biol. 1998, 8, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Fiorino, D.F.; Phillips, A.G. Facilitation of Sexual Behavior in Male Rats Following D-Amphetamine-Induced Behavioral Sensitization. Psychopharmacology 1999, 142, 200–208. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Wilkins, M.F.; DiPietro, N.; Benibgui, M.; Toledano, R.; Rowe, A.; Couch, M.C. Inhibitory and Disinhibitory Effects of Psychomotor Stimulants and Depressants on the Sexual Behavior of Male and Female Rats. Horm. Behav. 2010, 58, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Andretic, R.; Van Swinderen, B.; Greenspan, R.J. Dopaminergic Modulation of Arousal in Drosophila. Curr. Biol. 2005, 15, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadoni, C.; Solinas, M.; Di Chiara, G. Psychostimulant Sensitization: Differential Changes in Accumbal Shell and Core Dopamine. Eur. J. Pharmacol. 2000, 388, 69–76. [Google Scholar] [CrossRef]
- Chen, R.; Tilley, M.R.; Wei, H.; Zhou, F.; Zhou, F.; Ching, S.; Quan, N.; Stephens, R.L.; Hill, E.R.; Nottoli, T.; et al. Abolished Cocaine Reward in Mice with a Cocaine-Insensitive Dopamine Transporter. Proc. Natl. Acad. Sci. USA 2006, 103, 9333–9338. [Google Scholar] [CrossRef] [Green Version]
- Dluzen, D.E.; Bhatt, S.; McDermott, J.L. Differences in Reserpine-Induced Striatal Dopamine Output and Content between Female and Male Mice: Implications for Sex Differences in Vesicular Monoamine Transporter 2 Function. Neuroscience 2008, 154, 1488–1496. [Google Scholar] [CrossRef]
- Brennan, K.A.; Carati, C.; Lea, R.A.; Fitzmaurice, P.S.; Schenk, S. Effect of D1-like and D2-like Receptor Antagonists on Methamphetamine and 3,4-Methylenedioxymethamphetamine Self-Administration in Rats. Behav. Pharmacol. 2009, 20, 688–694. [Google Scholar] [CrossRef]
- Makos, M.A.; Han, K.A.; Heien, M.L.; Ewing, A.G. Using in Vivo Electrochemistry to Study the Physiological Effects of Cocaine and Other Stimulants on the Drosophila melanogaster Dopamine Transporter. ACS Chem. Neurosci. 2010, 1, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Bainton, R.J.; Tsai, L.T.Y.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine Modulates Acute Responses to Cocaine, Nicotine and Ethanol in Drosophila. Curr. Biol. 2000, 10, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Freyberg, Z.; Sonders, M.S.; Aguilar, J.I.; Hiranita, T.; Karam, C.S.; Flores, J.; Pizzo, A.B.; Zhang, Y.; Farino, Z.J.; Chen, A.; et al. Mechanisms of Amphetamine Action Illuminated through Optical Monitoring of Dopamine Synaptic Vesicles in Drosophila Brain. Nat. Commun. 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kanno, M.; Hiramatsu, S.; Kondo, S.; Tanimoto, H.; Ichinose, T. Voluntary Intake of Psychoactive Substances Is Regulated by the Dopamine Receptor Dop1R1 in Drosophila. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The Neural Basis of Drug Craving: An Incentive-Sensitization Theory of Addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- McClung, C.; Hirsh, J. The Trace Amine Tyramine Is Essential for Sensitization to Cocaine in Drosophila. Curr. Biol. 1999, 9, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Andretic, R.; Chaney, S.; Hirsh, J. Requirement of Circadian Genes for Cocaine Sensitization in Drosophila. Science 1999, 285, 1066–1068. [Google Scholar] [CrossRef]
- Kuribara, H. Effects of Interdose Interval on Ambulatory Sensitization to Methamphetamine, Cocaine and Morphine in Mice. Eur. J. Pharmacol. 1996, 316, 1–5. [Google Scholar] [CrossRef]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor Activity Level Monitoring Using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 5, 1238–1242. [Google Scholar] [CrossRef]
- Cichewicz, K.; Hirsh, J. ShinyR-DAM: A Program Analyzing Drosophila Activity, Sleep and Circadian Rhythms. Commun. Biol. 2018, 1, 25. [Google Scholar] [CrossRef]
- Dimitrijevic, N.; Dzitoyeva, S.; Manev, H. An Automated Assay of the Behavioral Effects of Cocaine Injections in Adult Drosophila. J. Neurosci. Methods 2004, 137, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Filošević, A.; Al-Samarai, S.; Andretić Waldowski, R. High Throughput Measurement of Locomotor Sensitization to Volatilized Cocaine in Drosophila melanogaster. Front. Mol. Neurosci. 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Rigo, F.; Filošević, A.; Petrović, M.; Jović, K.; Andretić Waldowski, R. Locomotor Sensitization Modulates Voluntary Self-Administration of Methamphetamine in Drosophila melanogaster. Addict. Biol. 2021, 26, e12963. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, J.; Pistovcakova, J.; Vrskova, D.; Dusek, L.; Sulcova, A. The Effects of Methamphetamine Self-Administration on Behavioural Sensitization in the Olfactory Bulbectomy Rat Model of Depression. Int. J. Neuropsychopharmacol. 2012, 15, 1503–1511. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Grygoruk, A.; Brooks, E.S.; Ackerson, L.C.; Maidment, N.T.; Bainton, R.J.; Krantz, D.E. Overexpression of the Drosophila Vesicular Monoamine Transporter Increases Motor Activity and Courtship but Decreases the Behavioral Response to Cocaine. Mol. Psychiatry 2006, 11, 99–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.F.; Daniels, R.; Romero-Calderón, R.; Grygoruk, A.; Chang, H.Y.; Najibi, R.; Shamouelian, D.; Salazar, E.; Solomon, M.; Ackerson, L.C.; et al. Drosophila Vesicular Monoamine Transporter Mutants Can Adapt to Reduced or Eliminated Vesicular Stores of Dopamine and Serotonin. Genetics 2009, 181, 525–541. [Google Scholar] [CrossRef] [Green Version]
- Branson, K.; Robie, A.A.; Bender, J.; Perona, P.; Dickinson, M.H. High-Throughput Ethomics in Large Groups of Drosophila. Nat. Methods 2009, 6, 451–457. [Google Scholar] [CrossRef]
- Scaplen, K.M.; Mei, N.J.; Bounds, H.A.; Song, S.L.; Azanchi, R.; Kaun, K.R. Automated Real-Time Quantification of Group Locomotor Activity in Drosophila melanogaster. Sci. Rep. 2019, 9, 4427. [Google Scholar] [CrossRef]
- Kabra, M.; Robie, A.A.; Rivera-Alba, M.; Branson, S.; Branson, K. JAABA: Interactive Machine Learning for Automatic Annotation of Animal Behavior. Nat. Methods 2013, 10, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Lebestky, T.; Chang, J.S.C.; Dankert, H.; Zelnik, L.; Kim, Y.C.; Han, K.A.; Wolf, F.W.; Perona, P.; Anderson, D.J. Two Different Forms of Arousal in Drosophila Are Oppositely Regulated by the Dopamine D1 Receptor Ortholog DopR via Distinct Neural Circuits. Neuron 2009, 64, 522–536. [Google Scholar] [CrossRef] [Green Version]
- Rothenfluh, A.; Threlkeld, R.J.; Bainton, R.J.; Tsai, L.T.Y.; Lasek, A.W.; Heberlein, U. Distinct Behavioral Responses to Ethanol Are Regulated by Alternate RhoGAP18B Isoforms. Cell 2006, 127, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, F.W.; Rodan, A.R.; Tsai, L.T.Y.; Heberlein, U. High-Resolution Analysis of Ethanol-Induced Locomotor Stimulation in Drosophila. J. Neurosci. 2002, 22, 11035–11044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.H.; Bae, J.E.; Lee, S.H.; Lee, S.D.; Chae, K.S. Effects of Gravity on Positive Phototaxis in Fruit Fly Drosophila melanogaster. Entomol. Res. 2016, 46, 272–277. [Google Scholar] [CrossRef]
- Fedele, G.; Green, E.W.; Rosato, E.; Kyriacou, C.P. An Electromagnetic Field Disrupts Negative Geotaxis in Drosophila via a CRY-Dependent Pathway. Nat. Commun. 2014, 5, 4391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejima, A.; Griffith, L.C. Courtship Initiation Is Stimulated by Acoustic Signals in Drosophila melanogaster. PLoS ONE 2008, 3, e3246. [Google Scholar] [CrossRef]
- Ribeiro, I.M.A.; Drews, M.; Bahl, A.; Machacek, C.; Borst, A.; Dickson, B.J. Visual Projection Neurons Mediating Directed Courtship in Drosophila. Cell 2018, 174, 607–621.e18. [Google Scholar] [CrossRef] [Green Version]
- Manoli, D.S.; Foss, M.; Villella, A.; Taylor, B.J.; Hall, J.C.; Baker, B.S. Male-Specific Fruitless Specifies the Neural Substrates of Drosophila Courtship Behaviour. Nature 2005, 436, 395–400. [Google Scholar] [CrossRef]
- Krstic, D.; Boll, W.; Noll, M. Sensory Integration Regulating Male Courtship Behavior in Drosophila. PLoS ONE 2009, 4, e4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzer, S. Behavioral Mutants of Drosophila Isolated by Countercurrent Distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Tsai, L.T.Y.; Bainton, R.J.; Blau, J.; Heberlein, U. Lmo Mutants Reveal a Novel Role for Circadian Pacemaker Neurons in Cocaine-Induced Behaviors. PLoS Biol. 2004, 2, e408. [Google Scholar] [CrossRef]
- George, R.; Lease, K.; Burnette, J.; Hirsh, J. A “Bottom-Counting” Video System for Measuring Cocaine-Induced Behaviors in Drosophila. Methods Enzymol. 2005, 393, 841–851. [Google Scholar] [CrossRef]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid Iterative Negative Geotaxis (RING): A New Method for Assessing Age-Related Locomotor Decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Ferveur, J.-F.; Greenspan, R.J. Courtship Behavior of Brain Mosaics in Drosophila. J. Neurogenet. 1998, 12, 205–226. [Google Scholar] [CrossRef]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; de la Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE Assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belovich, A.N.; Aguilar, J.I.; Mabry, S.J.; Cheng, M.H.; Zanella, D.; Hamilton, P.J.; Stanislowski, D.J.; Shekar, A.; Foster, J.D.; Bahar, I.; et al. A Network of Phosphatidylinositol (4,5)-Bisphosphate (PIP2) Binding Sites on the Dopamine Transporter Regulates Amphetamine Behavior in Drosophila melanogaster. Mol. Psychiatry 2021, 26, 4417–4430. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Carbone, M.A.; Huang, W.; Anholt, R.R.H.; Mackay, T.F.C. Genetic Basis of Variation in Cocaine and Methamphetamine Consumption in Outbred Populations of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2021, 118, e2104131118. [Google Scholar] [CrossRef] [PubMed]
- Highfill, C.A.; Baker, B.M.; Stevens, S.D.; Anholt, R.R.H.; Mackay, T.F.C. Genetics of Cocaine and Methamphetamine Consumption and Preference in Drosophila melanogaster. PLoS Genet. 2019, 15, e1007834. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Guo, J.; Liu, L.; Zhao, X.; Li, D.; Li, H.; Zhao, Q.; Wang, Y.; Qian, Q.; Wang, Y.; et al. The Neural Correlations of Spatial Attention and Working Memory Deficits in Adults with ADHD. NeuroImage Clin. 2019, 22, 101728. [Google Scholar] [CrossRef] [PubMed]
- Lev, A.; Braw, Y.; Elbaum, T.; Wagner, M.; Rassovsky, Y. Eye Tracking During a Continuous Performance Test: Utility for Assessing ADHD Patients. J. Atten. Disord. 2020, 26, 245–255. [Google Scholar] [CrossRef] [PubMed]
- van Swinderen, B. Attention-Like Processes in Drosophila Require Short-Term Memory Genes. Science 2007, 315, 1590–1593. [Google Scholar] [CrossRef]
- Van Swinderen, B.; Greenspan, R.J. Salience Modulates 20–30 Hz Brain Activity in Drosophila. Nat. Neurosci. 2003, 6, 579–586. [Google Scholar] [CrossRef]
- Wolf, R.; Heisenberg, M. On the Fine Structure of Yaw Torque in Visual Flight Orientation of Drosophila melanogaster—II. A Temporally and Spatially Variable Weighting Function for the Visual Field (‘visual Attention’). J. Comp. Physiol. A 1980, 140, 69–80. [Google Scholar] [CrossRef]
- Lehmann, F.O.; Dickinson, M.H. The Changes in Power Requirements and Muscle Efficiency during Elevated Force Production in the Fruit Fly Drosophila melanogaster. J. Exp. Biol. 1997, 200, 1133–1143. [Google Scholar] [CrossRef]
- Wolf, R.; Heisenberg, M. Visual Space from Visual Motion: Turn Integration in Tethered Flying Drosophila. Learn. Mem. 1997, 4, 318–327. [Google Scholar] [CrossRef] [Green Version]
- van Swinderen, B.; Brembs, B. Attention-Like Deficit and Hyperactivity in a Drosophila Memory Mutant. J. Neurosci. 2010, 30, 1003–1014. [Google Scholar] [CrossRef]
- Money, K.M.; Stanwood, G.D. Developmental Origins of Brain Disorders: Roles for Dopamine. Front. Cell. Neurosci. 2013, 7, 260. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sham, P.C.; Owen, M.J.; He, L. Meta-Analysis Shows Significant Association between Dopamine System Genes and Attention Deficit Hyperactivity Disorder (ADHD). Hum. Mol. Genet. 2006, 15, 2276–2284. [Google Scholar] [CrossRef]
- Dalsgaard, S.; Nielsen, H.S.; Simonsen, M. Five-Fold Increase in National Prevalence Rates of Attention-Deficit/ Hyperactivity Disorder Medications for Children and Adolescents with Autism Spectrum Disorder, Attention-Deficit/Hyperactivity Disorder, and Other Psychiatric Disorders: A Danish Regist. J. Child Adolesc. Psychopharmacol. 2013, 23, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voet, M.; Harich, B.; Franke, B.; Schenck, A. ADHD-Associated Dopamine Transporter, Latrophilin and Neurofibromin Share a Dopamine-Related Locomotor Signature in Drosophila. Mol. Psychiatry 2016, 21, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, A.B.; Karam, C.S.; Zhang, Y.; Yano, H.; Freyberg, R.J.; Karam, D.S.; Freyberg, Z.; Yamamoto, A.; McCabe, B.D.; Javitch, J.A. The Membrane Raft Protein Flotillin-1 Is Essential in Dopamine Neurons for Amphetamine-Induced Behavior in Drosophila. Mol. Psychiatry 2013, 18, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, P.J.; Campbell, N.G.; Sharma, S.; Erreger, K.; Herborg Hansen, F.; Saunders, C.; Belovich, A.N.; Daly, M.J.; Gibbs, R.A.; Boerwinkle, E.; et al. De Novo Mutation in the Dopamine Transporter Gene Associates Dopamine Dysfunction with Autism Spectrum Disorder. Mol. Psychiatry 2013, 18, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, N.G.; Shekar, A.; Aguilar, J.I.; Peng, D.; Navratna, V.; Yang, D.; Morley, A.N.; Duran, A.M.; Galli, G.; O’Grady, B.; et al. Structural, Functional, and Behavioral Insights of Dopamine Dysfunction Revealed by a Deletion in SLC6A3. Proc. Natl. Acad. Sci. USA 2019, 116, 3853–3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartier, E.; Hamilton, P.J.; Belovich, A.N.; Shekar, A.; Campbell, N.G.; Saunders, C.; Andreassen, T.F.; Gether, U.; Veenstra-Vanderweele, J.; Sutcliffe, J.S.; et al. Rare Autism-Associated Variants Implicate Syntaxin 1 (STX1 R26Q) Phosphorylation and the Dopamine Transporter (HDAT R51W) in Dopamine Neurotransmission and Behaviors. EBioMedicine 2015, 2, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, P.J.; Belovich, A.N.; Khelashvili, G.; Saunders, C.; Erreger, K.; Javitch, J.A.; Sitte, H.H.; Weinstein, H.; Matthies, H.J.G.; Galli, A. PIP2 Regulates Psychostimulant Behaviors through Its Interaction with a Membrane Protein. Nat. Chem. Biol. 2014, 10, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Karam, C.S.; Williams, B.L.; Jones, S.K.; Javitch, J.A. The Role of the Dopamine Transporter in the Effects of Amphetamine on Sleep and Sleep Architecture in Drosophila. Neurochem. Res. 2021. [Google Scholar] [CrossRef]
- Pizzo, A.B.; Karam, C.S.; Zhang, Y.; Ma, C.L.; Mccabe, B.D.; Javitch, J.A. Amphetamine-Induced Behavior Requires CaMKII-Dependent Dopamine Transporter Phosphorylation. Mol. Psychiatry 2014, 19, 279–281. [Google Scholar] [CrossRef] [Green Version]
- van Swinderen, B.; Flores, K.A. Attention-like Processes Underlying Optomotor Performance in a Drosophila Choice Maze. Dev. Neurobiol. 2007, 67, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, G.; John, V.; Kapahi, P.; Bredesen, D.E. Alzheimer’s Model Develops Early ADHD Syndrome. J. Neurol. Neurophysiol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bainton, R.J.; Tsai, L.T.Y.; Schwabe, T.; DeSalvo, M.; Gaul, U.; Heberlein, U. Moody Encodes Two GPCRs That Regulate Cocaine Behaviors and Blood-Brain Barrier Permeability in Drosophila. Cell 2005, 123, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Sedore, S.A.; Cronmiller, C.; Hirsh, J. Type II CAMP-Dependent Protein Kinase-Deficient Drosophila Are Viable but Show Developmental, Circadian, and Drug Response Phenotypes. J. Biol. Chem. 2000, 275, 20588–20596. [Google Scholar] [CrossRef] [Green Version]
- Vanderwerf, S.M.; Buck, D.C.; Wilmarth, P.A.; Sears, L.M.; David, L.L.; Morton, D.B.; Neve, K.A. Role for Rab10 in Methamphetamine-Induced Behavior. PLoS ONE 2015, 10, e0136167. [Google Scholar] [CrossRef] [Green Version]
- Zerón-Rugerio, M.F.; Carpio-Arias, T.V.; Ferreira-García, E.; Díez-Noguera, A.; Cambras, T.; Alda, J.Á.; Izquierdo-Pulido, M. ADHD Subtypes Are Associated Differently with Circadian Rhythms of Motor Activity, Sleep Disturbances, and Body Mass Index in Children and Adolescents: A Case–Control Study. Eur. Child Adolesc. Psychiatry 2020, 30, 1917–1927. [Google Scholar] [CrossRef]
- Coogan, A.N.; Baird, A.L.; Popa-Wagner, A.; Thome, J. Circadian Rhythms and Attention Deficit Hyperactivity Disorder: The What, the When and the Why. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 67, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarver, D.E.; Rapport, M.D.; Kofler, M.J.; Raiker, J.S.; Friedman, L.M. Hyperactivity in Attention-Deficit/Hyperactivity Disorder (ADHD): Impairing Deficit or Compensatory Behavior? J. Abnorm. Child Psychol. 2015, 43, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Kume, K. Dopamine Is a Regulator of Arousal in the Fruit Fly. J. Neurosci. 2005, 25, 7377–7384. [Google Scholar] [CrossRef] [Green Version]
- Hansen, F.H.; Skjørringe, T.; Yasmeen, S.; Arends, N.V.; Sahai, M.A.; Erreger, K.; Andreassen, T.F.; Holy, M.; Hamilton, P.J.; Neergheen, V.; et al. Missense Dopamine Transporter Mutations Associate with Adult Parkinsonism and ADHD. J. Clin. Investig. 2014, 124, 3107–3120. [Google Scholar] [CrossRef] [Green Version]
- Mazei-Robison, M.S.; Couch, R.S.; Shelton, R.C.; Stein, M.A.; Blakely, R.D. Sequence Variation in the Human Dopamine Transporter Gene in Children with Attention Deficit Hyperactivity Disorder. Neuropharmacology 2005, 49, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Friedel, S.; Saar, K.; Sauer, S.; Dempfle, A.; Walitza, S.; Renner, T.; Romanos, M.; Freitag, C.; Seitz, C.; Palmason, H.; et al. Association and Linkage of Allelic Variants of the Dopamine Transporter Gene in ADHD. Mol. Psychiatry 2007, 12, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Herborg, F.; Andreassen, T.F.; Berlin, F.; Loland, C.J.; Gether, U. Neuropsychiatric Disease–Associated Genetic Variants of the Dopamine Transporter Display Heterogeneous Molecular Phenotypes. J. Biol. Chem. 2018, 293, 7250–7262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, J.I.; Cheng, M.H.; Font, J.; Schwartz, A.C.; Ledwitch, K.; Duran, A.; Mabry, S.J.; Belovich, A.N.; Zhu, Y.; Carter, A.M.; et al. Psychomotor Impairments and Therapeutic Implications Revealed by a Mutation Associated with Infantile Parkinsonism-Dystonia. eLife 2021, 10, e68039. [Google Scholar] [CrossRef]
- Palacio, J.D.; Castellanos, F.X.; Pineda, D.A.; Lopera, F.; Arcos-Burgos, M.; Quiroz, Y.T.; Henao, G.C.; Puerta, I.C.; Ramírez, D.L.; Rapoport, J.L.; et al. Attention-Deficit/Hyperactivity Disorder and Comorbidities in 18 Paisa Colombian Multigenerational Families. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 1506–1515. [Google Scholar] [CrossRef]
- Arcos-Burgos, M.; Jain, M.; Acosta, M.T.; Shively, S.; Stanescu, H.; Wallis, D.; Domené, S.; Vélez, J.I.; Karkera, J.D.; Balog, J.; et al. A Common Variant of the Latrophilin 3 Gene, LPHN3, Confers Susceptibility to ADHD and Predicts Effectiveness of Stimulant Medication. Mol. Psychiatry 2010, 15, 1053–1066. [Google Scholar] [CrossRef] [Green Version]
- Wallis, D.; Hill, D.S.; Mendez, I.A.; Abbott, L.C.; Finnell, R.H.; Wellman, P.J.; Setlow, B. Initial Characterization of Mice Null for Lphn3, a Gene Implicated in ADHD and Addiction. Brain Res. 2012, 1463, 85–92. [Google Scholar] [CrossRef]
- Regan, S.L.; Hufgard, J.R.; Pitzer, E.M.; Sugimoto, C.; Hu, Y.C.; Williams, M.T.; Vorhees, C.V. Knockout of Latrophilin-3 in Sprague-Dawley Rats Causes Hyperactivity, Hyper-Reactivity, under-Response to Amphetamine, and Disrupted Dopamine Markers. Neurobiol. Dis. 2019, 130, 104494. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, Y.; Kuromi, H.; Delgado, R.; Maureira, C.; Oliva, C.; Labarca, P. Synaptic Vesicle Pools and Plasticity of Synaptic Transmission at the Drosophila Synapse. Brain Res. Rev. 2004, 47, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Wu, C.-F. Unraveling Synaptic GCaMP Signals: Differential Excitability and Clearance Mechanisms Underlying Distinct Ca2+ Dynamics in Tonic and Phasic Excitatory, and Aminergic Modulatory Motor Terminals in Drosophila. eNeuro 2018, 5, ENEURO.0362-17.2018. [Google Scholar] [CrossRef] [Green Version]
- Hyman, S.L.; Shores, A.; North, K.N. The Nature and Frequency of Cognitive Deficits in Children with Neurofibromatosis Type 1. Neurology 2005, 65, 1037–1044. [Google Scholar] [CrossRef]
- Mautner, V.F.; Granström, S.; Leark, R.A. Impact of ADHD in Adults with Neurofibromatosis Type 1: Associated Psychological and Social Problems. J. Atten. Disord. 2015, 19, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Mautner, V.F.; Kluwe, L.; Thakker, S.D.; Leark, R.A. Treatment of ADHD in Neurofibromatosis Type 1. Dev. Med. Child Neurol. 2002, 44, 164–170. [Google Scholar] [CrossRef]
- Diggs-Andrews, K.A.; Tokuda, K.; Izumi, Y.; Zorumski, C.F.; Wozniak, D.F.; Gutmann, D.H. Dopamine Deficiency Underlies Learning Deficits in Neurofibromatosis-1 Mice. Ann. Neurol. 2013, 73, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Levantini, V.; Muratori, P.; Inguaggiato, E.; Masi, G.; Milone, A.; Valente, E.; Tonacci, A.; Billeci, L. EYES Are the Window to the Mind: Eye-Tracking Technology as a Novel Approach to Study Clinical Characteristics of ADHD. Psychiatry Res. 2020, 290, 113135. [Google Scholar] [CrossRef] [PubMed]
- Gumenyuk, V.; Korzyukov, O.; Escera, C.; Hämäläinen, M.; Huotilainen, M.; Häyrinen, T.; Oksanen, H.; Näätänen, R.; Von Wendt, L.; Alho, K. Electrophysiological Evidence of Enhanced Distractibility in ADHD Children. Neurosci. Lett. 2005, 374, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.P.; Stordeur, C.; Septier, M.; Acquaviva, E.; Peyre, H.; Delorme, R. Oculomotor Abnormalities in Children with Attention-Deficit/Hyperactivity Disorder Are Improved by Methylphenidate. J. Child Adolesc. Psychopharmacol. 2017, 27, 274–280. [Google Scholar] [CrossRef]
- Gong, Z.F.; Xia, S.Z.; Liu, L.; Feng, C.H.; Guo, A.K. Operant Visual Learning and Memory in Drosophila Mutants Dunce, Amnesiac and Radish. J. Insect Physiol. 1998, 44, 1149–1158. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, R.K.; Martin, J.; Mattheisen, M.; Als, T.D.; Agerbo, E.; Baldursson, G.; Belliveau, R.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; et al. Discovery of the First Genome-Wide Significant Risk Loci for Attention Deficit/Hyperactivity Disorder. Nat. Genet. 2019, 51, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Rohde, P.D.; Jensen, I.R.; Sarup, P.M.; Ørsted, M.; Demontis, D.; Sørensen, P.; Kristensen, T.N. Genetic Signatures of Drug Response Variability in Drosophila melanogaster. Genetics 2019, 213, 633–650. [Google Scholar] [CrossRef]
- Kalda, A.; Zharkovsky, A. Epigenetic Mechanisms of Psychostimulant-Induced Addiction, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 120, ISBN 9780128029787. [Google Scholar]
- Bellosta, P.; Soldano, A. Dissecting the Genetics of Autism Spectrum Disorders: A Drosophila Perspective. Front. Physiol. 2019, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.; Peng, L.; Barnard-Brak, L. The Comorbidity of ADHD in Children Diagnosed with Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2016, 31, 11–18. [Google Scholar] [CrossRef]
- Binda, F.; Dipace, C.; Bowton, E.; Robertson, S.D.; Lute, B.J.; Fog, J.U.; Zhang, M.; Sen, N.; Colbran, R.J.; Gnegy, M.E.; et al. Syntaxin 1A Interaction with the Dopamine Transporter Promotes Amphetamine-Induced Dopamine Efflux. Mol. Pharmacol. 2008, 74, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooke, R.; Rasool, A.; Schneider, J.; Levine, J.D. Drosophila melanogaster Behaviour Changes in Different Social Environments Based on Group Size and Density. Commun. Biol. 2020, 3, 6–11. [Google Scholar] [CrossRef]
- Chen, S.; Lee, A.Y.; Bowens, N.M.; Huber, R.; Kravitz, E.A. Fighting Fruit Flies: A Model System for the Study of Aggression. Proc. Natl. Acad. Sci. USA 2002, 99, 5664–5668. [Google Scholar] [CrossRef] [Green Version]
- Lasbleiz, C.; Ferveur, J.F.; Everaerts, C. Courtship Behaviour of Drosophila melanogaster Revisited. Anim. Behav. 2006, 72, 1001–1012. [Google Scholar] [CrossRef]
- Simon, A.F.; Chou, M.T.; Salazar, E.D.; Nicholson, T.; Saini, N.; Metchev, S.; Krantz, D.E. A Simple Assay to Study Social Behavior in Drosophila: Measurement of Social Space within a Group. Genes Brain Behav. 2012, 11, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.M.; Everett, C.V.; Nelson, A.M.; Gulley, J.M.; Zahniser, N.R. Low and High Locomotor Responsiveness to Cocaine Predicts Intravenous Cocaine Conditioned Place Preference in Male Sprague-Dawley Rats. Pharmacol. Biochem. Behav. 2007, 86, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neckameyer, W.S.; White, K. Drosophila Tyrosine Hydroxylase Is Encoded by the Pale Locus. J. Neurogenet. 1993, 8, 189–199. [Google Scholar] [CrossRef]
- Cichewicz, K.; Garren, E.J.; Adiele, C.; Aso, Y.; Wang, Z.; Wu, M.; Birman, S.; Rubin, G.M.; Hirsh, J. A New Brain Dopamine-Deficient Drosophila and Its Pharmacological and Genetic Rescue. Genes, Brain Behav. 2017, 16, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, R.G.; Rasheed, A.; Sardina, T.; Tully, T.; Hillman, R. Effects of Tyrosine Hydroxylase Mutants on Locomotor Activity in Drosophila: A Study in Functional Genomics. Behav. Genet. 2002, 32, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Makos, M.A.; Kim, Y.C.; Han, K.A.; Heien, M.L.; Ewing, A.G. In Vivo Electrochemical Measurements of Exogenously Applied Dopamine in Drosophila melanogaster. Anal. Chem. 2009, 81, 1848–1854. [Google Scholar] [CrossRef] [Green Version]
- Fagan, R.R.; Kearney, P.J.; Luethi, D.; Bolden, N.C.; Sitte, H.H.; Emery, P.; Melikian, H.E. Dopaminergic Ric GTPase Activity Impacts Amphetamine Sensitivity and Sleep Quality in a Dopamine Transporter-Dependent Manner in Drosophila melanogaster. Mol. Psychiatry 2021, 24–27. [Google Scholar] [CrossRef]
- Fagan, R.R.; Kearney, P.J.; Sweeney, C.G.; Luethi, D.; Schoot Uiterkamp, F.E.; Schicker, K.; Alejandro, B.S.; O’Connor, L.C.; Sitte, H.H.; Melikian, H.E. Dopamine Transporter Trafficking and Rit2 GTPase: Mechanism of Action and in Vivo Impact. J. Biol. Chem. 2020, 295, 5229–5244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, C.G.; Kearney, P.J.; Fagan, R.R.; Smith, L.A.; Bolden, N.C.; Zhao-Shea, R.; Rivera, I.V.; Kolpakova, J.; Xie, J.; Gao, G.; et al. Conditional, Inducible Gene Silencing in Dopamine Neurons Reveals a Sex-Specific Role for Rit2 GTPase in Acute Cocaine Response and Striatal Function. Neuropsychopharmacology 2020, 45, 384–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, S.C.; Gu, H.; Rudnick, G. Biogenic Amine Flux Mediated by Cloned Transporters Stably Expressed in Cultured Cell Lines: Amphetamine Specificity for Inhibition and Efflux. Mol. Pharmacol. 1995, 47, 544–550. [Google Scholar]
- Berglund, E.C.; Makos, M.A.; Keighron, J.D.; Phan, N.; Heien, M.L.; Ewing, A.G. Oral Administration of Methylphenidate Blocks the Effect of Cocaine on Uptake at the Drosophila Dopamine Transporter. ACS Chem. Neurosci. 2013, 4, 566–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fog, J.U.; Khoshbouei, H.; Holy, M.; Owens, W.A.; Vaegter, C.B.; Sen, N.; Nikandrova, Y.; Bowton, E.; McMahon, D.G.; Colbran, R.J.; et al. Calmodulin Kinase II Interacts with the Dopamine Transporter C Terminus to Regulate Amphetamine-Induced Reverse Transport. Neuron 2006, 51, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Khoshbouei, H.; Sen, N.; Guptaroy, B.; Johnson, L.; Lund, D.; Gnegy, M.E.; Galli, A.; Javitch, J.A. N-Terminal Phosphorylation of the Dopamine Transporter Is Required for Amphetamine-Induced Efflux. PLoS Biol. 2004, 2, 387–393. [Google Scholar] [CrossRef]
- Cremona, M.L.; Matthies, H.J.G.; Pau, K.; Bowton, E.; Speed, N.; Lute, B.J.; Anderson, M.; Sen, N.; Robertson, S.D.; Vaughan, R.A.; et al. Flotillin-1 Is Essential for PKC-Triggered Endocytosis and Membrane Microdomain Localization of DAT. Nat. Neurosci. 2011, 14, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Du, F.; Tian, Q.B.; Zhang, J.; Endo, S. Ca2+/Calmodulin-Dependent Protein Kinase IIα Clusters Are Associated with Stable Lipid Rafts and Their Formation Traps PSD-95. J. Neurochem. 2008, 104, 596–610. [Google Scholar] [CrossRef]
- Tsui, J.; Inagaki, M.; Schulmann, H. Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII) Localization Acts in Concert with Substrate Targeting to Create Spatial Restriction for Phosphorylation. J. Biol. Chem. 2005, 280, 9210–9216. [Google Scholar] [CrossRef] [Green Version]
- Eiden, L.E.; Weihe, E. VMAT2: A Dynamic Regulator of Brain Monoaminergic Neuronal Function Interacting with Drugs of Abuse. Ann. N. Y. Acad. Sci. 2014, 1216, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Greer, C.L.; Grygoruk, A.; Patton, D.E.; Ley, B.; Romero-Calderon, R.; Chang, H.Y.; Houshyar, R.; Bainton, R.J.; DiAntonio, A.; Krantz, D.E. A Splice Variant of the Drosophila Vesicular Monoamine Transporter Contains a Conserved Trafficking Domain and Functions in the Storage of Dopamine, Serotonin, and Octopamine. J. Neurobiol. 2005, 64, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Randesi, M.; Van Den Brink, W.; Levran, O.; Blanken, P.; Van Ree, J.M.; Ott, J.; Kreek, M.J. VMAT2 Gene (SLC18A2) Variants Associated with a Greater Risk for Developing Opioid Dependence. Pharmacogenomics 2019, 20, 331–341. [Google Scholar] [CrossRef]
- Fehr, C.; Sommerlad, D.; Sander, T.; Anghelescu, I.; Dahmen, N.; Szegedi, A.; Mueller, C.; Zill, P.; Soyka, M.; Preuss, U.W. Association of VMAT2 Gene Polymorphisms with Alcohol Dependence. J. Neural Transm. 2013, 120, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the Vesicular Monoamine Transporter-2: A Novel Mechanism for Cocaine and Other Psychostimulants. J. Pharmacol. Exp. Ther. 2001, 296, 762–767. [Google Scholar] [PubMed]
- Karam, C.S.; Jones, S.K.; Javitch, J.A. Come Fly with Me: An Overview of Dopamine Receptors in Drosophila melanogaster. Basic Clin. Pharmacol. Toxicol. 2020, 126, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andretic, R.; Kim, Y.C.; Jones, F.S.; Han, K.A.; Greenspan, R.J. Drosophila D1 Dopamine Receptor Mediates Caffeine-Induced Arousal. Proc. Natl. Acad. Sci. USA 2008, 105, 20392–20397. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Chen, P.C.; Chiang, Y.C. Molecular Mechanisms of Psychostimulant Addiction. Chang. Gung Med. J. 2009, 32, 148–154. [Google Scholar] [PubMed]
- Abarca, C.; Albrecht, U.; Spanagel, R. Cocaine Sensitization and Reward Are under the Influence of Circadian Genes and Rhythm. Proc. Natl. Acad. Sci. USA 2002, 99, 9026–9030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClung, C.A.; Sidiropoulou, K.; Vitaterna, M.; Takahashi, J.S.; White, F.J.; Cooper, D.C.; Nestler, E.J. Regulation of Dopaminergic Transmission and Cocaine Reward by the Clock Gene. Proc. Natl. Acad. Sci. USA 2005, 102, 9377–9381. [Google Scholar] [CrossRef] [Green Version]
- Andretic, R.; Hirsh, J. Circadian Modulation of Dopamine Receptor Responsiveness in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 1873–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parekh, P.K.; Ozburn, A.R.; McClung, C.A. Circadian Clock Genes: Effects on Dopamine, Reward and Addiction. Alcohol 2015, 49, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majercak, J.; Kalderon, D.; Edery, I. Drosophila melanogaster Deficient in Protein Kinase A Manifests Behavior-Specific Arrhythmia but Normal Clock Function. Mol. Cell. Biol. 1997, 17, 5915–5922. [Google Scholar] [CrossRef] [Green Version]
- Scheggi, S.; Raone, A.; De Montis, M.G.; Tagliamonte, A.; Gambarana, C. Behavioral Expression of Cocaine Sensitization in Rats Is Accompanied by a Distinct Pattern of Modifications in the PKA/DARPP-32 Signaling Pathway. J. Neurochem. 2007, 103, 1168–1183. [Google Scholar] [CrossRef] [Green Version]
- Lee, D. Global and Local Missions of CAMP Signaling in Neural Plasticity, Learning, and Memory. Front. Pharmacol. 2015, 6, 161. [Google Scholar] [CrossRef] [Green Version]
- King, I.; Tsai, L.T.Y.; Pflanz, R.; Voigt, A.; Lee, S.; Jäckle, H.; Lu, B.; Heberlein, U. Drosophila Tao Controls Mushroom Body Development and Ethanol-Stimulated Behavior through Par-1. J. Neurosci. 2011, 31, 1139–1148. [Google Scholar] [CrossRef]
- Peineau, S.; Bradley, C.; Taghibiglou, C.; Doherty, A.; Bortolotto, Z.A.; Wang, Y.T.; Collingridge, G.L. The Role of GSK-3 in Synaptic Plasticity. Br. J. Pharmacol. 2008, 153, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, F.W.; Eddison, M.; Lee, S.; Cho, W.; Heberlein, U. GSK-3/Shaggy Regulates Olfactory Habituation in Drosophila. Proc. Natl. Acad. Sci. USA 2007, 104, 4653–4657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and Their Regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Underhill, S.M.; Stolz, D.B.; Murdoch, G.H.; Thiels, E.; Romero, G.; Amara, S.G. Amphetamine Activates Rho GTPase Signaling to Mediate Dopamine Transporter Internalization and Acute Behavioral Effects of Amphetamine. Proc. Natl. Acad. Sci. USA 2015, 112, E7138–E7147. [Google Scholar] [CrossRef] [Green Version]
- Dietz, D.M.; Sun, H.; Lobo, M.K.; Cahill, M.E.; Chadwick, B.; Gao, V.; Koo, J.W.; Mazei-Robison, M.S.; Dias, C.; Maze, I.; et al. Rac1 Is Essential in Cocaine-Induced Structural Plasticity of Nucleus Accumbens Neurons. Nat. Neurosci. 2012, 15, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Vallender, E.J.; Goswami, D.B.; Shinday, N.M.; Westmoreland, S.V.; Yao, W.D.; Rowlett, J.K. Transcriptomic Profiling of the Ventral Tegmental Area and Nucleus Accumbens in Rhesus Macaques Following Long-Term Cocaine Self-Administration. Drug Alcohol Depend. 2017, 175, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.S.; Soga, T.; Pandy, V.; Wu, Y.S.; Parhar, I.S.; Mohamed, Z. MicroRNA Expression Signature of Methamphetamine Use and Addiction in the Rat Nucleus Accumbens. Metab. Brain Dis. 2017, 32, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.M.; Wang, J.; Wu, P.; Zhu, W.L.; Li, Q.Q.; Xue, Y.X.; Zhai, H.F.; Shi, J.; Lu, L. Glycogen Synthase Kinase 3β in the Nucleus Accumbens Core Mediates Cocaine-Induced Behavioral Sensitization. J. Neurochem. 2009, 111, 1357–1368. [Google Scholar] [CrossRef]
- Wei, Y.M.; Li, S.X.; Shi, H.S.; Ding, Z.B.; Luo, Y.X.; Xue, Y.X.; Lu, L.; Yu, C.X. Protracted Cocaine Withdrawal Produces Circadian Rhythmic Alterations of Phosphorylated GSK-3β in Reward-Related Brain Areas in Rats. Behav. Brain Res. 2011, 218, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Wang, D.; Wang, L.; Fang, Q.; Wan, X.; Zhang, J.; Hu, Y.; Li, H.; Zhang, J.; et al. 4R Tau Modulates Cocaine-Associated Memory through Adult Dorsal Hippocampal Neurogenesis. J. Neurosci. 2021, 41, 6753–6774. [Google Scholar] [CrossRef]
- Gonzalez, D.A.; Jia, T.; Pinzón, J.H.; Acevedo, S.F.; Ojelade, S.A.; Xu, B.; Tay, N.; Desrivières, S.; Hernandez, J.L.; Banaschewski, T.; et al. The Arf6 Activator Efa6/PSD3 Confers Regional Specificity and Modulates Ethanol Consumption in Drosophila and Humans. Mol. Psychiatry 2018, 23, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Ojelade, S.A.; Jia, T.; Rodan, A.R.; Chenyang, T.; Kadrmas, J.L.; Cattrell, A. Rsu1 Regulates Ethanol Consumption in Drosophila and Humans. Proc. Natl. Acad. Sci. USA 2015, 112, E4085–E4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, D.L.; Traynor, J.R. Regulators of G Protein Signaling (RGS) Proteins as Drug Targets: Modulating G-Protein-Coupled Receptor (GPCR) Signal Transduction. J. Med. Chem. 2011, 54, 7433–7440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.J.; Fuchikami, M.; Dwyer, J.M.; Lepack, A.E.; Duman, R.S.; Aghajanian, G.K. GSK-3 Inhibition Potentiates the Synaptogenic and Antidepressant-like Effects of Subthreshold Doses of Ketamine. Neuropsychopharmacology 2013, 38, 2268–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devineni, A.V.; Heberlein, U. Preferential Ethanol Consumption in Drosophila Models Features of Addiction. Curr. Biol. 2009, 19, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- MacKay, T.F.C.; Richards, S.; Stone, E.A.; Barbadilla, A.; Ayroles, J.F.; Zhu, D.; Casillas, S.; Han, Y.; Magwire, M.M.; Cridland, J.M.; et al. The Drosophila melanogaster Genetic Reference Panel. Nature 2012, 482, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.M.; Mokashi, S.S.; Shankar, V.; Hatfield, J.S.; Hannah, R.C.; Mackay, T.F.C.; Anholt, R.R.H. The Drosophila Brain on Cocaine at Single-Cell Resolution. Genome Res. 2021, 31, 1927–1937. [Google Scholar] [CrossRef]
- Ro, J.; Harvanek, Z.M.; Pletcher, S.D. FLIC: High-Throughput, Continuous Analysis of Feeding Behaviors in Drosophila. PLoS ONE 2014, 9, e101107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.; Yang, S.E.; Montgomery, A.B.; Reed, A.R.; Rodan, A.R.; Rothenfluh, A. The Fly Liquid-Food Electroshock Assay (FLEA) Suggests Opposite Roles for Neuropeptide F in Avoidance of Bitterness and Shock. BMC Biol. 2021, 19, 31. [Google Scholar] [CrossRef]


| Gene | Homologue 1 | Gene Function 2 | Mutant 3 | SUD Related Behavior | Psychostimulant Response 4 | Disease Model |
|---|---|---|---|---|---|---|
| iav | TRPV6 | ion channel | LoF | sensitization | mutants do not sensitize to COC [62] | |
| Dop1R1 | DRD1, DRD5 | DA signaling | KD | consumption, preference | MB KD alters experience dependent change in consumption of COC and MA [93] | |
| LoF, KD | consumption, preference | mutation or MB KD disrupts acute and experience dependent MA preference [60] | ||||
| Dop1R2 | ADRB1 | DA signaling | LoF, KD | consumption, preference | reduced preference for MA [60] | |
| Dop2R | DRD2 | DA signaling | null | consumption, preference | reduced preference for MA [60] | |
| DopEcR | GPR21 | DA signaling | null | consumption, preference | increased preference for MA [60] | |
| DAT | DAT1 | DA reuptake | null | locomotion | dDATfmn flies do not exhibit hyperlocomotive response to AMPH [106] | |
| partial LoF | locomotion | DATfmn flies expressing hDAT-T356M have blunted locomotor response to AMPH [107] | ASD | |||
| partial LoF | locomotion | DATfmn flies expressing hDAT-ΔN336 are hyperactive and have impaired AMPH -induced reverse DA transport [108] | ASD | |||
| partial LoF | locomotion | DATfmn flies expressing the ASD-associated variant hDAT-R/W display a decrease in AMPH-induced locomotion [109] | ASD | |||
| partial LoF | locomotion | DATfmn flies expressing hDATK/A have blunted locomotor response to AMPH [110] | ||||
| partial LoF | locomotion | hDAT-R443A mutants have a blunted locomotor response to AMPH [91] | ||||
| partial LoF | consumption, preference | hDAT-R443A mutants do not develop preference in the CAFE [91] | ||||
| KD | sleep, arousal | MPH rescues sleep deficit in DAT pan-neuronal KD [105] | ADHD | |||
| null | sleep, arousal | AMPH decreases hyperactivity and induces sleep in DATfmn flies [111] | ADHD | |||
| CaMKII | CAMK2D | cell signaling | expression of inhibitor | locomotion | dopaminergic expression of CaMKII inhibitor abolishes AMPH-induced hyperlocomotion [112] | |
| Flo1 | FLOT1 | membrane protein | LoF | locomotion | Flotillin 1 mutants (Floe02554) have a blunted locomotor response to AMPH [106] | |
| dVMAT | VMAT2 | MOA transport | OE | motor- impairment | OE decreases COC-induced impairment of negative geotaxis [71] | |
| OE | locomotion | OE blunts COC-induced increases in locomotion [71] | ||||
| null | locomotion | reduced locomotor response to COC [72] | ||||
| null | locomotion | reduced locomotor response to AMPH [59] | ||||
| pharmaco- logical inhibition | locomotion | VMAT2 inhibitor reduces COC-induced motor activation [58] | ||||
| ple | TH | DA biosynthesis | null | locomotion | ple flies do not exhibit AMPH-induced increases in locomotion [106] | |
| DA biosynthesis | partial KO | locomotion | TH-deficient files have a blunted locomotor response to AMPH [111] | |||
| DA biosynthesis | targeted silencing, or activation | attention-like processes | acute MA exposure rescues optomotor response in flies expressing UAS-tnt or a truncated potassium channel (UAS-eagΔ932) in DA neurons [113] | |||
| LMO | LMO1 | circadian regulation | GoF | motor- impairment | mutants are resistant to COC-induced impairment of negative geotaxis [86] | |
| null, partial LoF | motor- impairment | mutants have increased sensitivity to COC-induced impairment of negative geotaxis [86] | ||||
| dbt | CSNK1D/E | circadian regulation | hypmorph, hypemorph | motor- activation | mutants have reduced sensitivity to initial COC exposure, and do not sensitize to repeated exposures [63] | |
| per | PER3 | circadian regulation | null, | motor-activation, motor- impairment | mutants are sensitive to initial COC exposure, but do not sensitize to repeated exposures at any dose [63,67,87] | |
| hypmorph, hypemorph | motor-activation | short and long period mutants display increase in behavioral score for initial COC exposure, but display limited sensitization to repeated exposures [63] | ||||
| null | sensitization | null mutants do not develop locomotor sensitization to vaporized MA [69] | ||||
| null | consumption | mutants do not self-administer MA [69] | ||||
| NA | circadian regulation | null | sensitization | mutants fail to develop sensitization to COC [68] | ||
| dClk | CLOCK | circadian regulation | hypomorph | sensitization | mutants are less likely to develop sensitization to COC [68] | |
| cyc | BMAL1 | circadian regulation | LoF | sensitization | mutants are less likely to develop sensitization to COC [68] | |
| tim | TIM | circadian regulation | LoF | locomotion | mutants have increased sensitivity to COC [68] | |
| msi | MSI2, MSI1, | development | targeted KD | consumption | MB KD increases COC preference [93] | |
| Snoo | SKI; SKIL | development | targeted KD | consumption, preference | MB KD increases initial COC preference in males and decreases initial MA preference in females [93] | |
| ed | NPHS1 | development | targeted KD | consumption, preference | MB KD increases initial MA preference in males, and decreases experience dependent MA preference in males and females [93] | |
| NA | APP; BACE1 | dysregulated in NDD | targeted expression | sleep, arousal | pan-neuronal expression of AβPP and hBACE1 produce ADHD-like phenotype rescued by MPH [114] | ADHD |
| Cirl | LPHN1 | cell adhesion, signaling | KD | sleep, arousal | methylphenidate rescues ADHD-like behavior in pan-neuronal knockdown [105] | ADHD |
| Nf1 | NF1 | GTPase activation | KD | sleep, arousal | MPH rescues ADHD-like behavior in pan-neuronal knockdown [105] | ADHD |
| moody | GPR84 | BBB permeability | partial LoF | motor- impairment | increased sensitivity to COC-induced impairment of negative geotaxis [115] | |
| pika-RII | PRKAR2A | cAMP signaling | severe LoF/null | motor-activation | reduced sensitivity to the motor-activating effects of COC; no sensitization to repeated exposure [116] | |
| whir | ARHGAP9 | GTPase activation | LoF | motor- impairment | resistant to the motor-impairing effects of COC on righting behavior [77] | |
| radish | GARNL3 | synaptic morphology, memory | LoF | attention-like processes | MPH rescues optomotor response, response to novel visual stimuli, and hyperactivity [101] | ADHD |
| Rab10 | RAB10 | GTPase | DN-Rab10 | locomotion | pan-neuronal expression of DN-Rab10 reduces MA-induced locomotion and MA-induced mortality [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philyaw, T.J.; Rothenfluh, A.; Titos, I. The Use of Drosophila to Understand Psychostimulant Responses. Biomedicines 2022, 10, 119. https://doi.org/10.3390/biomedicines10010119
Philyaw TJ, Rothenfluh A, Titos I. The Use of Drosophila to Understand Psychostimulant Responses. Biomedicines. 2022; 10(1):119. https://doi.org/10.3390/biomedicines10010119
Chicago/Turabian StylePhilyaw, Travis James, Adrian Rothenfluh, and Iris Titos. 2022. "The Use of Drosophila to Understand Psychostimulant Responses" Biomedicines 10, no. 1: 119. https://doi.org/10.3390/biomedicines10010119
APA StylePhilyaw, T. J., Rothenfluh, A., & Titos, I. (2022). The Use of Drosophila to Understand Psychostimulant Responses. Biomedicines, 10(1), 119. https://doi.org/10.3390/biomedicines10010119






