Sweet Taste Preference: Relationships with Other Tastes, Liking for Sugary Foods and Exploratory Genome-Wide Association Analysis in Subjects with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Baseline Anthropometric, Clinical and Biochemical Variables
2.3. Baseline Lifestyle Variables and Adherence to the Mediterranean Diet
2.4. Taste Preferences and Food Preferences
2.5. Taste Perception Tests
2.6. DNA Isolation and Genome-Wide Genotyping
2.7. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Preference for Sweet Taste and for Other Tastes
3.3. Perception of Sweet Taste and Other Tastes
3.4. Correlation of Sweet Taste Preference with the Other Tastes
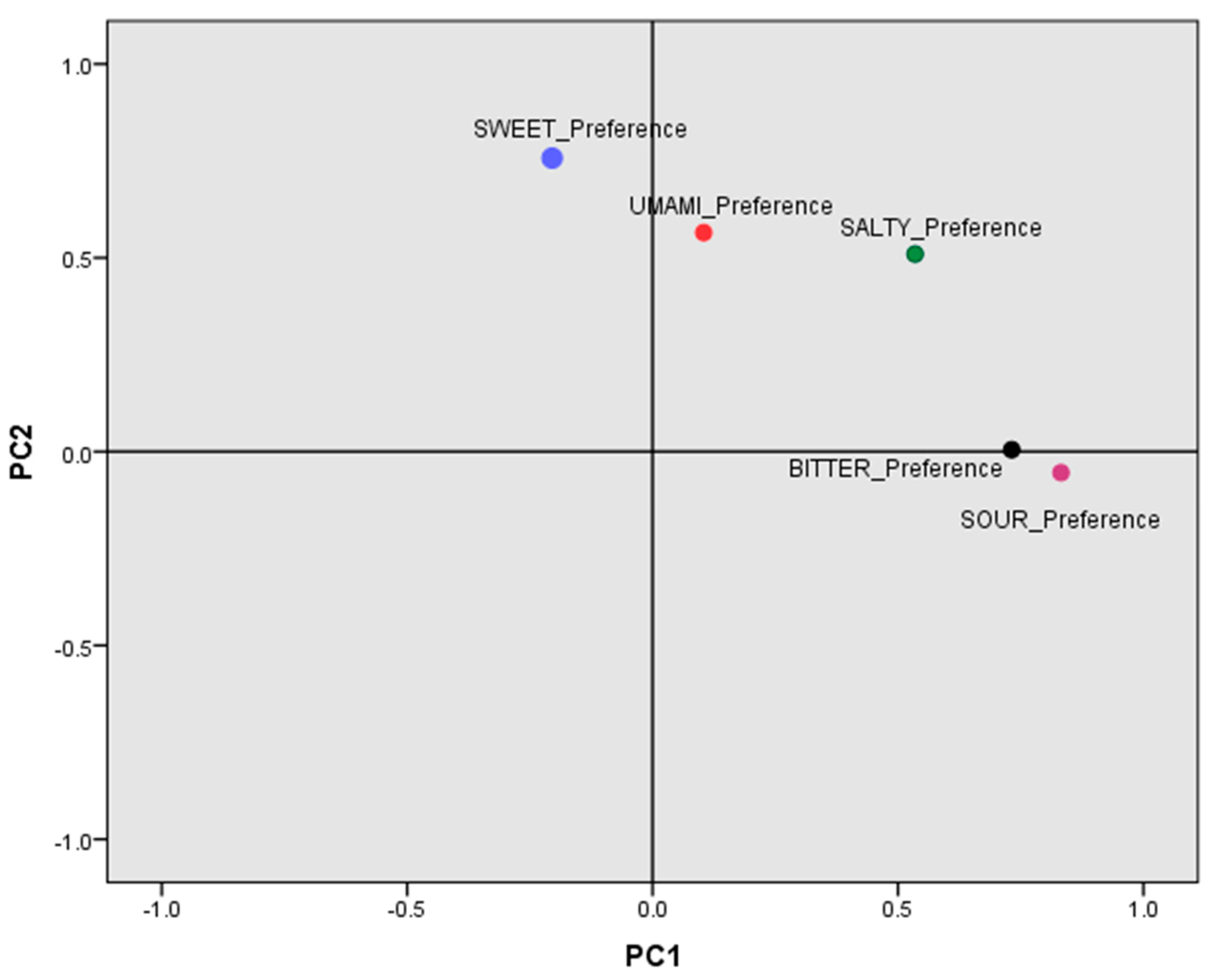
3.5. Factor Analysis of the Main Components for Taste Preferences
3.6. Association between Sweet Taste Preference and Liking for Sugary Foods
3.7. Association between Sweet Taste Preference and Sugar-Rich Food Intake
3.8. Exploratory GWASs to Study SNPs and Genes Associated with Sweet Taste Preference
3.8.1. GWASs for Sweet Taste Preference
SNP-Based GWAS for Sweet Taste Preference
Gene-Based GWAS for Sweet Taste Preference
3.8.2. GWAS for Factor 2
3.9. Association of rs2091718-PTPRN2 SNP with Sweet Taste Preference Variables and Other Related Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C. Associations between Dietary Patterns and the Incidence of Total and Fatal Cardiovascular Disease and All-Cause Mortality in 116,806 Individuals from the UK Biobank: A Prospective Cohort Study. BMC Med. 2021, 19, 83. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and Added Sugar Intakes, Sugar Types, and Cancer Risk: Results from the Prospective NutriNet-Santé Cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.P.; Collins, F.S. Precision Nutrition—The Answer to “What to Eat to Stay Healthy”. JAMA 2020, 324, 735. [Google Scholar] [CrossRef] [PubMed]
- Bush, C.L.; Blumberg, J.B.; El-Sohemy, A.; Minich, D.M.; Ordovás, J.M.; Reed, D.G.; Behm, V.A.Y. Toward the Definition of Personalized Nutrition: A Proposal by The American Nutrition Association. J. Am. Coll. Nutr. 2020, 39, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, R.; Fonseca, E.; Simon, S.A. The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity. Cell. Mol. Life Sci. 2020, 77, 3469–3502. [Google Scholar] [CrossRef]
- Murray, R.D. Savoring Sweet: Sugars in Infant and Toddler Feeding. Ann. Nutr. Metab. 2017, 70 (Suppl. S3), 38–46. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.A.; Bobowski, N.K.; Reed, D.R. The development of sweet taste: From biology to hedonics. Rev. Endocr. Metab. Disord. 2016, 17, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.-Y.; Tucker, R.M. Sweet Taste as a Predictor of Dietary Intake: A Systematic Review. Nutrients 2019, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, K.N.; Klikuszowian, E.; Schrader, L.; Siddique, J.; Van Horn, L.; Womack, V.Y.; Zenk, S.N. Assessment of the influence of food attributes on meal choice selection by socioeconomic status and race/ethnicity among women living in Chicago, USA: A discrete choice experiment. Appetite 2019, 139, 19–25. [Google Scholar] [CrossRef]
- Lee, K.-E. Students’ dietary habits, food service satisfaction, and attitude toward school meals enhance meal consumption in school food service. Nutr. Res. Pract. 2019, 13, 555–563. [Google Scholar] [CrossRef]
- Islam, M.R.; Trenholm, J.; Rahman, A.; Pervin, J.; Ekström, E.-C.; Rahman, S.M. Sociocultural Influences on Dietary Practices and Physical Activity Behaviors of Rural Adolescents-A Qualitative Exploration. Nutrients 2019, 11, 2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Mo, J. The Factors Influencing Meal Satisfaction in Older Adults: A Systematic Review and Meta-analysis. Asian Nurs. Res. Korean Soc. Nurs. Sci. 2019, 13, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartkiene, E.; Steibliene, V.; Adomaitiene, V.; Juodeikiene, G.; Cernauskas, D.; Lele, V.; Klupsaite, D.; Zadeike, D.; Jarutiene, L.; Guiné, R.P.F. Factors Affecting Consumer Food Preferences: Food Taste and Depression-Based Evoked Emotional Expressions with the Use of Face Reading Technology. Biomed. Res. Int. 2019, 2019, 2097415. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, C.B.M.; de Bekker-Grob, E.W.; van Lenthe, F.J. Factors affecting food choices of older adults from high and low socioeconomic groups: A discrete choice experiment. Am. J. Clin. Nutr. 2015, 101, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Mainland, J.D.; Arayata, C.J. Sensory nutrition: The role of taste in the reviews of commercial food products. Physiol. Behav. 2019, 209, 112579. [Google Scholar] [CrossRef] [PubMed]
- Bier, D.M. Dietary Sugars: Not as Sour as They Are Made Out to Be. Nestle Nutr. Inst. Workshop Ser. 2020, 95, 100–111. [Google Scholar] [PubMed]
- Petty, S.; Salame, C.; Mennella, J.A.; Pepino, M.Y. Relationship between Sucrose Taste Detection Thresholds and Preferences in Children, Adolescents, and Adults. Nutrients 2020, 12, 1918. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and food preference. J. Nutr. 2012, 142, 1142S–1148S. [Google Scholar] [CrossRef] [Green Version]
- Ventura, A.K.; Mennella, J.A. Innate and learned preferences for sweet taste during childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G.K. Why do we like sweet taste: A bitter tale? Physiol. Behav. 2016, 164, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Reed, D.R.; Knaapila, A. Genetics of taste and smell: Poisons and pleasures. Prog. Mol. Biol. Transl. Sci. 2010, 94, 213–240. [Google Scholar]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.A.; Bobowski, N.K. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol. Behav. 2015, 152, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Kavaliauskienė, I.; Domarkienė, I.; Ambrozaitytė, L.; Barauskienė, L.; Meškienė, R.; Arasimavičius, J.; Irnius, A.; Kučinskas, V. Association Study of Taste Preference: Analysis in the Lithuanian Population. Food Sci. Nutr. 2021, 9, 4310–4321. [Google Scholar] [CrossRef] [PubMed]
- Van Langeveld, A.W.B.; Teo, P.S.; de Vries, J.H.M.; Feskens, E.J.M.; de Graaf, C.; Mars, M. Dietary taste patterns by sex and weight status in the Netherlands. Br. J. Nutr. 2018, 119, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.; Keast, R.S.J. The Association between Sweet Taste Function, Anthropometry, and Dietary Intake in Adults. Nutrients 2016, 8, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicerale, S.; Riddell, L.J.; Keast, R.S.J. The association between perceived sweetness intensity and dietary intake in young adults. J. Food Sci. 2012, 77, H31–H35. [Google Scholar] [CrossRef] [PubMed]
- von Molitor, E.; Riedel, K.; Krohn, M.; Hafner, M.; Rudolf, R.; Cesetti, T. Sweet Taste Is Complex: Signaling Cascades and Circuits Involved in Sweet Sensation. Front. Hum. Neurosci. 2021, 15, 667709. [Google Scholar] [CrossRef] [PubMed]
- Desor, J.A.; Beauchamp, G.K. Longitudinal changes in sweet preferences in humans. Physiol. Behav. 1987, 39, 639–641. [Google Scholar] [CrossRef]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic Background of Taste Perception, Taste Preferences, and Its Nutritional Implications: A Systematic Review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Bachmanov, A.A.; Bosak, N.P.; Lin, C.; Matsumoto, I.; Ohmoto, M.; Reed, D.R.; Nelson, T.M. Genetics of taste receptors. Curr. Pharm. Des. 2014, 20, 2669–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolden, A.A.; Feeney, E.L. Genetic Differences in Taste Receptors: Implications for the Food Industry. Annu. Rev. Food Sci. Technol. 2020, 11, 183–204. [Google Scholar] [CrossRef] [Green Version]
- Smail, H.O. The roles of genes in the bitter taste. AIMS Genet. 2019, 6, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, M.; Liu, B. Current Progress in Understanding the Structure and Function of Sweet Taste Receptor. J. Mol. Neurosci. 2021, 71, 234–244. [Google Scholar] [CrossRef]
- Jang, J.H.; Kwon, O.; Moon, S.J.; Jeong, Y.T. Recent Advances in Understanding Peripheral Taste Decoding I: 2010 to 2020. Endocrinol. Metab. 2021, 36, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Ledda, M.; Kutalik, Z.; Souza Destito, M.C.; Souza, M.M.; Cirillo, C.A.; Zamboni, A.; Martin, N.; Morya, E.; Sameshima, K.; Beckmann, J.S.; et al. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum. Mol. Genet. 2014, 23, 259–267. [Google Scholar] [CrossRef]
- Hwang, L.-D.; Gharahkhani, P.; Breslin, P.A.S.; Gordon, S.D.; Zhu, G.; Martin, N.G.; Reed, D.R.; Wright, M.J. Bivariate genome-wide association analysis strengthens the role of bitter receptor clusters on chromosomes 7 and 12 in human bitter taste. BMC Genom. 2018, 19, 678. [Google Scholar] [CrossRef] [Green Version]
- Hwang, L.-D.; Lin, C.; Gharahkhani, P.; Cuellar-Partida, G.; Ong, J.-S.; An, J.; Gordon, S.D.; Zhu, G.; MacGregor, S.; Lawlor, D.A.; et al. New insight into human sweet taste: A genome-wide association study of the perception and intake of sweet substances. Am. J. Clin. Nutr. 2019, 109, 1724–1737. [Google Scholar] [CrossRef] [Green Version]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Fernández-Carrión, R.; Barragán, R.; Ortega-Azorín, C.; Estruch, R.; González, J.I.; Salas-Salvadó, J.; Lamon-Fava, S.; et al. Association between taste perception and adiposity in overweight or obese older subjects with metabolic syndrome and identification of novel taste-related genes. Am. J. Clin. Nutr. 2019, 109, 1709–1723. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Floriano, W.B.; Inoue, M.; Li, X.; Lin, C.; Murovets, V.O.; Reed, D.R.; Zolotarev, V.A.; Beauchamp, G.K. Genetics of sweet taste preferences. Flavour Fragr. J. 2011, 26, 286–294. [Google Scholar] [CrossRef]
- Park, S.; Liu, M.; Song, M.Y. Mental stress and physical activity interact with the genetic risk scores of the genetic variants related to sweetness preference in high sucrose-containing food and glucose tolerance. Food Sci. Nutr. 2020, 8, 3492–3503. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakamura, Y.; Doi, Y.; Narita, A.; Shimizu, A.; Imaeda, N.; Goto, C.; Matsui, K.; Kadota, A.; Miura, K.; et al. A Genome-Wide Association Study on Confection Consumption in a Japanese Population: The Japan Multi-Institutional Collaborative Cohort Study. Br. J. Nutr. 2021, 126, 1843–1851. [Google Scholar] [CrossRef]
- Kawafune, K.; Hachiya, T.; Nogawa, S.; Takahashi, S.; Jia, H.; Saito, K.; Kato, H. Strong association between the 12q24 locus and sweet taste preference in the Japanese population revealed by genome-wide meta-analysis. J. Hum. Genet. 2020, 65, 939–947. [Google Scholar] [CrossRef]
- Zhong, V.W.; Kuang, A.; Danning, R.D.; Kraft, P.; van Dam, R.M.; Chasman, D.I.; Cornelis, M.C. A genome-wide association study of bitter and sweet beverage consumption. Hum. Mol. Genet. 2019, 28, 2449–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novembre, J.; Johnson, T.; Bryc, K.; Kutalik, Z.; Boyko, A.R.; Auton, A.; Indap, A.; King, K.S.; Bergmann, S.; Nelson, M.R.; et al. Genes Mirror Geography within Europe. Nature 2008, 456, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Carli, L.; Gambino, R.; Lubrano, C.; Rosato, R.; Bongiovanni, D.; Lanfranco, F.; Broglio, F.; Ghigo, E.; Bo, S. Impaired taste sensation in type 2 diabetic patients without chronic complications: A case-control study. J. Endocrinol. Investig. 2018, 41, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Neiers, F.; Canivenc-Lavier, M.-C.; Briand, L. What Does Diabetes “Taste” Like? Curr. Diab. Rep. 2016, 16, 49. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Indurkar, A.; Degwekar, S.; Bhowate, R. Evaluation of gustatory function in patients with diabetes mellitus type 2. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2009, 108, 876–880. [Google Scholar] [CrossRef]
- Perros, P.; MacFarlane, T.W.; Counsell, C.; Frier, B.M. Altered taste sensation in newly-diagnosed NIDDM. Diabetes Care 1996, 19, 768–770. [Google Scholar] [CrossRef]
- Wasalathanthri, S.; Hettiarachchi, P.; Prathapan, S. Sweet taste sensitivity in pre-diabetics, diabetics and normoglycemic controls: A comparative cross sectional study. BMC Endocr. Disord. 2014, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Pugnaloni, S.; Alia, S.; Mancini, M.; Santoro, V.; Di Paolo, A.; Rabini, R.A.; Fiorini, R.; Sabbatinelli, J.; Fabri, M.; Mazzanti, L.; et al. A Study on the Relationship between Type 2 Diabetes and Taste Function in Patients with Good Glycemic Control. Nutrients 2020, 12, 1112. [Google Scholar] [CrossRef]
- Coltell, O.; Asensio, E.M.; Sorlí, J.V.; Barragán, R.; Fernández-Carrión, R.; Portolés, O.; Ortega-Azorín, C.; Martínez-LaCruz, R.; González, J.I.; Zanón-Moreno, V.; et al. Genome-Wide Association Study (GWAS) on Bilirubin Concentrations in Subjects with Metabolic Syndrome: Sex-Specific GWAS Analysis and Gene-Diet Interactions in a Mediterranean Population. Nutrients 2019, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program With Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.; Zomeño, M.D.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Tinahones, F.J.; Miranda, J.L.; et al. Validity of the Energy-Restricted Mediterranean Diet Adherence Screener. Clin. Nutr. 2021, 40, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Wichchukit, S.; O’Mahony, M. The 9-point hedonic scale and hedonic ranking in food science: Some reappraisals and alternatives. J. Sci. Food Agric. 2015, 95, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Rehm, C.D.; Monsivais, P.; Drewnowski, A. Importance of taste, nutrition, cost and convenience in relation to diet quality: Evidence of nutrition resilience among US adults using National Health and Nutrition Examination Survey (NHANES) 2007–2010. Prev. Med. 2016, 90, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardello, A.V.; Maller, O. Relationships Between Food Preferences and Food Acceptance Ratings. J. Food Sci. 1982, 47, 1553–1557. [Google Scholar] [CrossRef]
- Garland, R. The Mid-Point on a Rating Scale: Is it Desirable? Mark. Bull. 1991, 2, 66–70. [Google Scholar]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, R.; Zhu, M.; Qin, N.; Wang, Y.; Fan, J.; Sun, Q.; Ji, M.; Fan, X.; Xie, J.; et al. Integrated gene-based and pathway analyses using UK Biobank data identify novel genes for chronic respiratory diseases. Gene 2020, 767, 145287. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.d.O.; Gorgulho, B.M.; de Castro, M.A.; Fisberg, R.M.; Marchioni, D.M.; Baltar, V.T. Principal Component Analysis and Factor Analysis: Differences and similarities in Nutritional Epidemiology application. Rev. Bras. Epidemiol. 2019, 22, e190041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Turner, S.D. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv 2014, 005165. [Google Scholar] [CrossRef]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Umićević Mirkov, M.; de Leeuw, C.A.; van den Heuvel, M.P.; Posthuma, D. Genetic mapping of cell type specificity for complex traits. Nat. Commun. 2019, 10, 3222. [Google Scholar] [CrossRef] [Green Version]
- de Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef] [PubMed]
- Szkiba, D.; Kapun, M.; von Haeseler, A.; Gallach, M. SNP2GO: Functional analysis of genome-wide association studies. Genetics 2014, 197, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.; Jun, G.R.; Dupuis, J.; Farrer, L.A. Comparison of methods for multivariate gene-based association tests for complex diseases using common variants. Eur. J. Hum. Genet. 2019, 27, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Venditti, C.; Musa-Veloso, K.; Lee, H.Y.; Poon, T.; Mak, A.; Darch, M.; Juana, J.; Fronda, D.; Noori, D.; Pateman, E.; et al. Determinants of Sweetness Preference: A Scoping Review of Human Studies. Nutrients 2020, 12, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.; Wasserfall, C.; Wallstrom, G.; Wang, J.; Wang, H.; Barker, K.; Schatz, D.; Atkinson, M.; Qiu, J.; LaBaer, J. Tracking the Antibody Immunome in Type 1 Diabetes Using Protein Arrays. J. Proteome Res. 2017, 16, 195–203. [Google Scholar] [CrossRef]
- Suckale, J.; Solimena, M. The insulin secretory granule as a signaling hub. Trends Endocrinol. Metab. 2010, 21, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Digiacomo, L.; Lavagnino, Z.; Occhipinti, M.; Bugliani, M.; Cappello, V.; Caracciolo, G.; Marchetti, P.; Piston, D.W.; Cardarelli, F. Insulin secretory granules labelled with phogrin-fluorescent proteins show alterations in size, mobility and responsiveness to glucose stimulation in living β-cells. Sci. Rep. 2019, 9, 2890. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Kubosaki, A.; Ito, Y.; Notkins, A.L. Disturbances in the secretion of neurotransmitters in IA-2/IA-2beta null mice: Changes in behavior, learning and lifespan. Neuroscience 2009, 159, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-Z.; Han, S.; Kranzler, H.R.; Farrer, L.A.; Gelernter, J. A genomewide linkage scan of cocaine dependence and major depressive episode in two populations. Neuropsychopharmacology 2011, 36, 2422–2430. [Google Scholar] [CrossRef] [Green Version]
- Curtis, D.; Vine, A.E.; McQuillin, A.; Bass, N.J.; Pereira, A.; Kandaswamy, R.; Lawrence, J.; Anjorin, A.; Choudhury, K.; Datta, S.R.; et al. Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes. Psychiatr. Genet. 2011, 21, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Mosca, S.J.; Langevin, L.M.; Dewey, D.; Innes, A.M.; Lionel, A.C.; Marshall, C.C.; Scherer, S.W.; Parboosingh, J.S.; Bernier, F.P. Copy-number variations are enriched for neurodevelopmental genes in children with developmental coordination disorder. J. Med. Genet. 2016, 53, 812–819. [Google Scholar] [CrossRef]
- Linthorst, J.; Meert, W.; Hestand, M.S.; Korlach, J.; Vermeesch, J.R.; Reinders, M.J.T.; Holstege, H. Extreme enrichment of VNTR-associated polymorphicity in human subtelomeres: Genes with most VNTRs are predominantly expressed in the brain. Transl. Psychiatry 2020, 10, 369. [Google Scholar] [CrossRef]
- De Roeck, A.; Duchateau, L.; Van Dongen, J.; Cacace, R.; Bjerke, M.; Van den Bossche, T.; Cras, P.; Vandenberghe, R.; De Deyn, P.P.; Engelborghs, S.; et al. An intronic VNTR affects splicing of ABCA7 and increases risk of Alzheimer’s disease. Acta Neuropathol. 2018, 135, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Say, Y.-H. The association of insertions/deletions (INDELs) and variable number tandem repeats (VNTRs) with obesity and its related traits and complications. J. Physiol. Anthr. 2017, 36, 25. [Google Scholar] [CrossRef]
- Vincent, J.B. Unstable repeat expansion in major psychiatric disorders: Two decades on, is dynamic DNA back on the menu? Psychiatr. Genet. 2016, 26, 156–165. [Google Scholar] [CrossRef]
- Lee, S. The association of genetically controlled CpG methylation (cg158269415) of protein tyrosine phosphatase, receptor type N2 (PTPRN2) with childhood obesity. Sci. Rep. 2019, 9, 4855. [Google Scholar] [CrossRef] [Green Version]
- Ouni, M.; Saussenthaler, S.; Eichelmann, F.; Jähnert, M.; Stadion, M.; Wittenbecher, C.; Rönn, T.; Zellner, L.; Gottmann, P.; Ling, C.; et al. Epigenetic Changes in Islets of Langerhans Preceding the Onset of Diabetes. Diabetes 2020, 69, 2503–2517. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-H.; Lu, A.T.; Paul, K.C.; Folle, A.D.; Bronstein, J.M.; Bordelon, Y.; Horvath, S.; Ritz, B. Longitudinal Epigenome-Wide Methylation Study of Cognitive Decline and Motor Progression in Parkinson’s Disease. J. Parkinson’s Dis. 2019, 9, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Agha, G.; Mendelson, M.M.; Ward-Caviness, C.K.; Joehanes, R.; Huan, T.; Gondalia, R.; Salfati, E.; Brody, J.A.; Fiorito, G.; Bressler, J.; et al. Blood Leukocyte DNA Methylation Predicts Risk of Future Myocardial Infarction and Coronary Heart Disease. Circulation 2019, 140, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Rath, P.; Liu, J.; Ryu, D.; Pei, L.; Noonepalle, S.K.; Shull, A.Y.; Feng, Q.; Litofsky, N.S.; Miller, D.C.; et al. Identification of Global DNA Methylation Signatures in Glioblastoma-Derived Cancer Stem Cells. J. Genet. Genom. 2015, 42, 355–371. [Google Scholar] [CrossRef] [Green Version]
- Wielscher, M.; Vierlinger, K.; Kegler, U.; Ziesche, R.; Gsur, A.; Weinhäusel, A. Diagnostic Performance of Plasma DNA Methylation Profiles in Lung Cancer, Pulmonary Fibrosis and COPD. EBioMedicine 2015, 2, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, W.J.A.J.; Pulido, R. Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochim. Biophys. Acta 2013, 1832, 1673–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awamleh, Z.; Butcher, D.T.; Hanley, A.; Retnakaran, R.; Haertle, L.; Haaf, T.; Hamilton, J.; Weksberg, R. Exposure to Gestational Diabetes Mellitus (GDM) Alters DNA Methylation in Placenta and Fetal Cord Blood. Diabetes Res. Clin. Pract. 2021, 174, 108690. [Google Scholar] [CrossRef]
- Liang, F.; Lv, K.; Wang, Y.; Yuan, Y.; Lu, L.; Feng, Q.; Jing, X.; Wang, H.; Liu, C.; Rayner, S.; et al. Personalized Epigenome Remodeling Under Biochemical and Psychological Changes During Long-Term Isolation Environment. Front. Physiol. 2019, 10, 932. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, J.A.P.; Ono, R.K.; Cantão, M.E.; Ibelli, A.M.G.; Peixoto, J.d.O.; Moreira, G.C.M.; Godoy, T.F.; Coutinho, L.L.; Munari, D.P.; Ledur, M.C. Exploring the Genetic Architecture of Feed Efficiency Traits in Chickens. Sci. Rep. 2021, 11, 4622. [Google Scholar] [CrossRef] [PubMed]
- Vesnina, A.; Prosekov, A.; Kozlova, O.; Atuchin, V. Genes and Eating Preferences, Their Roles in Personalized Nutrition. Genes 2020, 11, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised Nutrition and Health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Characteristics | Total (n = 425) | Men (n = 183) | Women (n = 242) | p |
|---|---|---|---|---|
| Age (years) | 65.2 ± 4.7 | 64.0 ± 5.3 | 66.1 ± 4.1 | <0.001 |
| Weight (Kg) | 83.9 ± 13.7 | 92.5 ± 13.2 | 77.3 ± 9.8 | <0.001 |
| BMI (Kg/m2) | 32.2 ± 3.6 | 32.2 ± 3.4 | 32.2 ± 3.7 | 0.961 |
| Waist circumference (cm) | 105.6 ± 10.1 | 111.1 ± 8.7 | 101.4 ± 9.0 | <0.001 |
| SBP (mm Hg) | 141.3 ± 18.1 | 143.8 ± 18.6 | 139.5 ± 17.6 | 0.015 |
| DBP (mm Hg) | 80.9 ± 9.9 | 82.7 ± 10.4 | 79.5 ± 9.4 | 0.001 |
| Total cholesterol (mg/dL) | 196.8 ± 37.8 | 188.3 ± 39.0 | 203.3 ± 35.6 | <0.001 |
| LDL-C (mg/dL) | 130.9 ± 33.2 | 131.5 ± 33.7 | 130.7 ± 32.8 | 0.681 |
| HDL-C (mg/dL) | 59.9 ± 14.3 | 52.4 ± 11.3 | 64.8 ± 13.9 | <0.001 |
| Triglycerides (mg/dL) | 103.3 ± 58.3 | 117.7 ± 69.9 | 93.9 ± 47.1 | <0.001 |
| Fasting glucose (mg/dL) | 92.1 ± 16.9 | 94.0 ± 17.9 | 90.8 ± 16.2 | 0.001 |
| Physical Activity (MET.min/wk) | 1679 ± 1526 | 1947 ± 1797 | 1476 ± 1250 | 0.002 |
| Hours of sleep per night (h/day) 1 | 6.8 ± 1.1 | 6.9 ± 1.0 | 6.7 ± 1.1 | 0.014 |
| Adherence to MedDiet 2 | 8.0 ± 2.8 | 7.9 ± 2.8 | 8.1 ± 2.7 | 0.404 |
| Type 2 diabetes: n, % | 163 (38.4) | 71 (38.8) | 92 (38.0) | 0.870 |
| Obesity: n, % | 239 (56.2) | 110 (60.1) | 129 (53.3) | 0.422 |
| Current smokers: n, % | 48 (11.3) | 30 (16.4) | 18 (7.4) | <0.001 |
| Taste Preference | Total (n = 425) | Sex | Diabetes | ||||
|---|---|---|---|---|---|---|---|
| Men (n = 183) | Women (n = 242) | p1 | No (n = 262) | Yes (n = 163) | p2 | ||
| Sweet | 7.16 ± 0.09 | 7.03 ± 0.14 | 7.25 ± 0.12 | 0.245 | 6.99 ± 0.12 | 7.42 ± 0.14 | 0.021 |
| Salty | 7.56 ± 0.08 | 7.45 ± 0.13 | 7.65 ± 0.10 | 0.196 | 7.54 ± 0.10 | 7.60 ± 0.12 | 0.690 |
| Sour | 4.62 ± 0.10 | 4.71 ± 0.16 | 4.55 ± 0.14 | 0.445 | 4.66 ± 0.13 | 4.55 ± 0.17 | 0.614 |
| Umami | 5.95 ± 0.09 | 6.44 ± 0.13 | 5.58 ± 0.12 | <0.001 | 6.00 ± 0.11 | 5.87 ± 0.14 | 0.482 |
| Bitter | 4.31 ± 0.10 | 4.47 ± 0.16 | 4.19 ± 0.14 | 0.184 | 4.33 ± 0.13 | 4.28 ± 0.17 | 0.830 |
| Taste (Tastant) 1 | Total (n = 348) | Diabetes | ||||
|---|---|---|---|---|---|---|
| No (n = 220) | Yes (n = 128) | p2 | p3 | p4 | ||
| Sweet (Sucrose) (400 mM) | 2.27 ± 0.07 | 2.34 ± 0.08 | 2.16 ± 0.11 | 0.182 | 0.173 | 0.171 |
| Salty (NaCl) (200 mM) | 2.58 ± 0.07 | 2.65 ± 0.09 | 2.44 ± 0.13 | 0.182 | 0.168 | 0.118 |
| Sour (Citric acid) (34 mM) | 2.51 ± 0.07 | 2.58 ± 0.08 | 2.39 ± 0.12 | 0.197 | 0.191 | 0.132 |
| Umami ((MPG) (200 mM) | 1.99 ± 0.07 | 1.99 ± 0.09 | 1.99 ± 0.12 | 0.969 | 0.979 | 0.898 |
| Bitter (PTC) (5.6 mM) | 1.38 ± 0.07 | 1.51 ± 0.09 | 1.16 ± 0.11 | 0.024 | 0.023 | 0.011 |
| Total taste score 5 | 10.71 ± 0.23 | 11.10 ± 0.29 | 10.05 ± 0.39 | 0.034 | 0.026 | 0.019 |
| Taste 1 | Sweet | Salty | Sour | Umami | Bitter | |
|---|---|---|---|---|---|---|
| Salty | r | 0.119 | 1 | |||
| p | 0.014 | |||||
| Sour | r | −0.110 | 0.296 | 1 | ||
| p | 0.024 | <0.001 | ||||
| Umami | r | 0.017 | 0.122 | 0.041 | 1 | |
| p | 0.730 | 0.012 | 0.402 | |||
| Bitter | r | 0.032 | 0.196 | 0.371 | 0.018 | 1 |
| p | 0.508 | <0.001 | <0.001 | 0.714 |
| Sweet Taste Preference 1 | ||||||
|---|---|---|---|---|---|---|
| Whole Population | ||||||
| Sugary Foods 2 | Taste Preference 1 (%) | OR 3 and 95% CI | p4 | p5 | ||
| Food Liking 2 | Low | High | ||||
| Breakfast cereals | High | 24.3% | 34.4% | 1.63 (1.04–2.55) | 0.031 | 0.055 |
| Sweets-pastries and ice creams | High | 64.5% | 96.3% | 14.48 (7.10–29.58) | <0.001 | <0.001 |
| Chocolates | High | 76.3% | 93.8% | 4.67 (2.52–8.66) | <0.001 | <0.001 |
| Sugar | High | 50.7% | 70.7% | 2.35 (1.55–3.55) | <0.001 | <0.001 |
| Non Diabetic Subjects | ||||||
| Food Liking 2 | % | OR 3 and 95% CI | p4 | p5 | ||
| Low | High | |||||
| Breakfast cereals | High | 30.8% | 43.0% | 1.70 (1.01–2.67) | 0.045 | 0.074 |
| Sweets-pastries and ice creams | High | 63.5% | 96.8% | 17.62 (6.64–46.76) | <0.001 | <0.001 |
| Chocolates | High | 74.0% | 92.4% | 4.27 (2.05–8.89) | <0.001 | <0.001 |
| Sugar | High | 51.9% | 71.5% | 2.32 (1.39–3.90) | 0.001 | 0.003 |
| Type 2 Diabetic Subjects | ||||||
| Food Liking 2 | % | OR 3 and 95% CI | p4 | p5 | ||
| Low | High | |||||
| Breakfast cereals | High | 10.4% | 22.6% | 2.51 (0.90–6.99) | 0.071 | 0.108 |
| Sweets-pastries and ice creams | High | 66.7% | 95.7% | 11.00 (3.74–32.35) | <0.001 | <0.001 |
| Chocolates | High | 81.3% | 95.7% | 5.08 (1.60–16.80) | 0.003 | 0.015 |
| Sugar | High | 49.9% | 69.9% | 2.48 (1.24–4.96) | 0.009 | 0.015 |
| Sweet Taste Preference 1 | ||||||
|---|---|---|---|---|---|---|
| Whole Population | ||||||
| Intake of Sugary Foods in The Mediterranean Diet Scale 2 | Low Intake 3 (Med Diet Adherence) | Preference 1 | OR 4 and 95% CI | p5 | p6 | |
| Low | High | |||||
| Sugary beverages (I-6) | <1/week | 58.6% | 56.8% | 0.93 (0.62–1.39) | 0.723 | 0.476 |
| Pastries (I-9) | <3/week | 47.4% | 42.1% | 0.81 (0.54–1.21) | 0.297 | 0.385 |
| Added sugar (I-13) | No or NCS | 63.2% | 71.8% | 1.48 (0.97–2.26) | 0.066 | 0.105 |
| Non Diabetic Subjects | ||||||
| Low Intake 3 (Med Diet Adherence) | Preference 1 | OR 4 and 95% CI | p5 | p6 | ||
| Low | High | |||||
| Sugary beverages (I-6) | <1/week | 54.8% | 61.4% | 1.31 (0.79–2.17) | 0.289 | 0.299 |
| Pastries (I-9) | <3/week | 43.3% | 44.9% | 1.07 (0.65–1.76) | 0.790 | 0.418 |
| Added sugar (I-13) | No or NCS | 56.7% | 59.5% | 1.12 (0.68–1.85) | 0.657 | 0.694 |
| Type 2 Diabetic Subjects | ||||||
| Low Intake 3 (Med Diet Adherence) | Preference 1 | OR 4 and 95% CI | p5 | p6 | ||
| Low | High | |||||
| Sugary beverages (I-6) | <1/week | 66.7% | 50.4% | 0.51 (0.25–1.03) | 0.057 | 0.013 |
| Pastries (I-9) | <3/week | 56.3% | 38.3% | 0.48 (0.24–0.95) | 0.035 | 0.039 |
| Added sugar (I-13) | No or NCS | 77.1% | 88.7% | 2.33 (0.96–5.66) | 0.057 | 0.125 |
| Chr | SNP | BP | OR | P | Alleles | MAF | Strand | Gene |
|---|---|---|---|---|---|---|---|---|
| 7 | rs2091718 | 158304646 | 0.347 | 7.460 × 10−9 | G | 0.245 | − | PTPRN2 |
| 7 | rs10256091 | 158299094 | 0.352 | 1.054 × 10−8 | G | 0.342 | + | PTPRN2 |
| 7 | rs5016019 | 158279412 | 0.364 | 2.773 × 10−8 | G | 0.251 | + | PTPRN2 |
| 7 | rs10275533 | 158376086 | 0.399 | 1.111 × 10−7 | A | 0.281 | + | PTPRN2 |
| 7 | rs2335160 | 158350293 | 0.445 | 2.683 × 10−6 | G | 0.260 | − | PTPRN2 |
| 7 | rs6463205 | 5022223 | 4.078 | 3.347 × 10−6 | T | 0.105 | + | RNF216P1 |
| 9 | rs10963760 | 18787794 | 0.480 | 9.748 × 10−6 | G | 0.259 | + | ADAMTSL1 |
| 2 | rs354728 | 143944775 | 0.513 | 1.461 × 10−5 | T | 0.206 | − | ARHGAP15 |
| 17 | rs2694130 | 38747318 | 0.251 | 1.560 × 10−5 | T | 0.046 | + | __ |
| 13 | rs971604 | 97068019 | 3.888 | 1.710 × 10−5 | T | 0.186 | + | HS6ST3 |
| 2 | rs10178148 | 144000004 | 0.505 | 2.759 × 10−5 | G | 0.144 | + | ARHGAP15 |
| 1 | rs319978 | 49067379 | 0.430 | 2.796 × 10−5 | T | 0.168 | − | AGBL4 |
| 17 | rs8082554 | 78039867 | 0.510 | 3.642 × 10−5 | T | 0.181 | + | CCDC40 |
| 7 | rs12667108 | 5133936 | 0.419 | 3.661 × 10−5 | T | 0.144 | + | __ |
| 9 | rs10811261 | 19882156 | 2.258 | 4.086 × 10−5 | G | 0.384 | + | SLC24A2 |
| 11 | rs3763872 | 9593427 | 0.531 | 4.329 × 10−5 | T | 0.406 | − | WEE1 |
| 21 | rs2835220 | 37367098 | 1.954 | 4.769 × 10−5 | C | 0.283 | + | LOC101928269 |
| 14 | rs1286470 | 91059658 | 0.479 | 4.955 × 10−5 | C | 0.213 | − | TTC7B |
| 2 | rs10187143 | 34022970 | 0.500 | 5.180 × 10−5 | A | 0.326 | + | LINC01317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Carrión, R.; Sorlí, J.V.; Coltell, O.; Pascual, E.C.; Ortega-Azorín, C.; Barragán, R.; Giménez-Alba, I.M.; Alvarez-Sala, A.; Fitó, M.; Ordovas, J.M.; et al. Sweet Taste Preference: Relationships with Other Tastes, Liking for Sugary Foods and Exploratory Genome-Wide Association Analysis in Subjects with Metabolic Syndrome. Biomedicines 2022, 10, 79. https://doi.org/10.3390/biomedicines10010079
Fernández-Carrión R, Sorlí JV, Coltell O, Pascual EC, Ortega-Azorín C, Barragán R, Giménez-Alba IM, Alvarez-Sala A, Fitó M, Ordovas JM, et al. Sweet Taste Preference: Relationships with Other Tastes, Liking for Sugary Foods and Exploratory Genome-Wide Association Analysis in Subjects with Metabolic Syndrome. Biomedicines. 2022; 10(1):79. https://doi.org/10.3390/biomedicines10010079
Chicago/Turabian StyleFernández-Carrión, Rebeca, Jose V. Sorlí, Oscar Coltell, Eva C. Pascual, Carolina Ortega-Azorín, Rocío Barragán, Ignacio M. Giménez-Alba, Andrea Alvarez-Sala, Montserrat Fitó, Jose M. Ordovas, and et al. 2022. "Sweet Taste Preference: Relationships with Other Tastes, Liking for Sugary Foods and Exploratory Genome-Wide Association Analysis in Subjects with Metabolic Syndrome" Biomedicines 10, no. 1: 79. https://doi.org/10.3390/biomedicines10010079
APA StyleFernández-Carrión, R., Sorlí, J. V., Coltell, O., Pascual, E. C., Ortega-Azorín, C., Barragán, R., Giménez-Alba, I. M., Alvarez-Sala, A., Fitó, M., Ordovas, J. M., & Corella, D. (2022). Sweet Taste Preference: Relationships with Other Tastes, Liking for Sugary Foods and Exploratory Genome-Wide Association Analysis in Subjects with Metabolic Syndrome. Biomedicines, 10(1), 79. https://doi.org/10.3390/biomedicines10010079









