An Effect of Cyclosporin A in a Treatment of Temporal Bone Defect Using hBM-MSCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
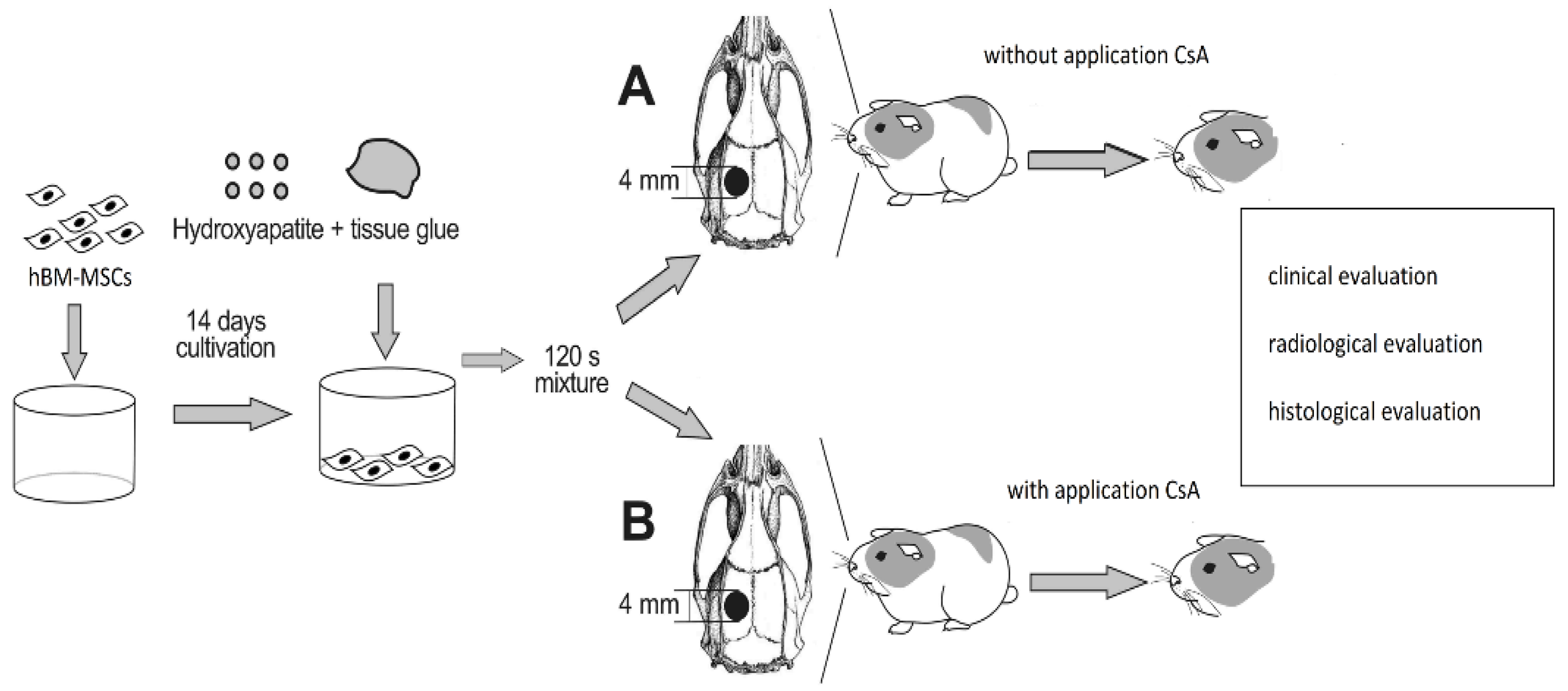
2.2. Experimental Design
2.3. Surgery
2.4. Cell Preparation
2.5. Scaffold Preparation
2.6. Histopathological Evaluation
2.7. Radiological Evaluation
2.8. Statistical Analysis
3. Results
3.1. Clinical Evaluation
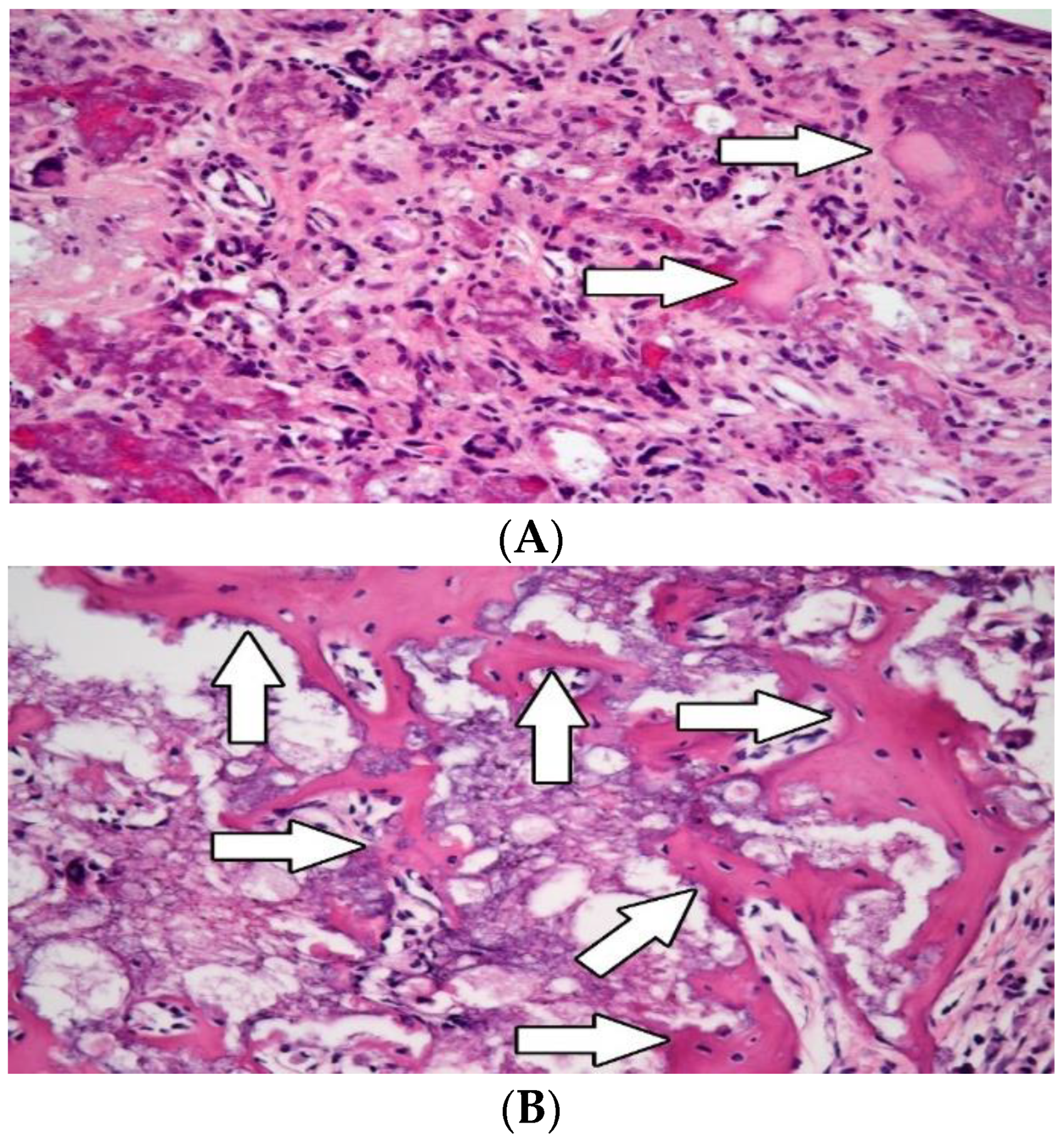
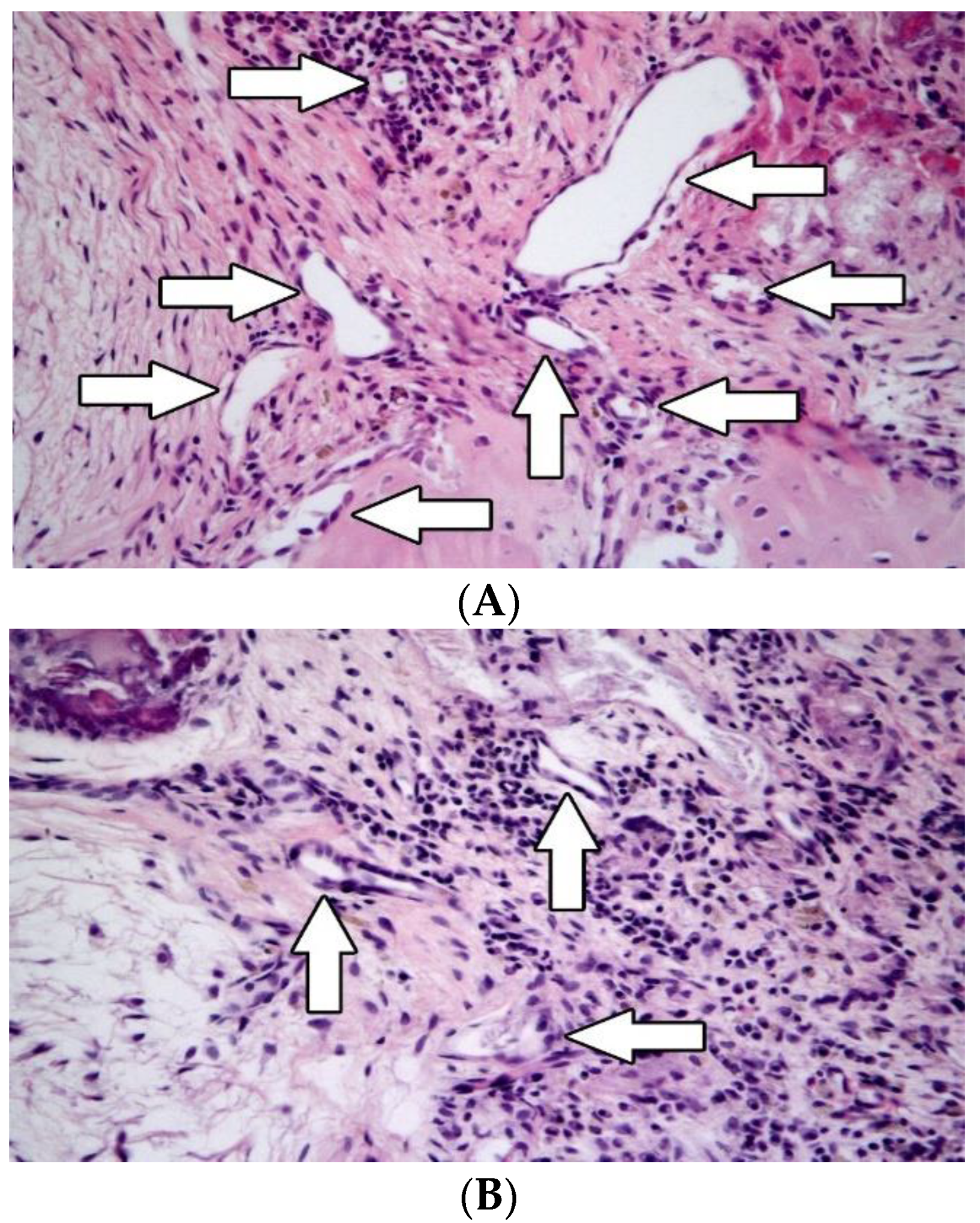
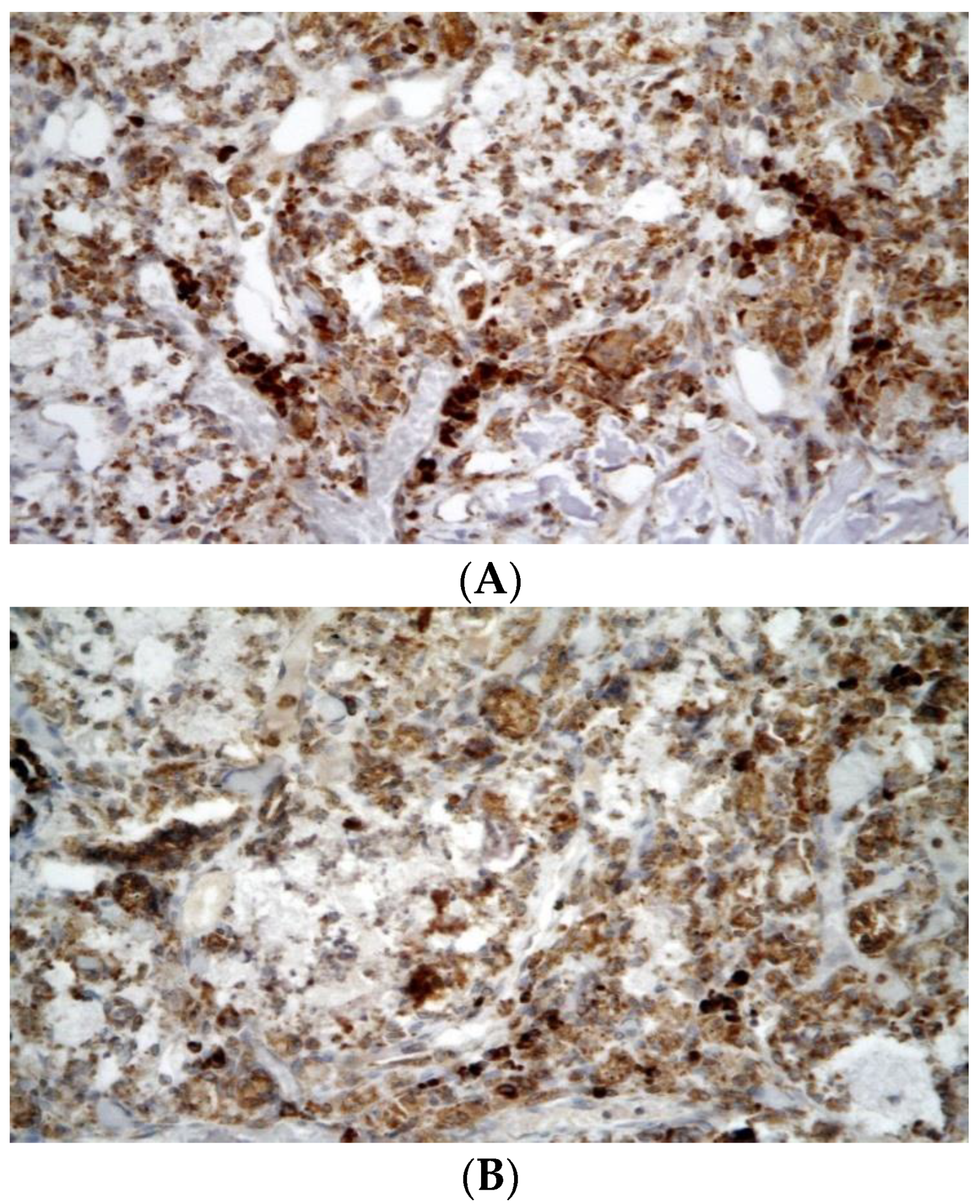
3.2. Histological Evaluation
3.2.1. Middle and Inner Ear Histology
3.2.2. Osteogenesis
3.2.3. Angiogenesis
3.2.4. Inflammation
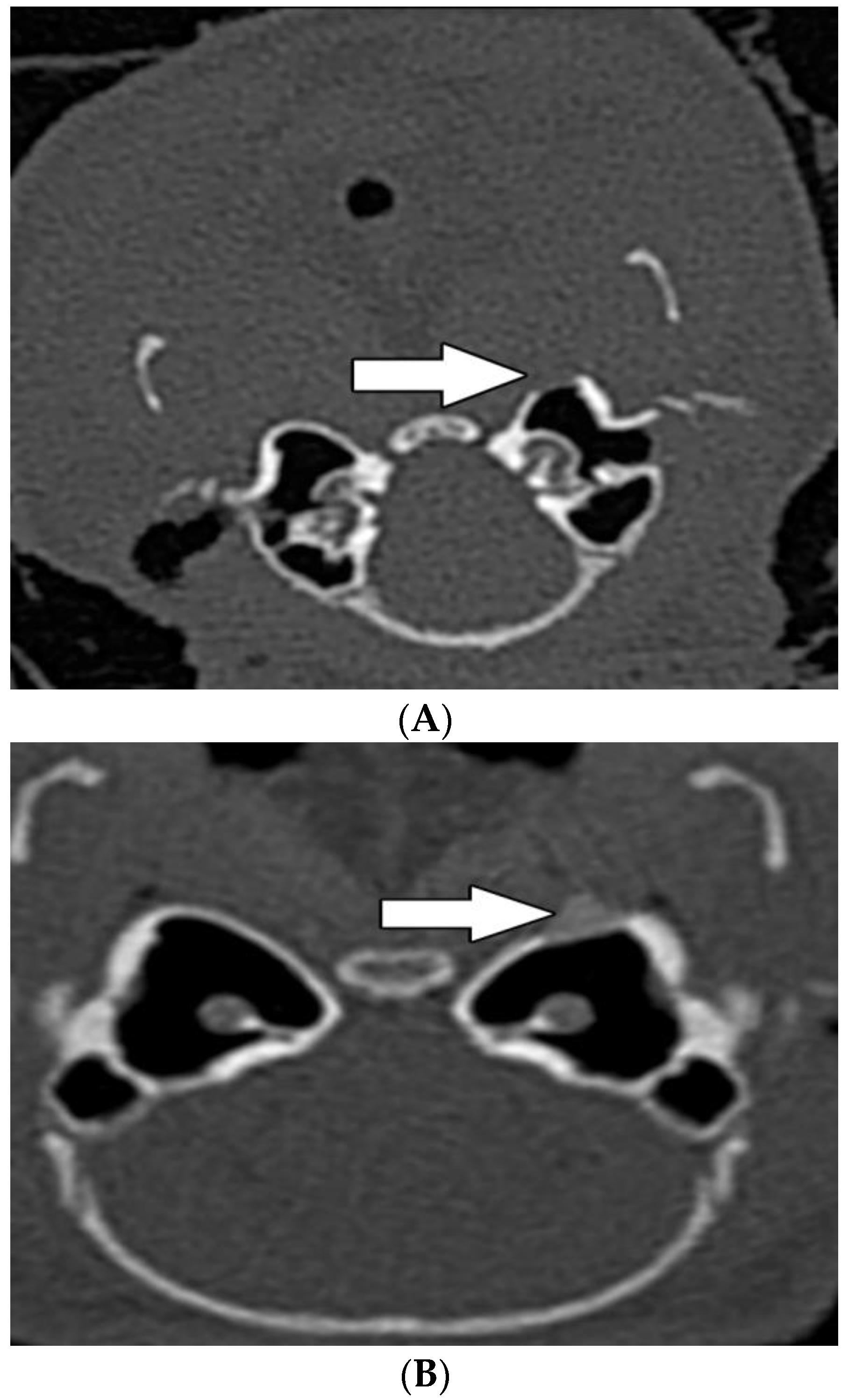
3.3. Radiological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maksimović, Z.; Rukovaniski, M. Intracranial complications of cholesteatoma. Acta Otorhinolaryngol. Belg. 1993, 47, 33–36. [Google Scholar]
- Klinickava, M.M.; Avnali, G.; Tuz, M.; Bagci, Ö. Is there a systematic inflammatory effect of cholesteatoma? Int. Arch. Otorhinolaryngol. 2017, 21, 42–45. [Google Scholar]
- Kim, C.W.; Oh, J.I.; Choi, K.Y.; Park, S.M.; Park, M.I. A technikue for concurrent procedure of mastoid obliteration and meatoplasty after cana wall down mastoidectomy. Auris Nasus Larynx 2012, 39, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Skoulakis, C.; Koltsidopoulos, P.; Iyer, A.; Kontorinis, G. Mastoid obliteration with systemic materials: A review of the literature. J. Int. Adv. Otol. 2019, 15, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Skoloudik, L.; Chrobok, V.; Kalfert, D.; Koci, Z.; Filip, S. Multipotent mesenchymal stromal cells in otorhinolaryngology. Med. Hypotheses 2014, 82, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Gamie, Z.; Mac Farlane, R.J.; Tomikson, A.; Moniaki, A.; Tran, G.T.; Gamie, Y.; Mantaralis, A.; Tsiridis, E. Skeletal tissue engineering using mesenchymal or embryonic stem cells: Clinical and experimental data. Expert Opin. Biol. Ther. 2014, 14, 1611–1639. [Google Scholar] [CrossRef]
- Hogan, W.J.; Storb, R. Use of cyclosporine in hematopoietic cell transplantation. Transplant. Proc. 2004, 36, 367–371. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, O.; Yuan, Y.; Sun, Z.; Li, P.; Wang, F.; Jiang, H.; Chen, T. Cyclosporin A inhibits adipogenic differentiation and regulates immunomodulatory functions of murine mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 498, 516–522. [Google Scholar]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 2. [Google Scholar] [CrossRef]
- Liu, S.; Liu, F.; Zhou, Y.; Jin, B.; Sun, O.; Guo, S. Immunosuppressive property of MSCs mediated by cell surface receptors. Front. Immunol. 2020, 11, e1076. [Google Scholar] [CrossRef]
- Skoloudik, L.; Chrobok, V.; Koci, Z.; Popelar, J.; Syka, J.; Laco, J.; Filipova, A.; Sykova, E.; Filip, S. The transplantation of hBM-MSCs increase bone neo-formation and proserves hearing function in the treatment of temporal bone defects—On the experience of two month follow up. Stem Cell Rev. Rep. 2018, 14, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ikezaki, S.; Imazato, K.; Koizumi, H.; Ohbuchi, T.; Hohei, N.; Hasihada, K. Partial mastoid obliteration combined with soft-wall reconstruction for modele ear cholesteatoma. Ann. Otol. Rhinol. Laryngol. 2014, 123, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.M.; Bächinger, D.; Botzen, J.; Grosmann, W.; Mlynski, R. Mastoid cavity obliteration leads to a clinically significant improvement in health-related quality of life. Eur. Arch. Otorhinolaryngol. 2020, 277, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Lim, H.J.; Cho, M.; Yang, S.M.; Park, K.; Park, H.Y.; Choung, Y.H. Clinival significance of β-tricalcium phosphate and plyposphate for mastoid cavity obliteration during middle ear surgery: Human and animal study. Clin. Exp. Otorhinolaryngol. 2013, 6, 127–134. [Google Scholar] [CrossRef]
- Sahli-Vivicorsi, S.; Alavi, Z.; Bran, W.; Cadieu, R.; Meriot, P.; Leelere, J.C.; Marianowski, R. Mid-term outcomes of mastoid obliteration with biological hydroxyapatite versus bioglass: A radiological and clinical study. Eur. Arch. Otorhinolaryngol. 2022, 279, 4379–4388. [Google Scholar] [CrossRef]
- Camernik, K.; Barlic, A.; Drobnic, M.; Marc, J.; Jeras, M.; Zupan, J. Mesenchymal stem cells in the musculoskeletal system: From animal models in human tissue regeneration? Stem Cell Rev Rep. 2018, 14, 346–369. [Google Scholar] [CrossRef]
- Skoloudik, L.; Chrobok, V.; Kalfert, D.; Koci, Z.; Sykova, E.; Chumak, T.; Popelar, J.; Syka, J.; Laco, J.; Dedkova, J.; et al. Human multipotent mesenchymal cells in the treatment of postoperative temporal bone defect: An animal model. Cell Transpl. 2016, 25, 1405–1414. [Google Scholar] [CrossRef]
- Corbett, J.M.; Hawthorne, I.; Dunbar, H.; Coulter, I.; Chonghaile, M.N.; Flyn, C.M.; English, K. Cyclosporine A and INFγ licencin enhances human mesenchymal stromal cell potency in a humamised mouse model of acute graft versus host disease. Stem Cell Res. Ther. 2021, 12, 238. [Google Scholar] [CrossRef]
- Hajkova, M.; Jaburek, F.; Porubska, B.; Bohacova, P.; Holan, V.; Krulova, M. Cyclosporine a promotes the therapeutic effect of mesenchymal stem cells on transplantation reaction. Clin. Sci. 2019, 133, 2143–2157. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dhalke, M.H. Immunomodulationm by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef]
- Shrestha, M.; Nguyen, T.T.; Park, J.; Chjoi, J.U.; Yook, S.; Jeong, J.H. Immunomodulation effect of mesenchymal stem cells in islet transplantation. Biomed. Pharmacother. 2021, 142, 112042. [Google Scholar] [CrossRef] [PubMed]
- Hajkova, M.; Javorkova, E.; Zajicova, A.; Trosan, P.; Holan, V.; Krulova, M. Alocal application of mesenchymal stem cells and cyclosporine A attenuates immune response by a switch in macrophage phenotype. J. Tissue Eng. Regen. Med. 2017, 11, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiva, T. The immunomodulatory function of mesenchymal stromal/stem cells mediated via paracrine activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Joel, M.D.M.; Yuan, J.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. MSC: Immunoregulatory effects roles on neutrophils and ecolving clinical potentials. Am. J. Transl. Res. 2019, 11, 3890–3904. [Google Scholar] [PubMed]
- Mezey, H. Human mesenchymal stem/stromal cells in immune regulation and therapy. Stem Cells Transl. Med. 2022, 11, 114–134. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Wilhelm, K.; Barker, J.H.; Marzi, I. Endothelial progenitor cell improve directly and indirectly early vasularization of mesenchymal stem cell derived bone regeneration in a critical bone defect in rats. Cell Transplant. 2012, 21, 1667–1677. [Google Scholar] [CrossRef]
- Rafiee, P.; Heidemann, J.; Ogawa, H.; Johnson, N.A.; Fisher, P.; Li, M.S.; Otterson, M.F.; Jonson, C.P.; Binio, D. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun. Signal. 2004, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Diomede, F.; Marconiu, D.G.; Fonticoli, L.; Pizzicanella, J.; Mericiaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional relationship between osteogenesis and angiogenesis in tissue regeneratrion. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Medhat, D.; Rodriguez, C.I.; Infante, A. Immunomodulatory effevts of MSCs in bone healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef]

| Group A (Mean Values ± SEM) | Group B (Mean Values ± SEM) | Statistical Significance | |
|---|---|---|---|
| New bone formation (in 1 mm2) | 25,041 (SEM ± 10,259) | 808,172 (SEM ± 48,834) | p = 0.000041 |
| New bone formation (volume %) | 2.4 (SEM ± 1) | 81 (SEM ± 5) | p = 0.000041 |
| Angiogenesis | 9 (SEM ± 2.4) | 7 (SEM ± 1.6) | p = 0.0152 |
| CD3 | 60 (SEM ± 5.9) | 16 (SEM ± 6.3) | p = 0.00573 |
| CHAE | 0 | 0 | |
| CD20 | 0 | 0 |
| Group A (Mean Values ± SEM) | Group B (Mean Values ± SEM) | Statistical Significance | |
|---|---|---|---|
| Areal density (HU) | 394 (SEM ± 73) | 1473 (SEM ± 146) | p = 0.00001 |
| Hot spot density (HU) | 958 (SEM ± 126) | 1643 (SEM ± 119) | p = 0.00276 |
| Mucosal thickening | 3 | 1 | p = 0.576 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoloudik, L.; Chrobok, V.; Laco, J.; Dedkova, J.; Diaz Garcia, D.; Filip, S. An Effect of Cyclosporin A in a Treatment of Temporal Bone Defect Using hBM-MSCs. Biomedicines 2022, 10, 2918. https://doi.org/10.3390/biomedicines10112918
Skoloudik L, Chrobok V, Laco J, Dedkova J, Diaz Garcia D, Filip S. An Effect of Cyclosporin A in a Treatment of Temporal Bone Defect Using hBM-MSCs. Biomedicines. 2022; 10(11):2918. https://doi.org/10.3390/biomedicines10112918
Chicago/Turabian StyleSkoloudik, Lukas, Viktor Chrobok, Jan Laco, Jana Dedkova, Daniel Diaz Garcia, and Stanislav Filip. 2022. "An Effect of Cyclosporin A in a Treatment of Temporal Bone Defect Using hBM-MSCs" Biomedicines 10, no. 11: 2918. https://doi.org/10.3390/biomedicines10112918
APA StyleSkoloudik, L., Chrobok, V., Laco, J., Dedkova, J., Diaz Garcia, D., & Filip, S. (2022). An Effect of Cyclosporin A in a Treatment of Temporal Bone Defect Using hBM-MSCs. Biomedicines, 10(11), 2918. https://doi.org/10.3390/biomedicines10112918






