Characteristics of Placental Histopathology in Women with Uncomplicated Pregnancies Affected by SARS-CoV-2 Infection at the Time of Delivery: A Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Histological Examination
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Population
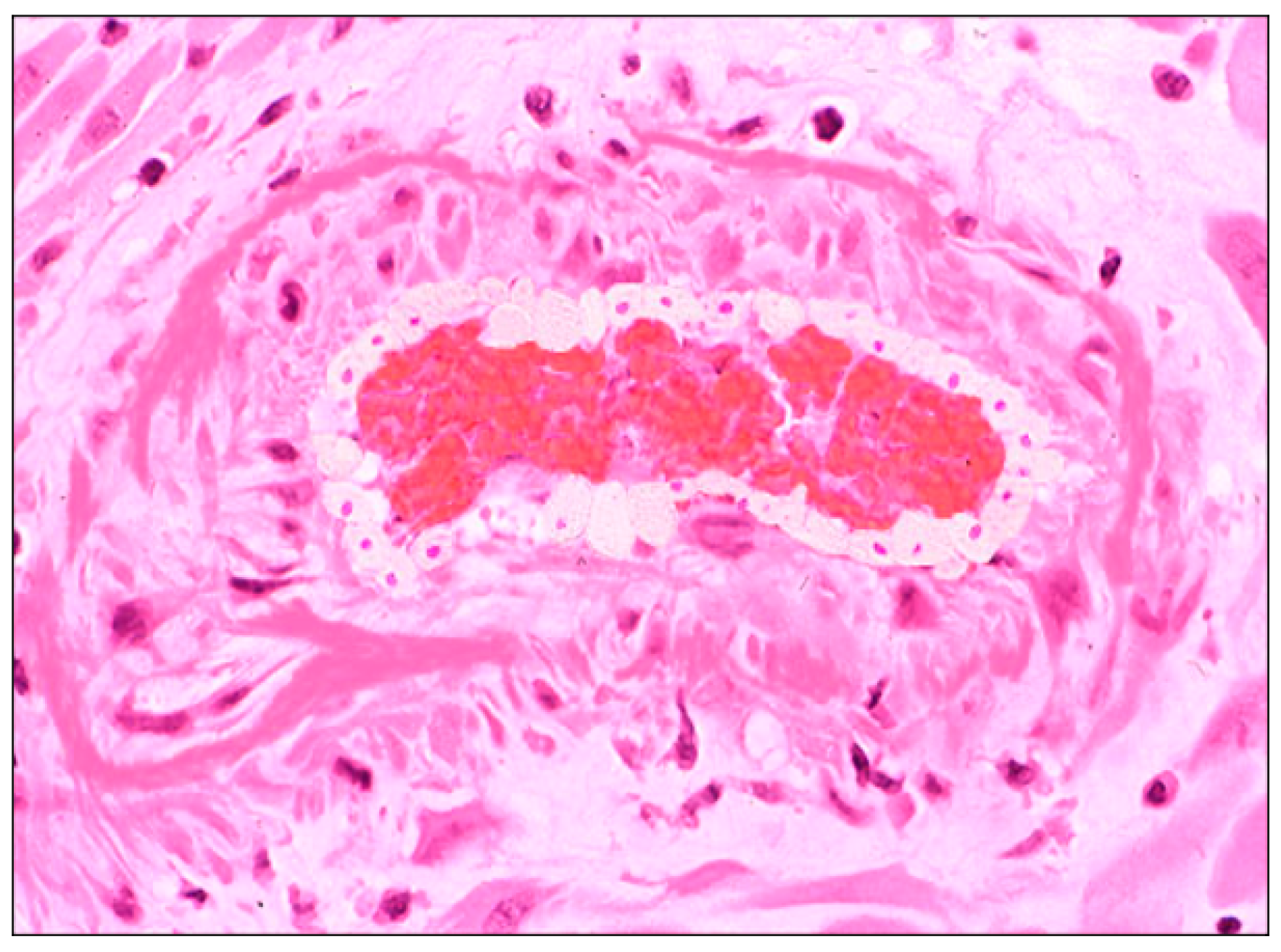
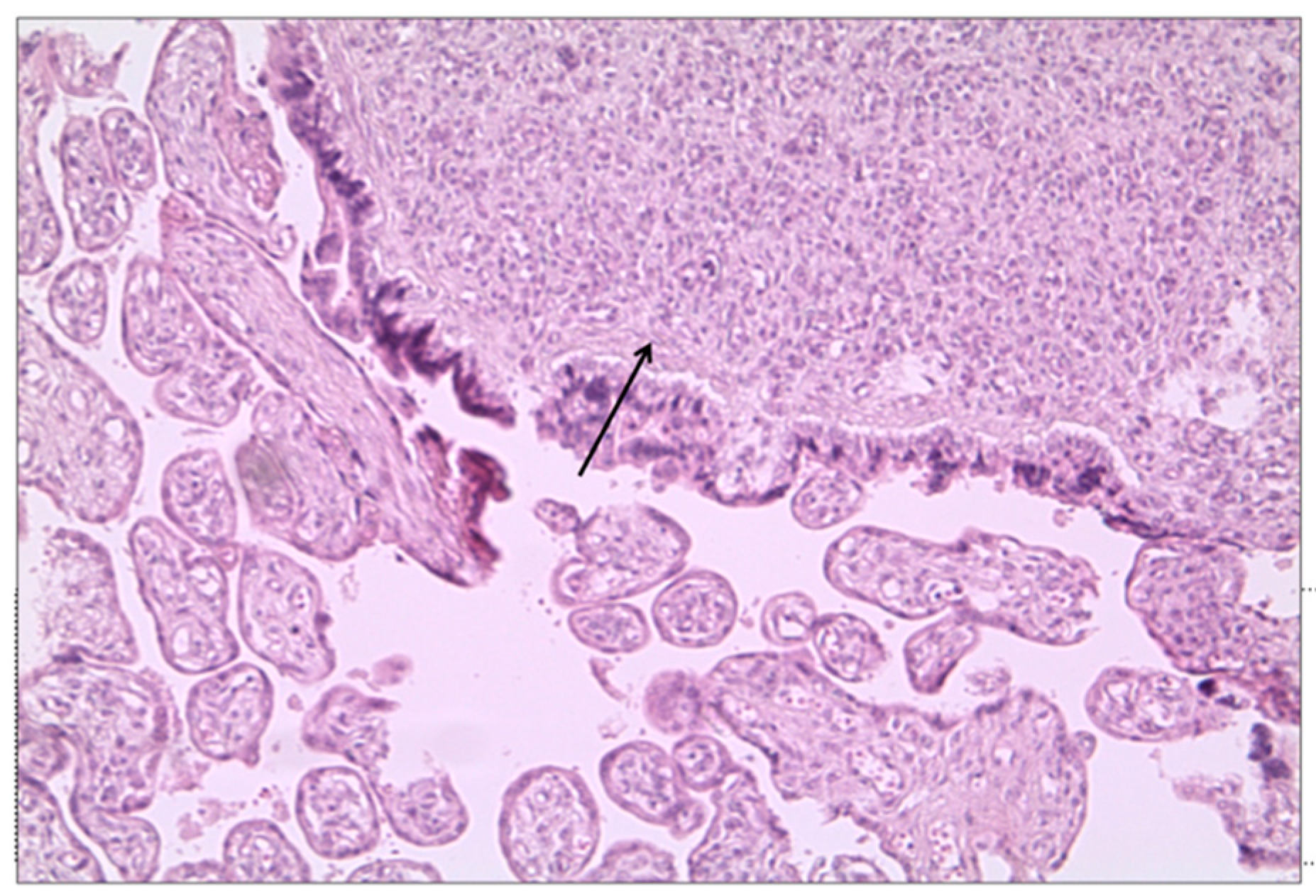
3.2. Histological Examination
4. Discussion
4.1. Summary of Study Findings
4.2. Interpretation of Study Findings and Comparison with the Literature
4.3. Study Strength and Limitation
4.4. Clinical and Research Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- Yang, J.; D’Souza, R.; Kharrat, A.; Fell, D.B.; Snelgrove, J.W.; Murphy, K.E.; Shah, P.S. COVID-19 pandemic and population-level pregnancy and neonatal outcomes: A living systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; von Dadelszen, P.; Kalafat, E.; Sebghati, M.; Ladhani, S.; Ugwumadu, A.; Draycott, T.; O’Brien, P.; Magee, L. PregnaCOVID3 study group. Change in obstetric attendance and activities during the COVID-19 pandemic. Lancet Infect. Dis. 2021, 21, e115. [Google Scholar] [CrossRef]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; APerry, A.; Bourne, T.; Lees, C.C.; et al. Pregnancy and neonatal outcomes of COVID-19: Coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021, 57, 573. [Google Scholar] [CrossRef] [PubMed]
- Karimi, L.; Vahedian-Azimi, A.; Makvandi, S.; Sahebkar, A. A Systematic Review of 571 Pregnancies Affected by COVID-19. Adv. Exp. Med. Biol. 2021, 1321, 287–298. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Silva do Vale, M.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The Intercovid Multinational Cohort Study. JAMA Pediatr. 2021, 175, 1. [Google Scholar] [CrossRef]
- Zaigham, M.; Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020, 99, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; von Dadelszen, P.; Draycott, T.; Ugwumadu, A.; O’Brien, P.; Magee, L. Change in the Incidence of Stillbirth and Preterm Delivery during the COVID-19 Pandemic. JAMA 2020, 324, 705. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: National cohort study. Am. J. Obstet. Gynecol. 2021, 225, e1–e522. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, M.; Kumar, M.; Khodor, S. COVID-19 Infection during Pregnancy: Risk of Vertical Transmission, Fetal, and Neonatal Outcomes. J. Pers. Med. 2021, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Shuo, C.; Bo, H.; Danju, L.; Li, X.; Yang, F.; Zhao, Y.; Nie, X.; Huang, B.X. Pregnancy with new coronavirus infection: Clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020, 49, 418–423. [Google Scholar] [CrossRef]
- di Girolamo, R.; Khalil, A.; Alameddine, S.; D’Angelo, E.; Galliani, C.; Matarrelli, B.; Buca, D.; Liberati, M.; Rizzo, G.; D’Antonio, F. Placental histopathology after SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100468. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.A.; Corsi Decenti, E.; Bonasoni, M.P.; Botta, G.; Castiglione, F.; D’Armiento, M.; Fulcheri, E.; Nebuloni, M.; Donati, S. The ItOSS COVID-Working Group. Placental Characteristics of a Large Italian Cohort of SARS-CoV-2-Positive Pregnant Women. Microorganisms 2022, 10, 1435. [Google Scholar] [CrossRef]
- Suhren, J.T.; Meinardus, A.; Hussein, K.; Schaumann, N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta 2022, 117, 72. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Dhaliwal, A. Infections in pregnancy with COVID-19 and other respiratory RNA virus diseases are rarely, if ever, transmitted to the fetus: Experiences with coronaviruses, HPIV, hMPV RSV, and Influenza. Arch. Pathol. Lab. Med. 2020, 144, 920–928. [Google Scholar] [CrossRef]
- Zaigham, M.; Gisselsson, D.; Sand, A.; Wikström, A.; von Wowern, E.; Schwartz, D.A.; Iorizzo, L.; Nelander, M.; Blomberg, M.; Papadogiannakis, N.; et al. Clinical-pathological features in placentas of pregnancies with SARS-CoV-2 infection and adverse outcome: Case series with and without congenital transmission. BJOG 2022, 129, 1361. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [Green Version]
- Travaglino, A.; Raffone, A.; Saccone, G.; Migliorini, S.; Maruotti, G.M.; Esposito, G.; Mollo, A.; Martinelli, P.; Zullo, F.; D’Armiento, M. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early-onset preeclampsia. Eur. J. Obstet. Gynecol. 2019, 234, 200–206. [Google Scholar] [CrossRef]
- Pinar, H.; Sung, C.J.; Oyer, C.E.; Singer, D.B. Reference values for singleton and twin placental weights. Pediatr. Pathol. Lab. Med. 1996, 16, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ville, Y. The placenta in COVID-19 infection in pregnancy. BJOG 2022, 129, 1375. [Google Scholar] [CrossRef] [PubMed]
- Bustamante Helfrich, B.; Chilukuri, N.; He, H.; Cerda, S.R.; Hong, X.; Wang, G.; Pearson, C.; Burd, I.; Wang, X. Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort. Placenta 2017, 52, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scifres, C.M.; Parks, W.T.; Feghali, M.; Caritis, S.N.; Catov, J.M. Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus. Placenta 2017, 49, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Assibey-Mensah, V.; Parks, W.T.; Gernand, A.D.; Catov, J.M. Race and risk of maternal vascular malperfusion lesions in the placenta. Placenta 2018, 69, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Ravishankar, S.; Luo, G.; Redline, R.W. Predictors of High Grade and Other Clinically Significant Placental Findings by Indication for Submission in Singleton Placentas from Term Births. Pediatr. Dev. Pathol. 2020, 23, 274–284. [Google Scholar] [CrossRef]
- Romero, R.; Kim, Y.M.; Pacora, P.; Chong, J.K.; Benshalom-Tirosh, N.; Jaiman, S.; Bhatti, G.; Kim, J.; Qureshi, F.; Jacques, S.M.; et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J. Perinat. Med. 2018, 46, 613–630. [Google Scholar] [CrossRef] [Green Version]
- Watkins, J.C.; Torous, V.F.; Roberts, D.J. Defining Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Placentitis. Arch. Pathol. Lab. Med. 2021, 145, 1341–1349. [Google Scholar] [CrossRef]
- Baergen, R.N.; Heller, D.S. Placental Pathology in COVID-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef]
- Taglauer, E.; Benarroch, Y.; Rop, K.; Barnett, E.; Sabharwal, V.; Yarrington, C.; Wachman, E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta 2020, 100, 69–74. [Google Scholar] [CrossRef]
- Konstantinidou, A.E.; Angelidou, S.; Havaki, S.; Paparizou, K.; Spanakis, N.; Chatzakis, C.; Sotiriadis, A.; Theodora, M.; Donoudis, C.; Daponte, A.; et al. Stillbirth due to SARS-CoV-2 placentitis without evidence of intrauterine transmission to fetus: Association with maternal risk factors. Ultrasound Obstet. Gynecol. 2022, 59, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Hyg, M.; Avvad-Portari, E.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Special Articles Placental Tissue Destruction and Insufficiency From COVID-19 Causes Stillbirth and Neonatal Death from Hypoxic-Ischemic Injury A Study of 68 Cases With SARS-CoV-2 Placentitis From 12 Countries. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Cagino, K.; Matthews, K.C.; Friedlander, R.L.; Glynn, S.M.; Kubiak, J.M.; Yang, Y.J.; Zhao, Z.; Baergen, R.N.; DiPace, J.I.; et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG 2020, 127, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Smithgall, M.C.; Liu-Jarin, X.; Hamele-Bena, D.; Cimic, A.; Mourad, M.; Debelenko, L.; Chen, X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: Histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020, 77, 994. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092. [Google Scholar] [CrossRef]
- Zhang, P.; Salafia, C.; Heyman, T.; Salafia, C.; Lederman, S.; Dygulska, B. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am. J. Obstet. Gynecol. MFM 2020, 2, 100197. [Google Scholar] [CrossRef] [PubMed]
- Gulersen, M.; Prasannan, L.; Tam Tam, H.; Metz, C.N.; Rochelson, B.; Meirowitz, N.; Shan, W.; Edelman, M.; AMillington, K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM 2020, 2, 100211. [Google Scholar] [CrossRef]
- He, M.; Skaria, P.; Kreutz, K.; Chen, L.; Hagemann, I.S.; Carter, E.B.; Mysorekar, I.U.; Nelson, D.; Pfeifer, J.; Dehner, L.P. Histopathology of Third Trimester Placenta from SARS-CoV-2-Positive Women. Fetal Pediatr. Pathol. 2022, 41, 403–412. [Google Scholar] [CrossRef]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1. [Google Scholar] [CrossRef]
- Adhikari, E.H.; Moreno, W.; Zofkie, A.C.; MacDonald, L.; McIntire, D.D.; Collins RR, J.; Spong, C.Y. Pregnancy Outcomes Among Women with and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw. Open 2020, 3, e2029256. [Google Scholar] [CrossRef] [PubMed]
- Debelenko, L.; Katsyv, I.; Chong, A.M.; Peruyero, L.; Szabolcs, M.; Uhlemann, A. Trophoblast damage with acute and chronic intervillositis: Disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum. Pathol. 2021, 109, 69. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef] [PubMed]

| Variables | |
|---|---|
| Maternal age (years) | 29.87 ± 4.9 |
| Gestational age at admission (weeks) | 39.102 ± 1.16 |
| Gestational age at delivery (weeks) | 39.316 ± 1.09 |
| Interval between first positive swab and delivery (days) | 3416 ± 3315 |
| Parity | |
| Primiparity | 12 (26) |
| Multiparity | 34 (74) |
| Gravidity | 2.25 ± 1.04 |
| S02 at admission | 97.2 ± 2.5 |
| Needing for respiratory support | 1 (2.2) |
| Diastolic blood pressure at admission (mmHg) | 70.6 ± 8.89 |
| Systolic blood pressure at admission (mmHg) | 122.17 ± 4.67 |
| Type of delivery (n (%)) | |
| Spontaneous | 21 (46) |
| Cesarean section | 25 (54) |
| Indication for CS (n%) | |
| Previous CS | 13 (52) |
| Failure of induction | 3 (12) |
| Labor dystocia | 6 (24) |
| Maternal request | 2 (8) |
| Breech presentation | 1 (4) |
| Birthweight (grams) | 3234.2 ± 405 |
| APGAR at 1 min | 7.75 ± 1.05 |
| APGAR at 5 min | 8.94 ± 0.39 |
| Fetal sex (n (%)) | |
| Female | 22 (47.8) |
| Male | 24 (52.2) |
| Head circumference (cm) | 33.52 ± 1.27 |
| Length (cm) | 48.7 ± 2.7 |
| Characteristics | |
|---|---|
| Placental weight (grams) | 460.46 ± 91.69 |
| Maximum thickness (cm) | 2.86 ± 0.60 |
| Minimum thickness (cm) | 1.78 ± 0.60 |
| Low/Moderate Blood Circulation Disease | Severe Blood Circulation Disease | Absent Blood Circulation Disease | p-Value | |
|---|---|---|---|---|
| n = 25 | n = 17 | n = 4 | ||
| Maternal age | 30.32 ± 6.04 | 29.58 ± 4.29 | 28.25 ± 1.92 | 0.853 |
| Gestational age at admission | 38.633 ± 2.577 | 39.05 ± 1.5 | 38.99 ± 1.74 | 0.349 |
| Gestational age at delivery | 39.107 ± 1.01 | 39.4 ± 1.24 | 39.36 ± 1.55 | 0.248 |
| Interval between first positive swab and delivery (days) | 1.48 ± 0.897 | 2.31 ± 1.79 | 4.5 ± 4.09 | 0.08 |
| Parity | 1.5 ± 0.9 | 1.7 ± 1.2 | 2.3 ± 1.2 | 0.123 |
| Gravidity | 2.08 ± 0.9 | 2.06 ± 1.02 | 3 ± 1 | 0.197 |
| S02 at admission | 95.6 ± 3.44 | 97.33 ± 1.37 | 98.25 ± 0.43 | 0.298 |
| Needing for respiratory support | 1 (4%) | 0 (0%) | 0 (0%) | -- |
| Diastolic blood pressure at admission (mmHg) | 68.8 ± 7.81 | 71.25 ± 8.56 | 70 ± 7.07 | 0.748 |
| Systolic blood pressure at admission (mmHg) | 121.93 ± 4.01 | 123.07 ± 6.05 | 122.5 ± 4.33 | 0.963 |
| Presence of mild symptoms | 7 (28) | 4 (23.5) | 1 (25) | 0.129 |
| Type of delivery | ||||
| Spontaneous | 10 (40%) | 8 (47%) | 4 (100%) | 0.06 |
| Cesarean section | 15 (60%) | 9 (53%) | - | |
| Birthweight (grams) | 3265.23 ± 393.84 | 3172.3 ± 445.64 | 3242.5 ± 299.02 | 0.903 |
| APGAR at 5 min | 8.9 ± 0.436 | 9 ± 0.39 | 9 ± 0.11 | 0.953 |
| Head circumference (cm) | 34.09 ± 0.94 | 33 ± 1.31 | 33 ± 1.0 | 0.296 |
| Length (cm) | 49.33 ± 1.43 | 48.11 ± 3.6 | 49.21 ± 2.45 | 0.727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarno, L.; Locci, M.; Fulgione, C.; Perillo, F.; Dell’Isola, A.; Mantelli, D.; Sibillo, C.; Saccone, G.; Maruotti, G.M.; Terracciano, D.; et al. Characteristics of Placental Histopathology in Women with Uncomplicated Pregnancies Affected by SARS-CoV-2 Infection at the Time of Delivery: A Single-Center Experience. Biomedicines 2022, 10, 3003. https://doi.org/10.3390/biomedicines10123003
Sarno L, Locci M, Fulgione C, Perillo F, Dell’Isola A, Mantelli D, Sibillo C, Saccone G, Maruotti GM, Terracciano D, et al. Characteristics of Placental Histopathology in Women with Uncomplicated Pregnancies Affected by SARS-CoV-2 Infection at the Time of Delivery: A Single-Center Experience. Biomedicines. 2022; 10(12):3003. https://doi.org/10.3390/biomedicines10123003
Chicago/Turabian StyleSarno, Laura, Mariavittoria Locci, Caterina Fulgione, Francesca Perillo, Angela Dell’Isola, Dalila Mantelli, Cristina Sibillo, Gabriele Saccone, Giuseppe Maria Maruotti, Daniela Terracciano, and et al. 2022. "Characteristics of Placental Histopathology in Women with Uncomplicated Pregnancies Affected by SARS-CoV-2 Infection at the Time of Delivery: A Single-Center Experience" Biomedicines 10, no. 12: 3003. https://doi.org/10.3390/biomedicines10123003







