Three-Dimensional Bioprinted Controlled Release Scaffold Containing Mesenchymal Stem/Stromal Lyosecretome for Bone Regeneration: Sterile Manufacturing and In Vitro Biological Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lyosecretome Preparation and Characterization
2.2.1. MSC Expansion and Secretome Collection
2.2.2. MSC Secretome Ultrafiltration and Lyophilization
2.2.3. Lyosecretome Characterization
2.2.4. Lyosecretome Microbiological Controls
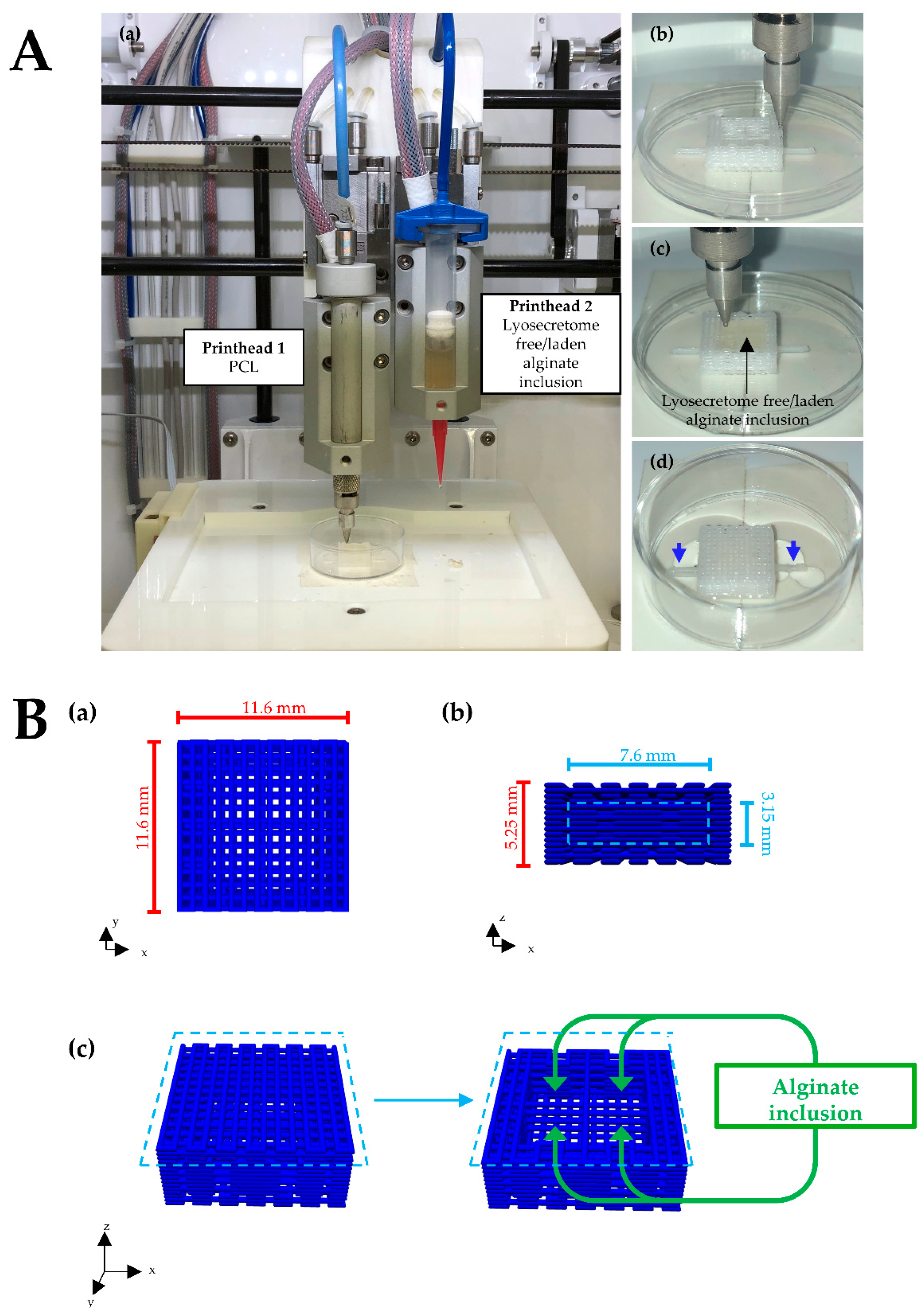
2.3. Scaffolds Design and Fabrication
2.4. Scaffold Microbiological Controls
2.5. Biological Assessment
2.5.1. Seeding of AD-MSCs
2.5.2. Evaluation of cell Adhesion by Scanning Electron Microscopy (SEM)
2.5.3. Osteogenic Differentiation
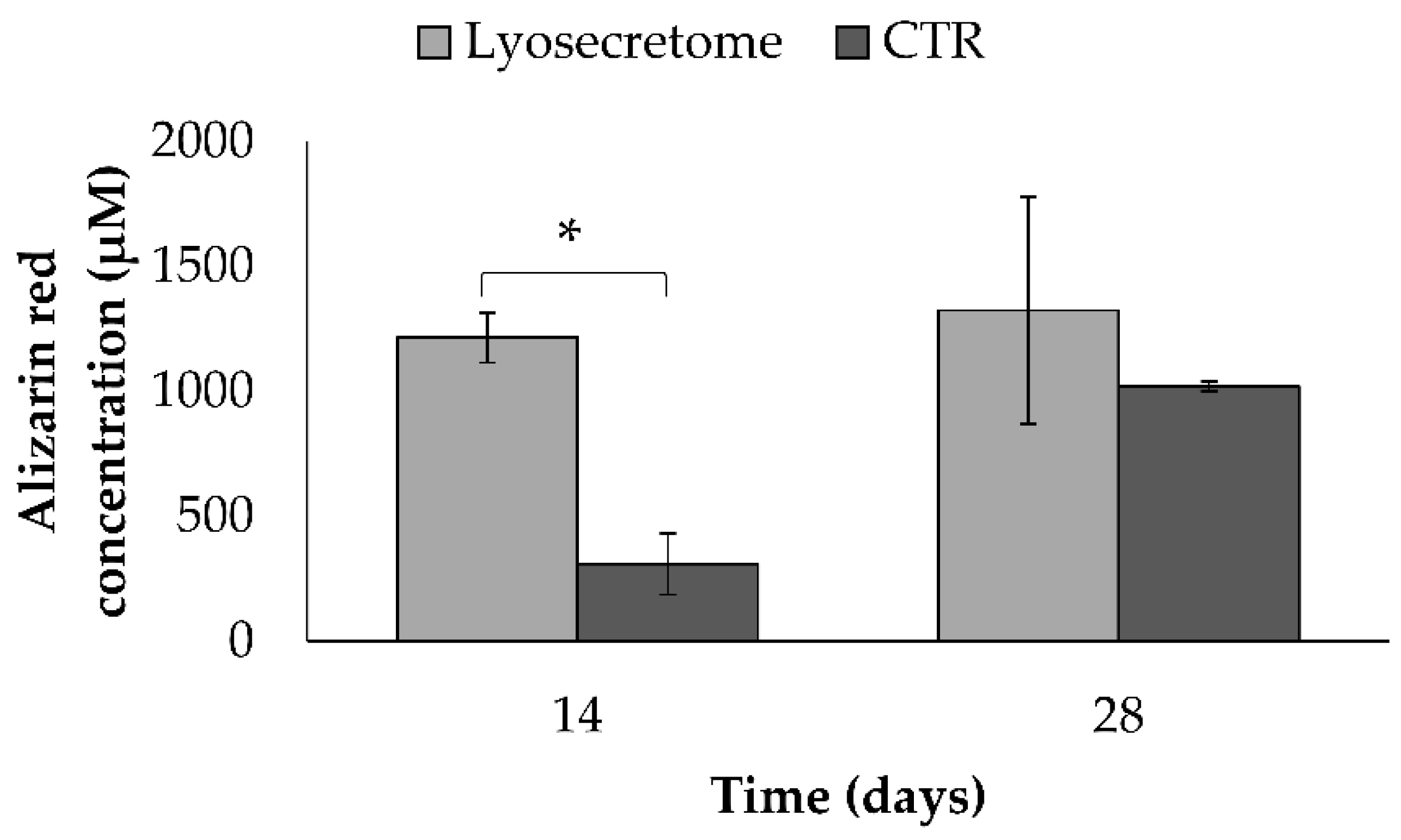
Alizarin Red Staining
Confocal Microscopy
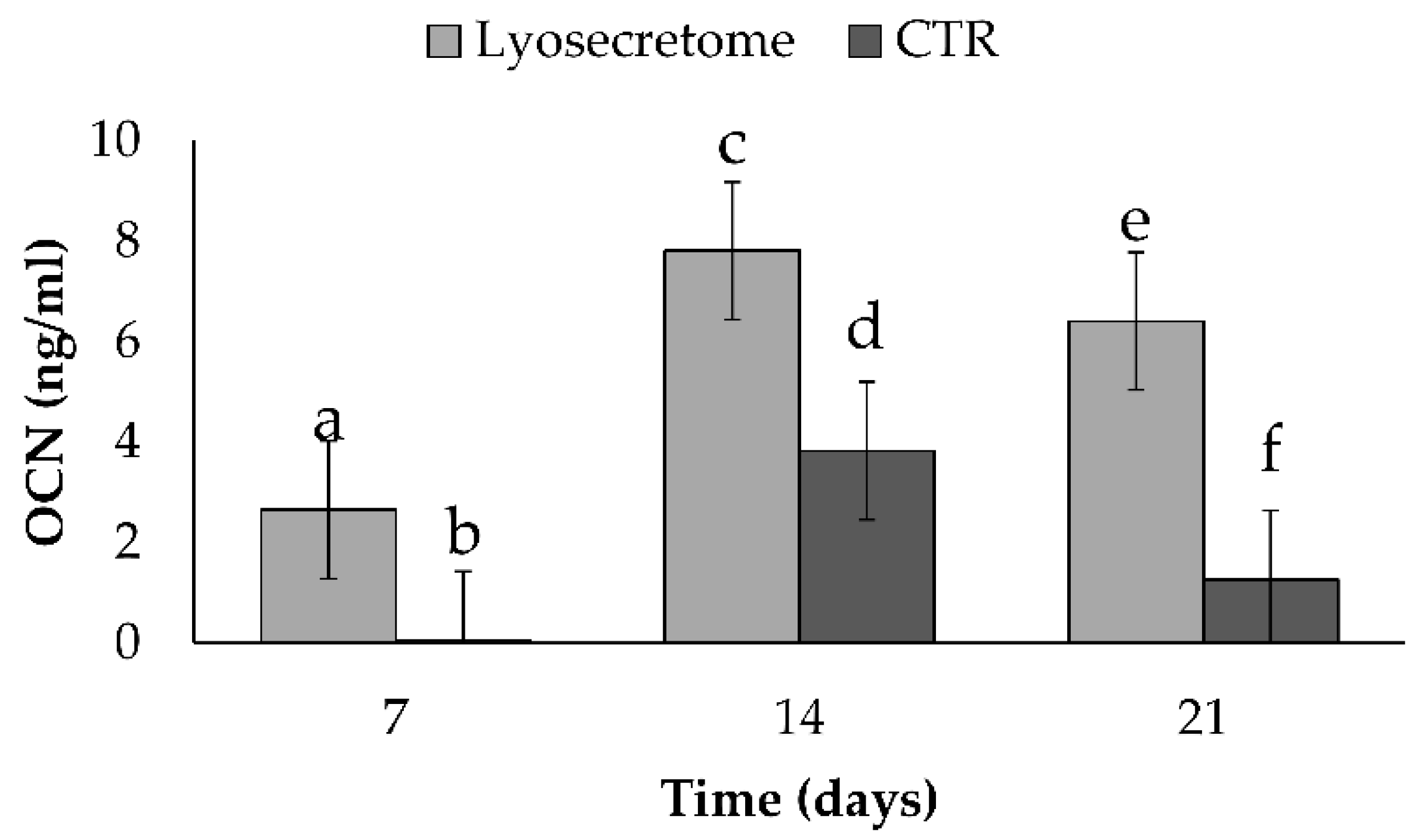
Dosage of Osteocalcin (OCN) by ELISA
2.5.4. Statistical Analysis
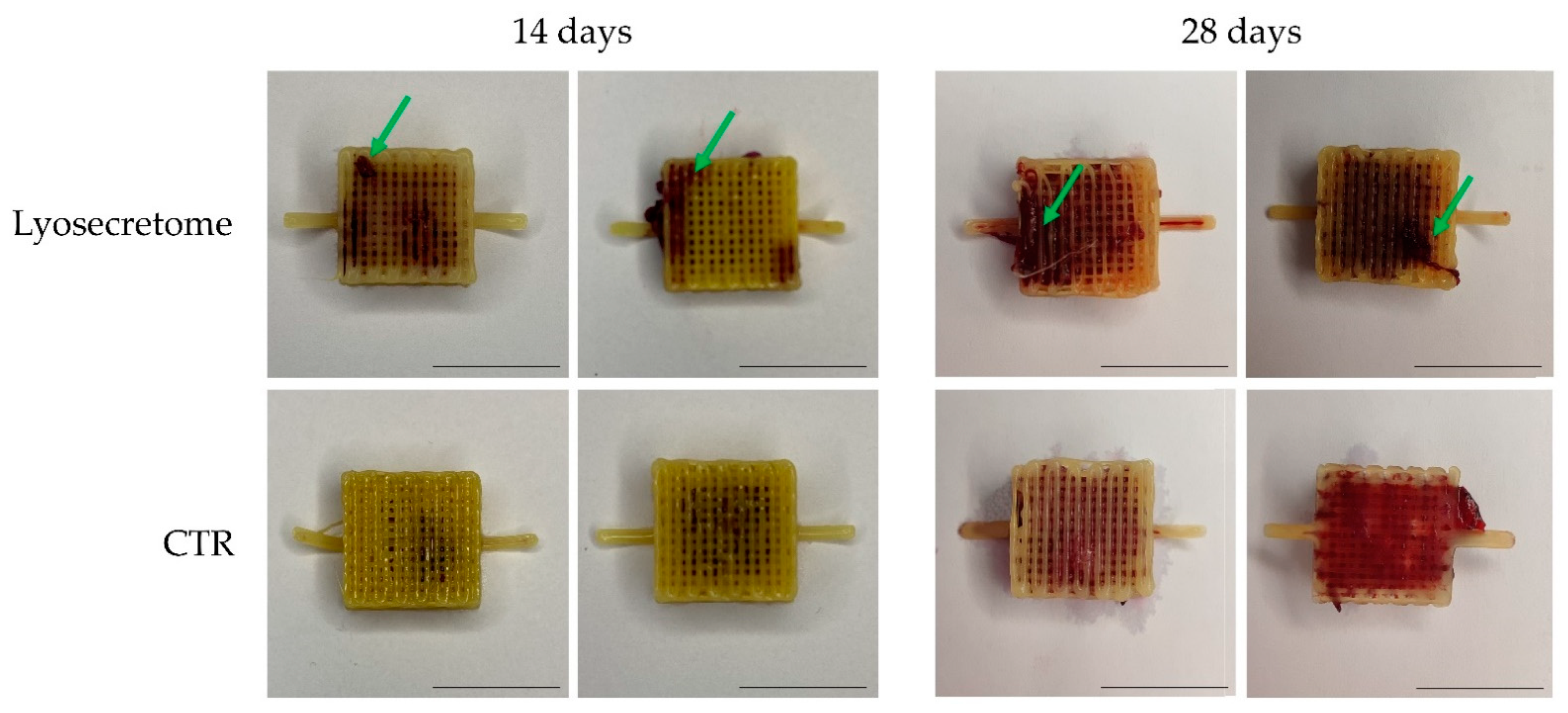
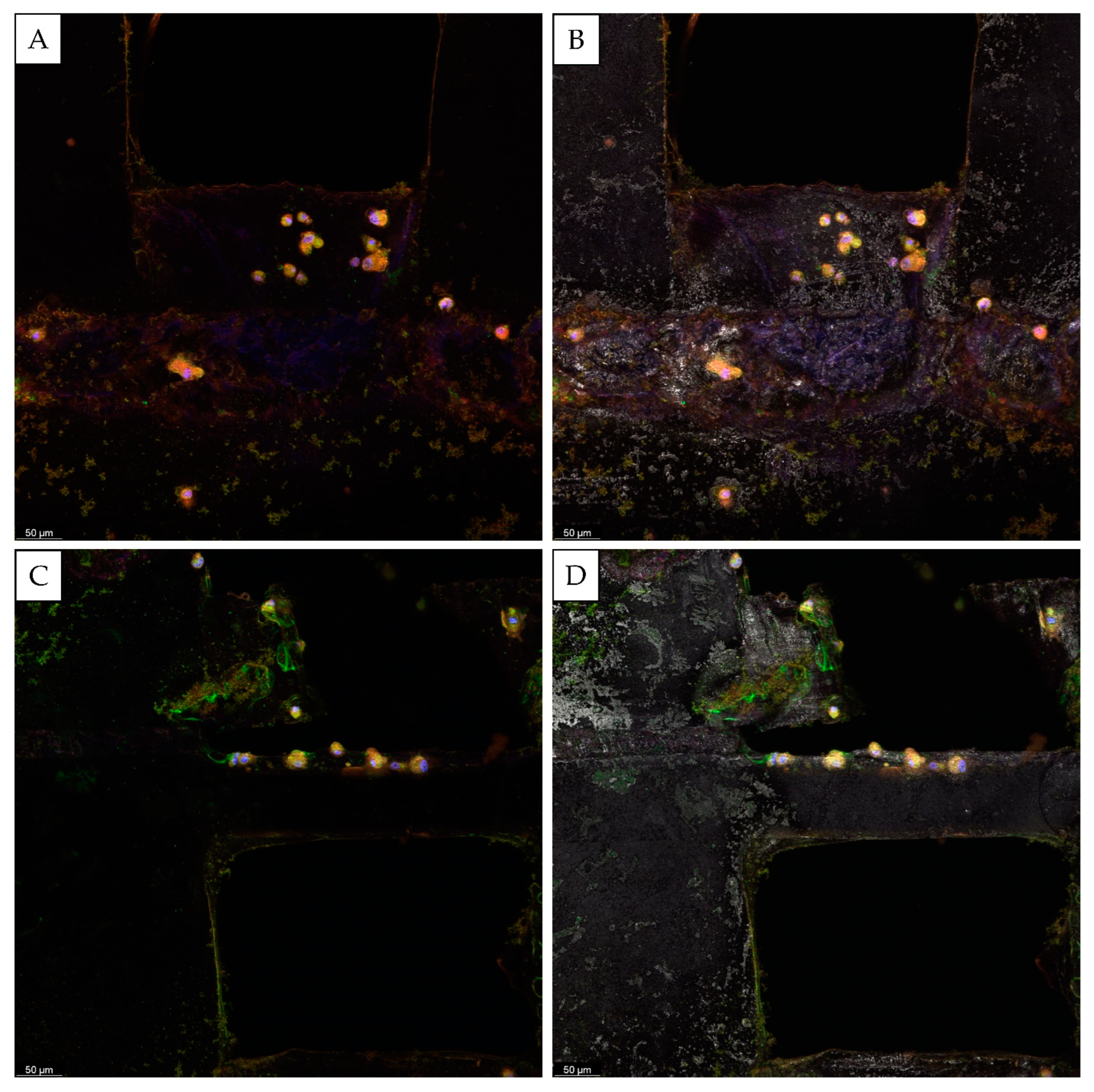
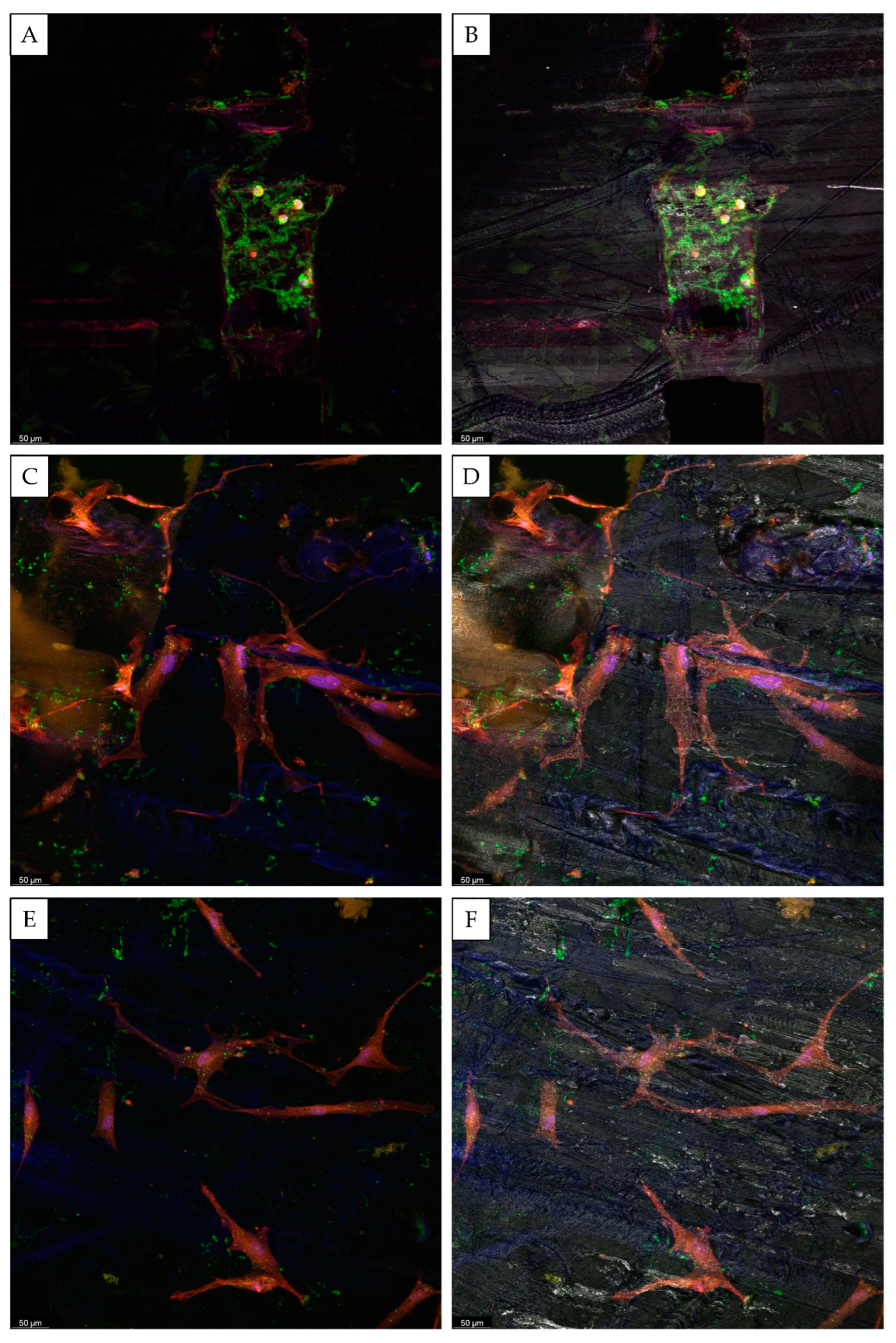
3. Results and Discussion
4. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering bioinks for 3D bioprinting. Biofabrication 2021, 13, 032001. [Google Scholar] [CrossRef] [PubMed]
- Kacarevic, Z.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanisevic, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D bioprinting processes: A perspective on classification and terminology. Int. J. Bioprint. 2018, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.G.; Stapleton, E.J.; Sgaglione, J.; Sgaglione, M.; Goldstein, T.; Sgaglione, N.A.; Grande, D.A. Three-Dimensional Bioprinting in Orthopaedics. JBJS Rev. 2020, 8, e0204. [Google Scholar] [CrossRef]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D bioprinting in tissue engineering: Advantages, deficiencies, improvements, and future perspectives. J. Mater. Chem. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef]
- He, Y.; Gu, Z.; Xie, M.; Fu, J.; Lin, H. Why choose 3D bioprinting? Part II: Methods and bioprinters. Bio-Des. Manuf. 2020, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Huang, Y.; Schilling, A.F.; Hubbell, K.; Cui, X. Organ Bioprinting: Are We There Yet? Adv. Healthc. Mater. 2018, 7, 1701018. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Baji, A.; Wong, S.-C.; Srivatsan, T.S.; Njus, G.O.; Mathur, G. Processing methodologies for polycaprolactone-hydroxyapatite composites: A review. Mater. Manuf. Process. 2006, 21, 211–218. [Google Scholar] [CrossRef]
- Raghunath, J.; Rollo, J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Biomaterials and scaffold design: Key to tissue-engineering cartilage. Biotechnol. Appl. Biochem. 2007, 46, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed] [Green Version]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-S.; Hsu, G.C.-Y.; Sono, T.; Lee, M.; James, A.W. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef]
- Fan, D.; Staufer, U.; Accardo, A. Engineered 3D polymer and hydrogel microenvironments for cell culture applications. Bioengineering 2019, 6, 113. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Soufivand, A.A.; Abolfathi, N.; Hashemi, A.; Lee, S.J. The effect of 3D printing on the morphological and mechanical properties of polycaprolactone filament and scaffold. Polym. Adv. Technol. 2020, 31, 1038–1046. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-L.; Kobayashi, E.; Liu, K.; Li, Q. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine 2016, 12, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Scocozza, F.; Sakaj, M.; Auricchio, F.; Marconi, S.; Riello, P.; Ferrari, C.; Cansolino, L.; Catenacci, L.; Sorrenti, M.; Scatto, M. Shape fidelity and sterility assessment of 3D printed polycaprolactone and hydroxyapatite scaffolds. J. Polym. Res. 2021, 28, 327. [Google Scholar] [CrossRef]
- The United States Pharmacopeia, USP 40/The National Formulary, NF 35; United States Pharmacopeial Convention: North Bethesda, MA, USA, 2017.
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. 2019, 9, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolagas, S.C. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020, 16, e1008714. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, E.; Tartara, F.; Cofano, F.; Di Perna, G.; Garbossa, D.; Perteghella, S.; Sorlini, M.; Mandracchia, D.; Giovannelli, L.; Gaetani, P.; et al. Freeze-Dried Secretome (Lyosecretome) from Mesenchymal Stem/Stromal Cells Promotes the Osteoinductive and Osteoconductive Properties of Titanium Cages. Int. J. Mol. Sci. 2021, 22, 8445. [Google Scholar] [CrossRef]
- Li, J.P.; Habibovic, P.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef]
- Hung, J.; Han, A.C.; Cai, E.; Chye, L.T. Comparison of titanium mesh versus polycaprolactone-tricalcium phosphate implant in orbital fracture reconstruction. Int. J. Oral Maxillofac. Surg. 2017, 46, 82. [Google Scholar] [CrossRef]
- Shim, J.H.; Won, J.Y.; Sung, S.J.; Lim, D.H.; Yun, W.S.; Jeon, Y.C.; Huh, J.B. Comparative Efficacies of a 3D-Printed PCL/PLGA/beta-TCP Membrane and a Titanium Membrane for Guided Bone Regeneration in Beagle Dogs. Polymers 2015, 7, 2061–2077. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Roato, I.; Perale, G.; Rossi, F.; Genova, T.; Mussano, F.; Ferracini, R.; Sorlini, M.; Torre, M.L.; Perteghella, S. Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 4064. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, E.; Scocozza, F.; Perteghella, S.; Segale, L.; Sorlini, M.; Auricchio, F.; Conti, M.; Torre, M.L. Three-Dimensional Bioprinted Controlled Release Scaffold Containing Mesenchymal Stem/Stromal Lyosecretome for Bone Regeneration: Sterile Manufacturing and In Vitro Biological Efficacy. Biomedicines 2022, 10, 1063. https://doi.org/10.3390/biomedicines10051063
Bari E, Scocozza F, Perteghella S, Segale L, Sorlini M, Auricchio F, Conti M, Torre ML. Three-Dimensional Bioprinted Controlled Release Scaffold Containing Mesenchymal Stem/Stromal Lyosecretome for Bone Regeneration: Sterile Manufacturing and In Vitro Biological Efficacy. Biomedicines. 2022; 10(5):1063. https://doi.org/10.3390/biomedicines10051063
Chicago/Turabian StyleBari, Elia, Franca Scocozza, Sara Perteghella, Lorena Segale, Marzio Sorlini, Ferdinando Auricchio, Michele Conti, and Maria Luisa Torre. 2022. "Three-Dimensional Bioprinted Controlled Release Scaffold Containing Mesenchymal Stem/Stromal Lyosecretome for Bone Regeneration: Sterile Manufacturing and In Vitro Biological Efficacy" Biomedicines 10, no. 5: 1063. https://doi.org/10.3390/biomedicines10051063
APA StyleBari, E., Scocozza, F., Perteghella, S., Segale, L., Sorlini, M., Auricchio, F., Conti, M., & Torre, M. L. (2022). Three-Dimensional Bioprinted Controlled Release Scaffold Containing Mesenchymal Stem/Stromal Lyosecretome for Bone Regeneration: Sterile Manufacturing and In Vitro Biological Efficacy. Biomedicines, 10(5), 1063. https://doi.org/10.3390/biomedicines10051063







