Single Application of Low-Dose, Hydroxyapatite-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation and Biomechanical Stabilization of a Bone Defect in a Senile Sheep Lumbar Osteopenia Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Recombinant Human BMP-2 and GDF-5 in E. coli
2.2. In Vitro Evaluation of the Dosages for BMP-2 and GDF-5 Combined with Bone Graft Substitutes (HA) for Application in Animal Experiments
2.3. In Vitro Release of BMP-2 and GDF-5 from the HA Particles
2.4. Animal Experiments
2.4.1. Surgical Procedure
2.4.2. Excision of the Samples
2.5. Digital Osteodensitometry
2.6. Histology/Histomorphometry
2.7. Dynamic Histomorphometrical Measurements
2.8. Biomechanical Testing (Compressive Strength)
2.9. Statistical Methods
3. Results
3.1. In Vitro Evaluation of Therapeutic Dosages for BMP-2 and GDF-5
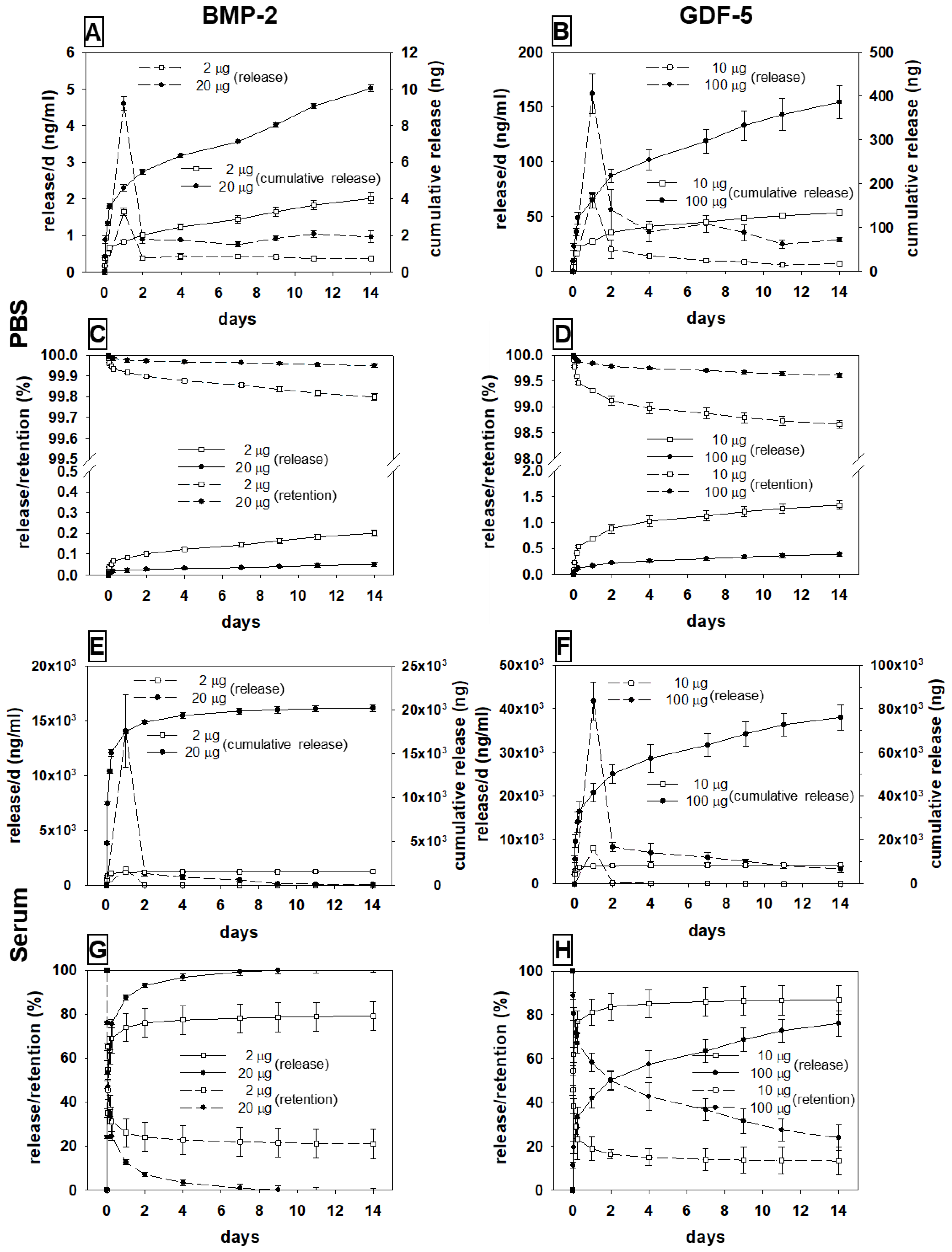
3.2. In Vitro Release of BMP-2 and GDF-5 from the HA Particles
3.2.1. BMP-2
Release in PBS
Release in Sheep Serum
3.2.2. GDF-5
Release in PBS
Release in Sheep Serum
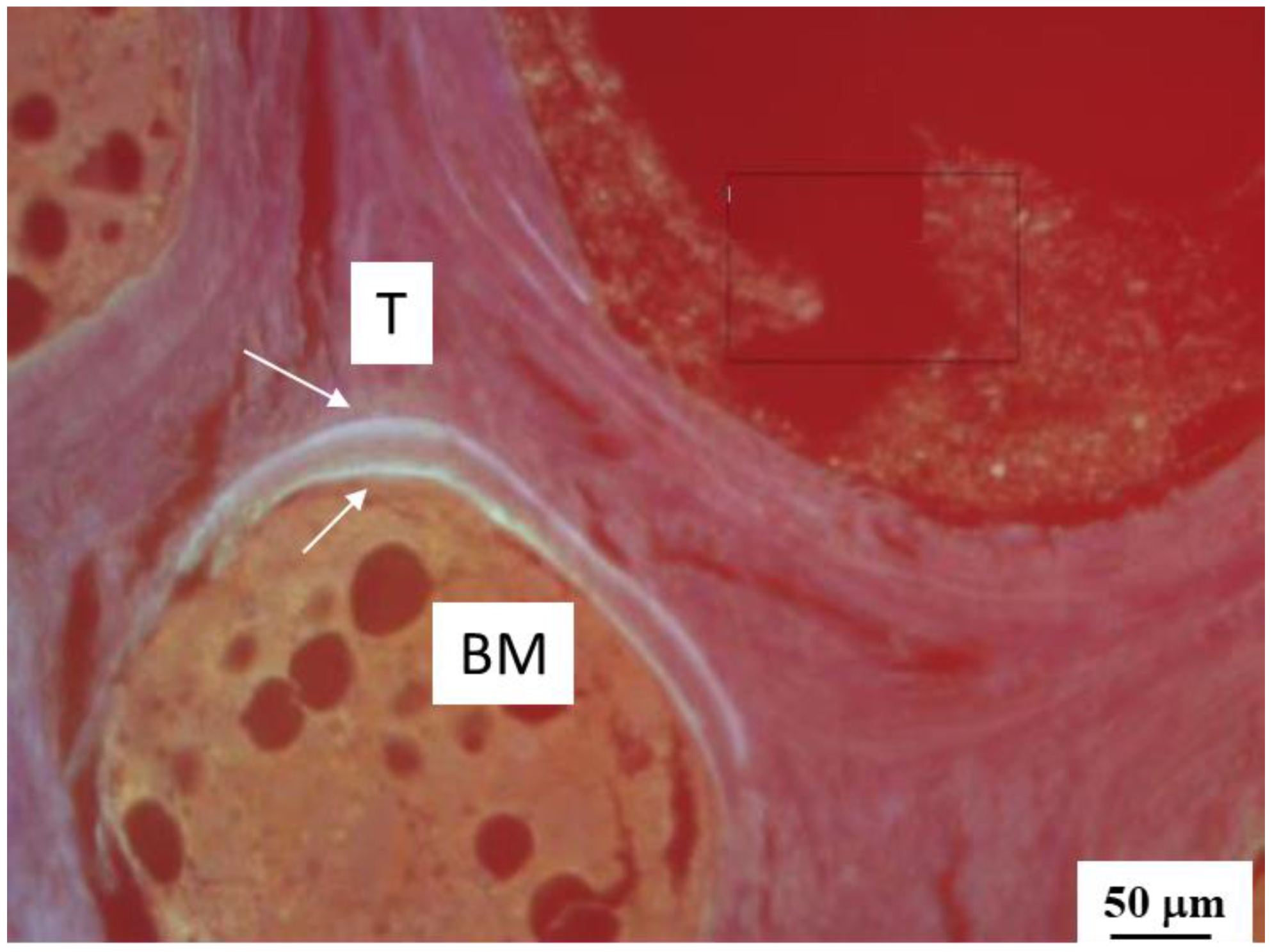
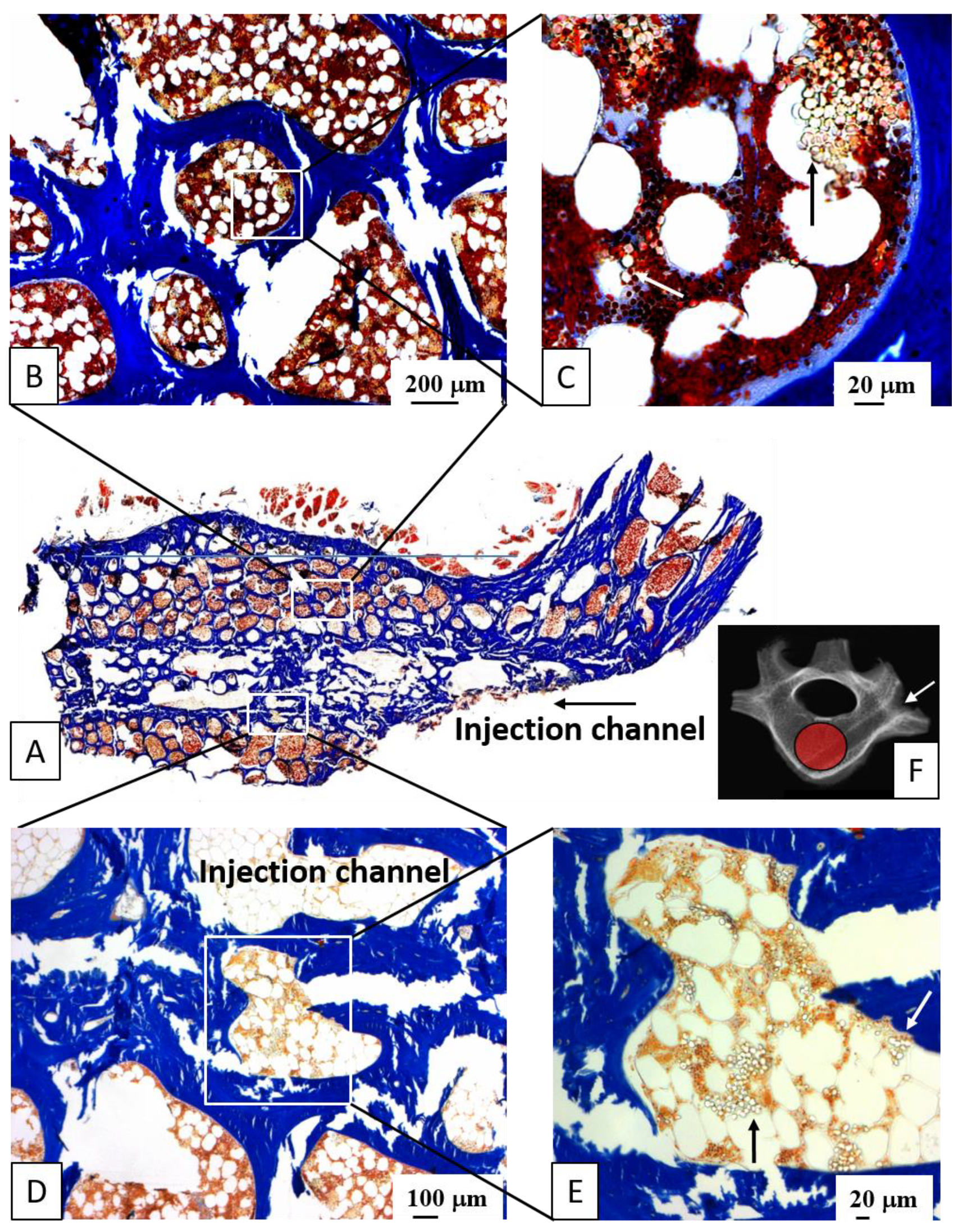
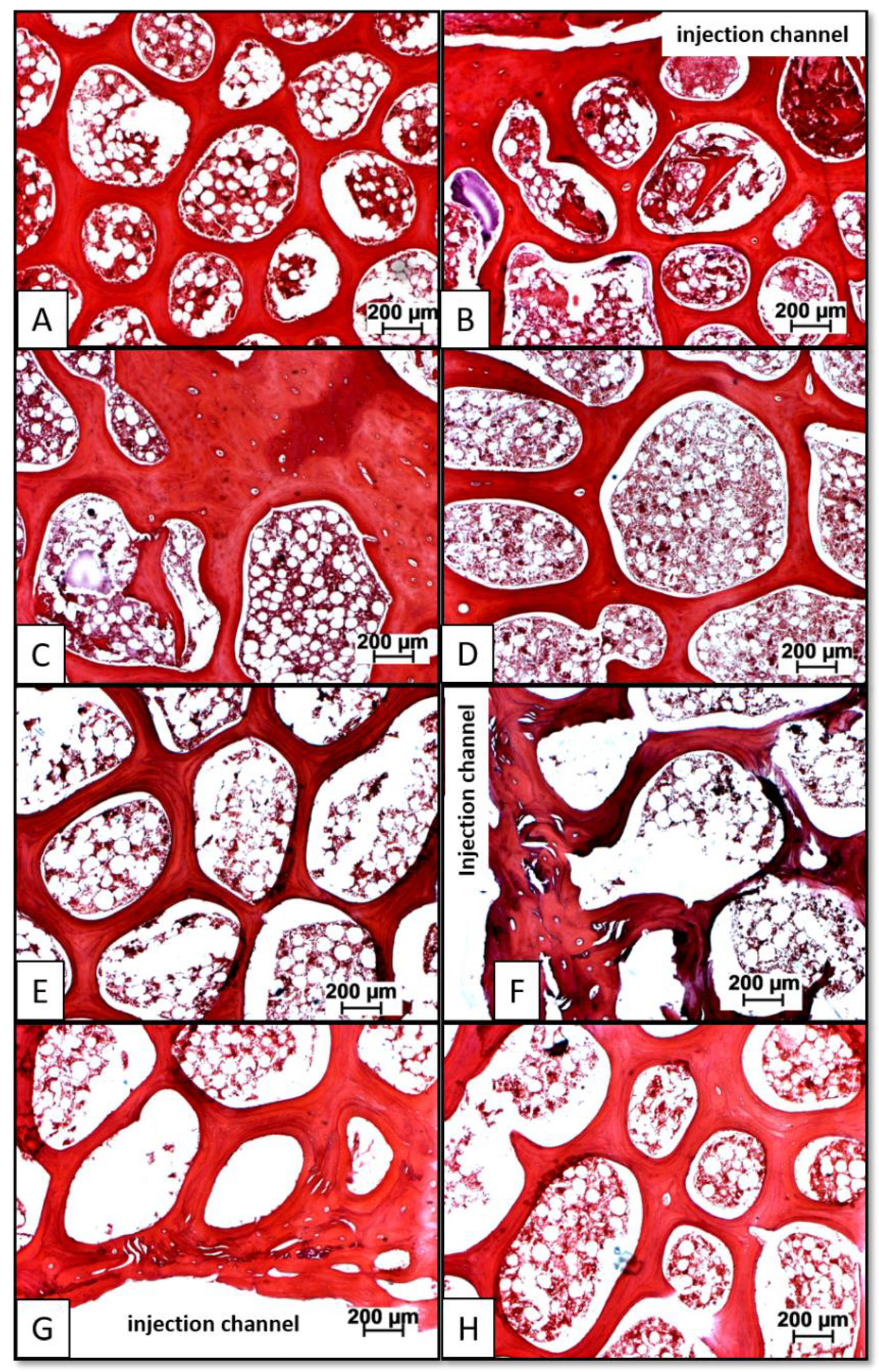
3.3. Distribution of Injected HA Particles; Potential Side Effects of BMP Therapy
3.4. Single Injection of HA-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation—Global Analysis of the Therapeutic Effects of Carrier HA or BMP-Coated Carrier
3.5. Bone Mineral Density
3.6. Static Histology/Histomorphometry
3.6.1. Structural Parameters
Bone Volume
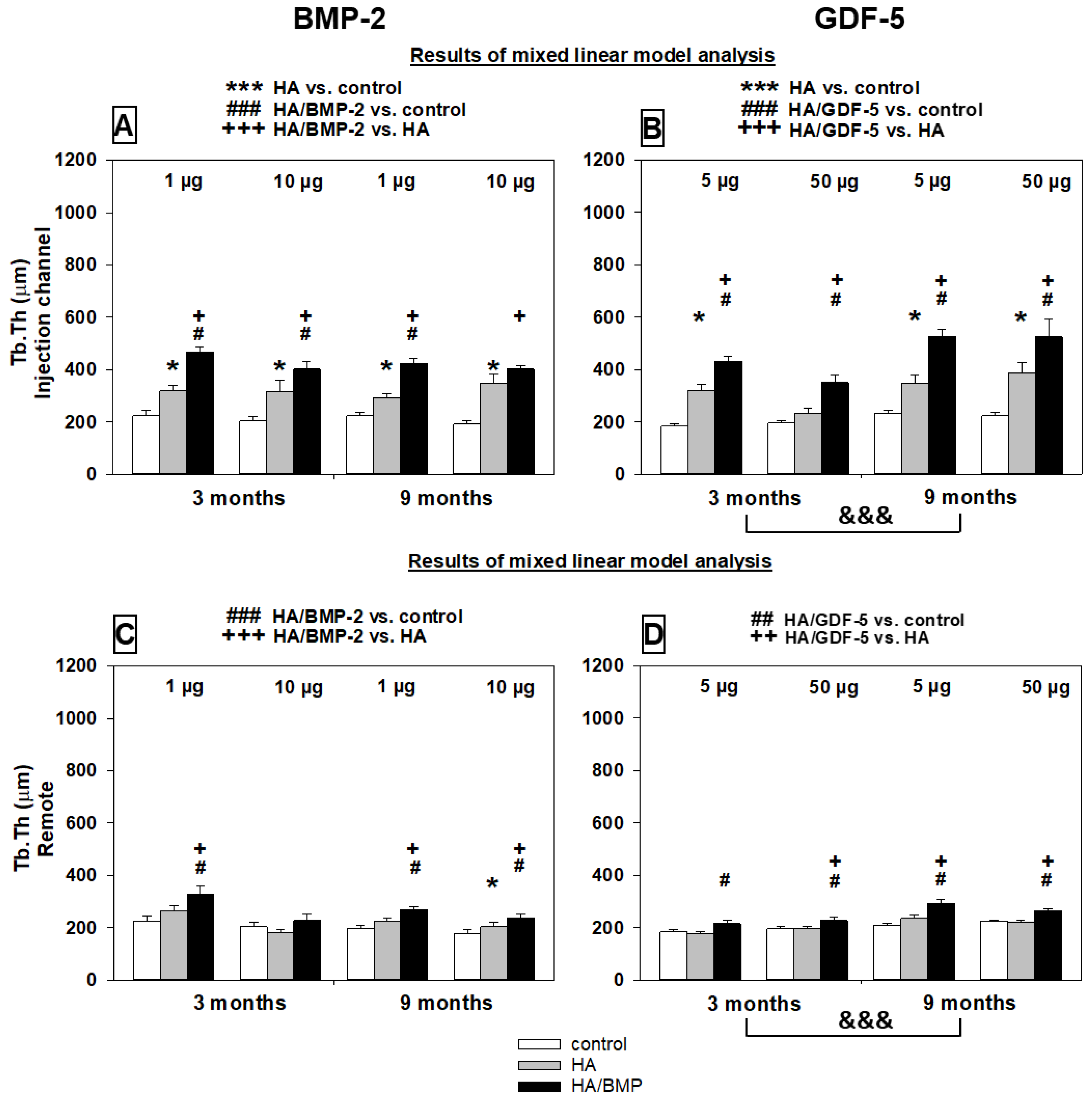
Trabecular Thickness
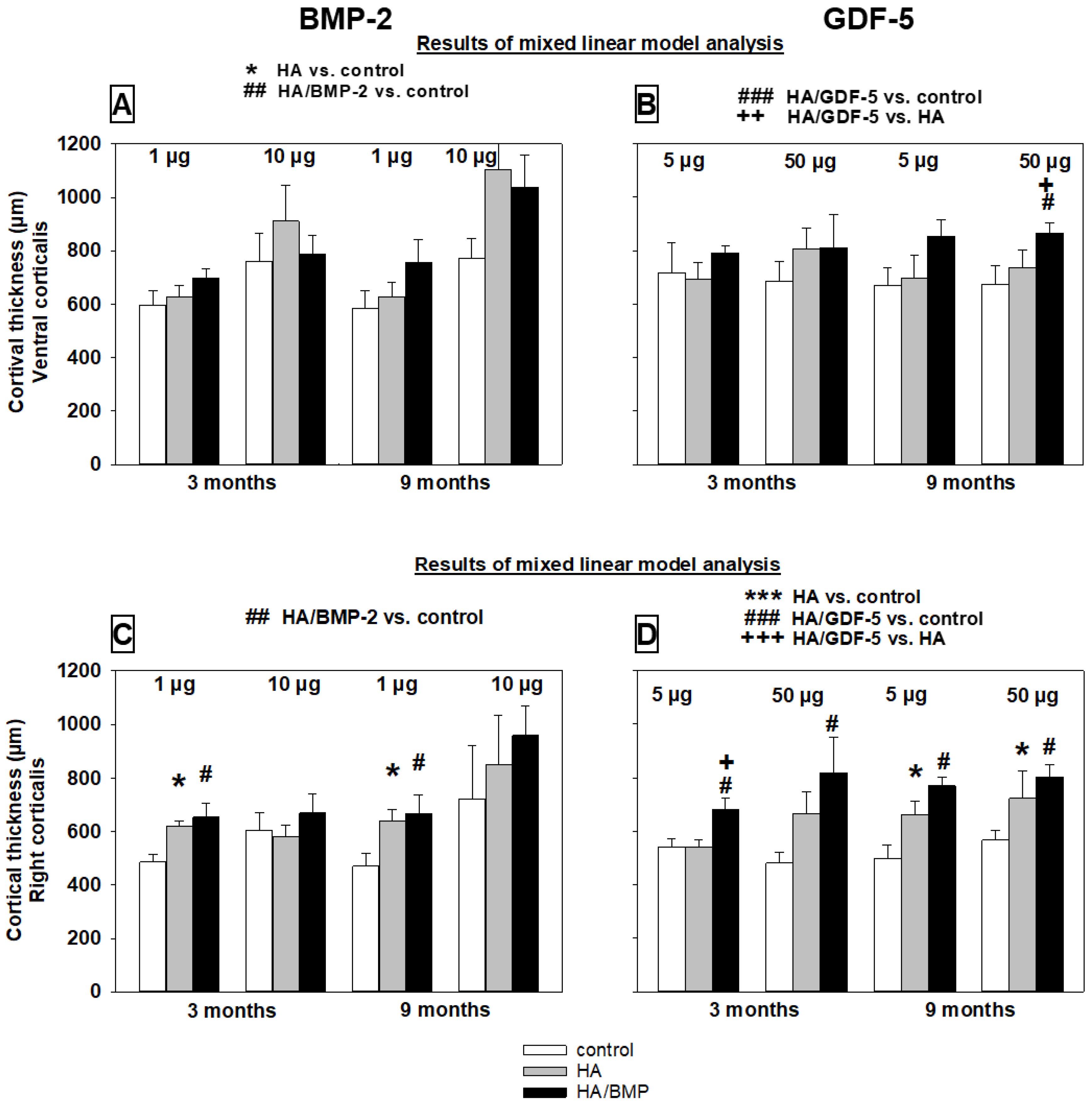
Cortical Thickness
3.6.2. Bone Formation Parameters (Static/Dynamic Histomorphometry)
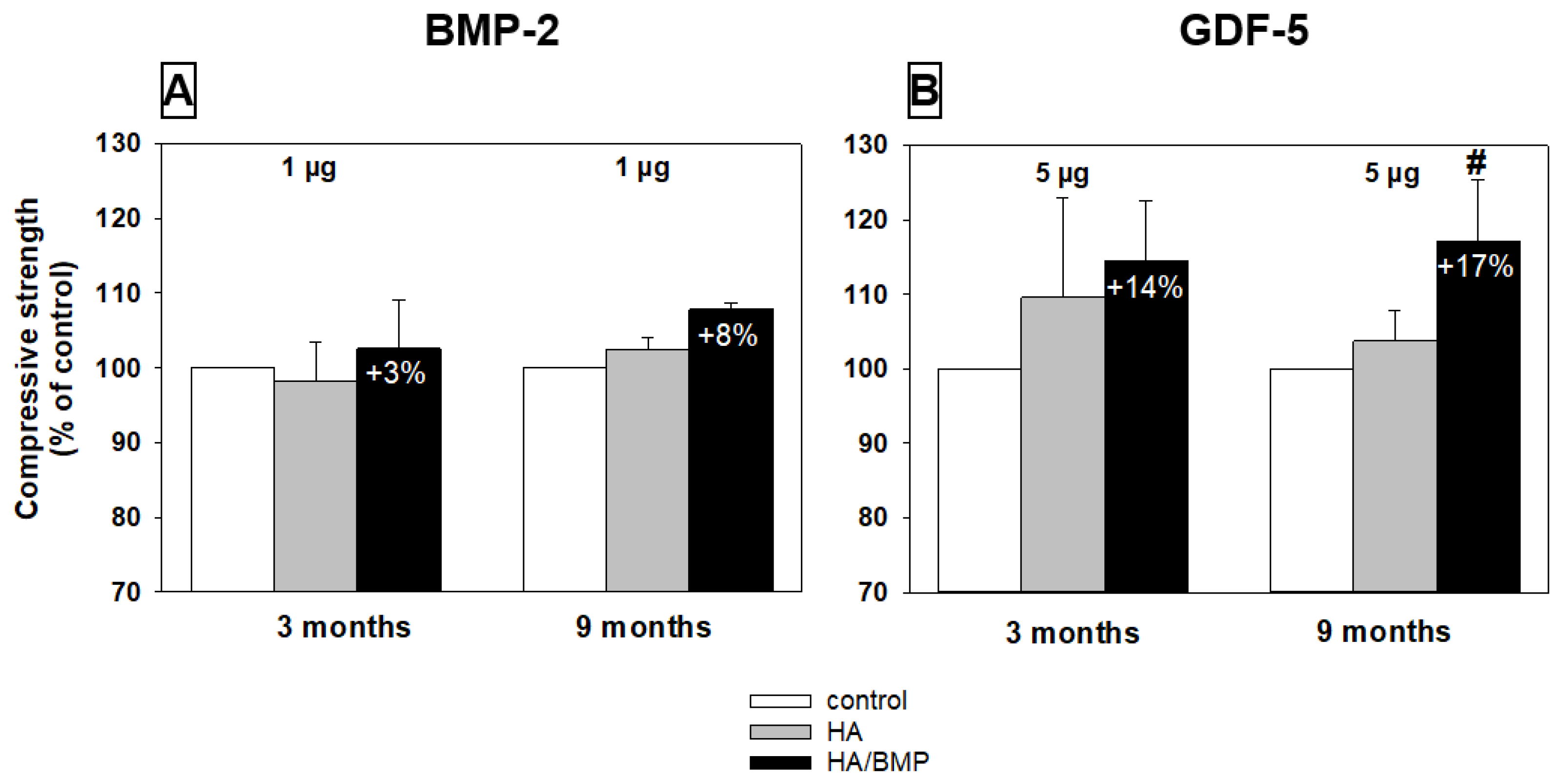
3.7. Biomechanical Testing (Compressive Strength)
4. Discussion
4.1. Animal Model
4.2. In Vitro Growth Factor Release from the HA Particles
4.3. Induction of Bone Formation
4.4. Effects of Very Low Doses of Growth Factors
4.5. Long-Term Efficacy of Growth-Factor-Coated HA
4.6. Remote Effects of the Therapy with Growth-Factor-Coated HA
4.7. Direct Comparison of BMP-2 and GFD-5
4.8. Limitations of the Study
5. Conclusions
Potential Clinical Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosman, F.; Krege, J.H.; Looker, A.C.; Schousboe, J.T.; Fan, B.; Sarafrazi Isfahani, N.; Shepherd, J.A.; Krohn, K.D.; Steiger, P.; Wilson, K.E.; et al. Spine fracture prevalence in a nationally representative sample of US women and men aged >/=40 years: Results from the National Health and Nutrition Examination Survey (NHANES) 2013-2014. Osteoporos. Int. 2017, 28, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. A Comparison of Kyphoplasty, Vertebroplasty, or Non-Surgical Treatment of Traumatic/Atraumatic Osteoporotic Vertebral Compression Fractures: A Short Review. Surg. Neurol. Int. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, J.; Yin, J.; Zhang, Z.; Liu, C.; Hao, D. Therapeutic effect of kyphoplasty and balloon vertebroplasty on osteoporotic vertebral compression fracture: A systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17810. [Google Scholar] [CrossRef] [PubMed]
- Yasko, A.W.; Lane, J.M.; Fellinger, E.J.; Rosen, V.; Wozney, J.M.; Wang, E.A. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J. Bone Jt. Surg. 1992, 74, 659–670. [Google Scholar] [CrossRef]
- Murakami, N.; Saito, N.; Takahashi, J.; Ota, H.; Horiuchi, H.; Nawata, M.; Okada, T.; Nozaki, K.; Takaoka, K. Repair of a proximal femoral bone defect in dogs using a porous surfaced prosthesis in combination with recombinant BMP-2 and a synthetic polymer carrier. Biomaterials 2003, 24, 2153–2159. [Google Scholar] [CrossRef]
- Simank, H.G.; Manggold, J.; Sebald, W.; Ries, R.; Richter, W.; Ewerbeck, V.; Sergi, C. Bone morphogenetic protein-2 and growth and differentiation factor-5 enhance the healing of necrotic bone in a sheep model. Growth Factors 2001, 19, 247–257. [Google Scholar] [CrossRef]
- Spiro, R.C.; Thompson, A.Y.; Poser, J.W. Spinal fusion with recombinant human growth and differentiation factor-5 combined with a mineralized collagen matrix. Anat. Rec. 2001, 263, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Jahng, T.A.; Fu, T.S.; Cunningham, B.W.; Dmitriev, A.E.; Kim, D.H. Endoscopic instrumented posterolateral lumbar fusion with Healos and recombinant human growth/differentiation factor-5. Neurosurgery 2004, 54, 171–181. [Google Scholar] [CrossRef]
- Sampath, T.K.; Reddi, A.H. Discovery of bone morphogenetic proteins-A historical perspective. Bone 2020, 140, 115548. [Google Scholar] [CrossRef]
- Gunnella, F.; Kunisch, E.; Bungartz, M.; Maenz, S.; Horbert, V.; Xin, L.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. Low-dose BMP-2 is sufficient to enhance the bone formation induced by an injectable, PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2017, 17, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Bungartz, M.; Kunisch, E.; Maenz, S.; Horbert, V.; Xin, L.; Gunnella, F.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. GDF5 significantly augments the bone formation induced by an injectable, PLGA-fiber reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2017, 17, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Rengachary, S.S. Bone morphogenetic proteins: Basic concepts. Neurosurg. Focus 2002, 13, e2. [Google Scholar] [CrossRef] [Green Version]
- Harwood, P.J.; Giannoudis, P.V. Application of bone morphogenetic proteins in orthopaedic practice: Their efficacy and side effects. Expert Opin. Drug Saf. 2005, 4, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- King, W.J.; Krebsbach, P.H. Growth factor delivery: How surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012, 64, 1239–1256. [Google Scholar] [CrossRef] [Green Version]
- Firkowska-Boden, I.; Adjiski, R.; Bautista, A.C.; Borowski, A.; Matziolis, G.; Jandt, K.D.; Kinne, R.W.; Bossert, J. Biopolymer surface modification of PLGA fibers enhances interfacial shear strength and supports immobilization of rhGDF-5 in fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2020, 115, 104285. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The family of bone morphogenetic proteins. Kidney Int. 2000, 57, 2207–2214. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jia, L.; Zhang, S.; Zheng, Y.; Zhou, Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res. Ther. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Scarfi, S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J. Stem Cells 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin. Orthop. Relat. Res. 1998, 11, 26–37. [Google Scholar] [CrossRef]
- Coleman, C.M.; Tuan, R.S. Growth/differentiation factor 5 enhances chondrocyte maturation. Dev. Dyn. 2003, 228, 208–216. [Google Scholar] [CrossRef]
- Depprich, R.H.J.; Sebald, W.; Kübler, N.R.; Würzler, K.K. Vergleich der osteogenen Potenz gentechnisch modifizierter BMP. Mund Kiefer Gesichtschirurgie 2005, 9, 363–368. [Google Scholar] [CrossRef]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [Green Version]
- Groeneveld, E.H.; Burger, E.H. Bone morphogenetic proteins in human bone regeneration. Eur. J. Endocrinol. 2000, 142, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Urist, M.R.; Lietze, A.; Dawson, E. Beta-tricalcium phosphate delivery system for bone morphogenetic protein. Clin. Orthop. Relat. Res. 1984, 187, 277–280. [Google Scholar] [CrossRef]
- Gunnella, F.; Kunisch, E.; Maenz, S.; Horbert, V.; Xin, L.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; Sachse, A.; et al. The GDF5 mutant BB-1 enhances the bone formation induced by an injectable, poly(l-lactide-co-glycolide) acid (PLGA) fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2018, 18, 357–369. [Google Scholar] [CrossRef]
- Spiro, R.C.; Liu, L.; Heidaran, M.A.; Thompson, A.Y.; Ng, C.K.; Pohl, J.; Poser, J.W. Inductive activity of recombinant human growth and differentiation factor-5. Biochem. Soc. Trans. 2000, 28, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kuniyasu, H.; Hirose, Y.; Ochi, M.; Yajima, A.; Sakaguchi, K.; Murata, M.; Pohl, J. Bone augmentation using rhGDF-5-collagen composite. Clin. Oral Implant. Res. 2003, 14, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Sachse, A.; Wagner, A.; Keller, M.; Wagner, O.; Wetzel, W.D.; Layher, F.; Venbrocks, R.A.; Hortschansky, P.; Pietraszczyk, M.; Wiederanders, B.; et al. Osteointegration of hydroxyapatite-titanium implants coated with nonglycosylated recombinant human bone morphogenetic protein-2 (BMP-2) in aged sheep. Bone 2005, 37, 699–710. [Google Scholar] [CrossRef]
- Raina, D.B.; Qayoom, I.; Larsson, D.; Zheng, M.H.; Kumar, A.; Isaksson, H.; Lidgren, L.; Tagil, M. Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials 2019, 188, 38–49. [Google Scholar] [CrossRef]
- Saito, N.; Takaoka, K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar] [CrossRef]
- Lee, Y.M.; Nam, S.H.; Seol, Y.J.; Kim, T.I.; Lee, S.J.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Han, S.B.; Choi, S.M. Enhanced bone augmentation by controlled release of recombinant human bone morphogenetic protein-2 from bioabsorbable membranes. J. Periodontol. 2003, 74, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Kübler, R.N.W.K. Bone morphogenetic proteins. Implantologie 2002, 10, 177–192. [Google Scholar]
- Maenz, S.; Brinkmann, O.; Hasenbein, I.; Braun, C.; Kunisch, E.; Horbert, V.; Gunnella, F.; Sachse, A.; Bischoff, S.; Schubert, H.; et al. The old sheep: A convenient and suitable model for senile osteopenia. J. Bone Miner. Metab. 2020, 38, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hortschansky, P.S.V.; Fahnert, B.; Riesenberg, D. Verfahren Zur Herstellung Von Dimeren, Biologisch Aktiven Knochenmorphologischen Proteinen. Patent DE 199 44 626 A1, 17 September 1999. [Google Scholar]
- Hortschansky, P.S.V.; Schmuck, K.D. Verwendung und Verfahren Zum Nachweis Von Biologisch Aktivem BMP Auf Einem Substrat. Patent DE 103 37 176 B3, 13 August 2003. [Google Scholar]
- Naqvi, S.B.; Mirza, T.; Sheikh, D.; Abbas, T. Application of Limulus Amebocyte Lysate (LAL) test for detecting endotoxin (pyrogen) in large volume parenterals. Pak. J. Pharm. Sci. 2004, 17, 89–94. [Google Scholar] [PubMed]
- Testing on bacterial endotoxin (2.6.14.). In European Pharmakopoeia, 5th ed.; Council of Europe: Strasbourg, France.
- Zaugg, M.; Zaborosch, C. Downstream-Processing Eines Rekombinanten Hepatitis B Impfstoffes; Biotechnet (Extract from Bio World) 03: Wädenswil, Switzerland, 2005. [Google Scholar]
- Behnam, K.; Murray, S.S.; Brochmann, E.J. BMP stimulation of alkaline phosphatase activity in pluripotent mouse C2C12 cells is inhibited by dermatopontin, one of the most abundant low molecular weight proteins in demineralized bone matrix. Connect. Tissue Res. 2006, 47, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Shukunami, C.; Nakamura, T.; Hiraki, Y. Differential expressions of BMP family genes during chondrogenic differentiation of mouse ATDC5 cells. Cell Struct. Funct. 2000, 25, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, P.; Schwappacher, R.; Kjaer, K.W.; Krakow, D.; Lehmann, K.; Dawson, K.; Stricker, S.; Pohl, J.; Plöger, F.; Staub, E.; et al. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J. Clin. Investig. 2005, 115, 2373–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnella, F.; Kunisch, E.; Horbert, V.; Maenz, S.; Bossert, J.; Jandt, K.D.; Ploger, F.; Kinne, R.W. In vitro release of bioactive bone morphogenetic proteins (GDF5, BB-1, and BMP-2) from a PLGA fiber-reinforced, brushite-forming calcium phosphate cement. Pharmaceutics 2019, 11, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Ellenberger, C. Funktionelle Pathologie des equinen Ovars und daraus resultierende endometriale Differenzierungsstörungen. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2003. [Google Scholar]
- Syed, Z.; Khan, A. Bone densitometry: Applications and limitations. J. Obstet. Gynaecol. Can. 2002, 24, 476–484. [Google Scholar] [CrossRef]
- Turner, A.S. The sheep as a model for osteoporosis in humans. Vet. J. 2002, 163, 232–239. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Parfitt, A.M. Bone histomorphometry: Proposed system for standardization of nomenclature, symbols, and units. Calcif. Tissue Res. 1988, 42, 284–286. [Google Scholar] [CrossRef]
- Parfitt, A.M. Bone histomorphometry: Standardization of nomenclature, symbols and units. Summary of proposed system. Bone Miner. 1988, 9, 67–69. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delling, G. Endokrine Osteopathien. In Veröffentlichungen aus der Pathologie; Büngeler, W.E.M., Lennert, K., Peters, G., Sandritter, W., Seifert, G., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1975. [Google Scholar]
- Protze, A. Einschätzung der Knochenqualität am Proximalen Femur bei Fortgeschrittener Koxarthrose und Deren Einfluss auf die Postoperative Periprothetische Knochenmineraldichteänderung. Ph.D. Thesis, Jena University Hospital, Gina, Germany, 2004. [Google Scholar]
- Chavassieux, P.; Buffet, A.; Vergnaud, P.; Garnero, P.; Meunier, P.J. Short-term effects of corticosteroids on trabecular bone remodeling in old ewes. Bone 1997, 20, 451–455. [Google Scholar] [CrossRef]
- Newman, E.; Turner, A.S.; Wark, J.D. The potential of sheep for the study of osteopenia: Current status and comparison with other animal models. Bone 1995, 16, 277S–284S. [Google Scholar] [CrossRef]
- Chhabra, A.; Zijerdi, D.; Zhang, J.; Kline, A.; Balian, G.; Hurwitz, S. BMP-14 deficiency inhibits long bone fracture healing: A biochemical, histologic, and radiographic assessment. J. Orthop. Trauma 2005, 19, 629–634. [Google Scholar] [CrossRef]
- Xin, L.; Bungartz, M.; Maenz, S.; Horbert, V.; Hennig, M.; Illerhaus, B.; Günster, J.; Bossert, J.; Bischoff, S.; Borowski, J.; et al. Decreased extrusion of calcium phosphate cement versus high viscosity PMMA cement into spongious bone marrow-an ex vivo and in vivo study in sheep vertebrae. Spine J. 2016, 16, 1468–1477. [Google Scholar] [CrossRef]
- Maenz, S.; Brinkmann, O.; Kunisch, E.; Horbert, V.; Gunnella, F.; Bischoff, S.; Schubert, H.; Sachse, A.; Xin, L.; Günster, J.; et al. Enhanced bone formation in sheep vertebral bodies after minimally invasive treatment with a novel, PLGA fiber-reinforced brushite cement. Spine J. 2017, 17, 709–719. [Google Scholar] [CrossRef]
- Eschler, A.; Roepenack, P.; Roesner, J.; Herlyn, P.K.; Martin, H.; Reichel, M.; Rotter, R.; Vollmar, B.; Mittlmeier, T.; Gradl, G. Cementless Titanium Mesh Fixation of Osteoporotic Burst Fractures of the Lumbar Spine Leads to Bony Healing: Results of an Experimental Sheep Model. Biomed. Res. Int. 2016, 2016, 4094161. [Google Scholar] [CrossRef] [Green Version]
- Haidekker, M.A.; Andresen, R.; Werner, H.J. Relationship between structural parameters, bone mineral density and fracture load in lumbar vertebrae, based on high-resolution computed tomography, quantitative computed tomography and compression tests. Osteoporos. Int. 1999, 9, 433–440. [Google Scholar] [CrossRef]
- Ito, M.; Nishida, A.; Koga, A.; Ikeda, S.; Shiraishi, A.; Uetani, M.; Hayashi, K.; Nakamura, T. Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone 2002, 31, 351–358. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 17 February 2022).
- Münzenberg, K.J.; Gebhardt, M. Tetracycline and bone collagen. Arch. Orthop. Unfallchir. 1970, 67, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.M.; Turner, A.S.; Seim, H.B., 3rd; MacLeay, J.; Toth, C.A.; Pierce, A.R.; Wheeler, D.L. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006, 6, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Kaymakci, B.; Wark, J.D. Precise accurate mineral measurements of excised sheep bones using X-ray densitometry. Bone Miner. 1994, 25, 231–246. [Google Scholar] [CrossRef]
- Ruhe, P.Q.; Boerman, O.C.; Russel, F.G.; Mikos, A.G.; Spauwen, P.H.; Jansen, J.A. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J. Mater. Sci. Mater. Med. 2006, 17, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Pobloth, A.M.; Bormann, N.; Kolarczik, N.; Schmidt-Bleek, K.; Schell, H.; Schwabe, P.; Duda, G.N.; Wildemann, B. Demineralized Bone Matrix as a Carrier for Bone Morphogenetic Protein-2: Burst Release Combined with Long-Term Binding and Osteoinductive Activity Evaluated In Vitro and In Vivo. Tissue Eng. Part A 2017, 23, 1321–1330. [Google Scholar] [CrossRef]
- Habraken, W.J.; Boerman, O.C.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res. A 2009, 91, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Strecker, S.E.; Unterman, S.; Charles, L.F.; Pivovarchick, D.; Maye, P.F.; Edelman, E.R.; Artzi, N. Osterix-mCherry Expression Allows for Early Bone Detection in a Calvarial Defect Model. Adv. Biosyst. 2019, 3, e1900184. [Google Scholar] [CrossRef]
- Olthof, M.G.L.; Kempen, D.H.R.; Liu, X.; Dadsetan, M.; Tryfonidou, M.A.; Yaszemski, M.J.; Dhert, W.J.A.; Lu, L. Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel. J. Tissue Eng. Regen. Med. 2018, 12, 1339–1351. [Google Scholar] [CrossRef]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Klosterhoff, B.S.; Stevens, H.Y.; Tran, L.; Guldberg, R.E. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017, 49, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, Z.; Ma, X.; Duan, Z.; Hui, J.; Zhu, C.; Zhang, D.; Fan, D.; Shang, L.; Chen, F. Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects. Biomolecules 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Burkus, J.K.; Transfeldt, E.E.; Kitchel, S.H.; Watkins, R.G.; Balderston, R.A. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 2002, 27, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Bouxsein, M.L.; Blake, C.A.; D’Augusta, D.; Kim, H.; Li, X.J.; Wozney, J.M.; Seeherman, H.J. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J. Orthop. Res. 2003, 21, 997–1004. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Malhotra, A.; Habibovic, P. Calcium Phosphates and Angiogenesis: Implications and Advances for Bone Regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Vehof, J.W.; Mahmood, J.; Takita, H.; van’t Hof, M.A.; Kuboki, Y.; Spauwen, P.H.; Jansen, J.A. Ectopic bone formation in titanium mesh loaded with bone morphogenetic protein and coated with calcium phosphate. Plast. Reconstr. Surg. 2001, 108, 434–443. [Google Scholar] [CrossRef]
- Gerhart, T.N.; Kirker-Head, C.A.; Kriz, M.J.; Holtrop, M.E.; Hennig, G.E.; Hipp, J.; Schelling, S.H.; Wang, E. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clin. Orthop. Relat. Res. 1993, 293, 317–326. [Google Scholar] [CrossRef]
- Bragdon, C.R.; Doherty, A.M.; Rubash, H.E.; Jasty, M.; Li, X.J.; Seeherman, H.; Harris, W.H. The efficacy of BMP-2 to induce bone ingrowth in a total hip replacement model. Clin. Orthop. Relat. Res. 2003, 417, 50–61. [Google Scholar]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Schmoekel, H.; Schense, J.C.; Weber, F.E.; Gratz, K.W.; Gnagi, D.; Muller, R.; Hubbell, J.A. Bone healing in the rat and dog with nonglycosylated BMP-2 demonstrating low solubility in fibrin matrices. J. Orthop. Res. 2004, 22, 376–381. [Google Scholar] [CrossRef]
- Wozney, J.M. Overview of bone morphogenetic proteins. Spine 2002, 27, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Trémollieres, F.A.; Pouillès, J.M.; Drewniak, N.; Laparra, J.; Ribot, C.A.; Dargent-Molina, P. Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: Sensitivity of the WHO FRAX tool. J. Bone Miner. Res. 2010, 25, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Merz, W.A. Die Streckenmessung an gerichteten Strukturen im Mikroskop und ihre Anwendung zur Bestimmung von Oberflächen-Volumen-Relationen im Knochengewebe. Mikroskopie 1967, 22, 132–142. [Google Scholar]
- Delling, G. Endokrine Osteopathien; Fischer: Stuttgart, Germany, 1975. [Google Scholar]
- Ashton, B.A.; Kuhlencordt, F.; Mohr, L.; Bergmann, G. Handbuch der inneren Medizin; Springer Berlin: Berlin, Germany, 1980. [Google Scholar]



| Growth Factor | Duration | Dose | BMP-2/GDF-5 Dosage |
|---|---|---|---|
| BMP-2 | Short term * (3 months) | low | 1 µg BMP-2/2.5 mg HA/50 µL serum (n = 12) |
| high | 10 µg BMP-2/2.5 mg HA/50 µL serum (n = 6) | ||
| Long term * (9 months) | low | 1 µg BMP-2/2.5 mg HA/50 µL serum (n = 12) | |
| high | 10 µg BMP-2/2.5 mg HA/50 µL serum (n = 6) | ||
| GDF-5 | Short term * (3 months) | low | 5 µg GDF-5/2.5 mg HA/50 µL serum (n = 12) |
| high | 50 µg GDF-5/2.5 mg HA/50 µL serum (n = 6) | ||
| Long term * (9 months) | low | 5 µg GDF-5/2.5 mg HA/50 µL serum (n = 12) | |
| high | 50 µg GDF-5/2.5 mg HA/50 µL serum (n = 6) |
| Parameter | Treatment (Adjacent Area 1) | Treatment (Remote Area 2) | Group | Time | Dose | Area | Group * Area | ||||||
| HA vs. Control | HA-BMP-2 vs. Control | HA-BMP-2 vs. HA | HA vs. Control | HA-BMP-2 vs. Control | HA-BMP-2 vs. HA | ||||||||
| BMD [%] (area 1) | P (ηp2) | n.s. | 0.001 | n.s. | n.a. | n.a. | n.a. | 0.001 (0.285) | n.s. | n.s. | n.a. | n.a. | |
| BV/TV [%] | P (ηp2) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.032 | <0.001 (0.749) | n.s. | n.s. | <0.001 (0.551) | <0.001 (0.375) | |
| Tb.Th [µm] | P (ηp2) | <0.001 | <0.001 | <0.001 | n.s. | <0.001 | <0.001 | <0.001 (0.730) | n.s. | n.s. | <0.001 (0.629) | <0.001 (0.442) | |
| Cort. Th [µm] | P (ηp2) | n.s. | 0.002 | n.s. | 0.014 | 0.007 | n.s. | <0.001 (0.160) | n.s. | 0.011 (0.291) | 0.001 (0.114) | n.s. | |
| Ob.S/BS [%] | P (ηp2) | 0.004 | <0.001 | n.s. | n.s. | n.s. | n.s. | <0.001 (0.164) | 0.006 (0.340) | 0.047 (0.192) | n.s. | n.s. | |
| OS/BS [%] | P (ηp2) | 0.014 | 0.001 | n.s. | n.s. | n.s. | n.s. | 0.002 (0.137) | n.s. | n.s. | 0.045 (0.046) | n.s. | |
| MS/BS [X] (area 1) | P (ηp2) | n.s. | n.s. | 0.017 | n.a. | n.a. | n.a. | 0.015 (0.260) | n.s. | n.s. | n.a. | n.a. | |
| MAR [X] (area 1) | P (ηp2) | 0.043 | n.s. | 0.014 | n.a. | n.a. | n.a. | 0.015 (0.259) | 0.038 (0.273) | n.s. | n.a. | n.a. | |
| BFR/BS [X] (area 1) | P (ηp2) | 0.030 | n.s. | 0.019 | n.a. | n.a. | n.a. | 0.012 (0.270) | n.s. | n.s. | n.a. | n.a. | |
| ES/BS [%] | P (ηp2) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.030 (0.262) | n.s. | n.s. | n.s. | |
| Parameter | Treatment (Adjacent Area 1) | Treatment (Remote Area 2) | Group | Time | Dose | Area | Group * Area | ||||||
| HA vs. Control | HA-GDF-5 vs. Control | HA-GDF-5 vs. HA | HA vs. Control | HA-GDF-5 vs. Control | HA-GDF-5 vs. HA | ||||||||
| BMD [%] (area 1) | P (ηp2) | n.s. | <0.001 | 0.003 | n.a. | n.a. | n.a. | <0.001 (0.332) | n.s. | n.s. | n.a. | n.a. | |
| BV/TV [%] | P (ηp2) | <0.001 | <0.001 | <0.001 | n.s. | <0.001 | <0.001 | <0.001 (0.805) | <0.001 (0.662) | n.s. | <0.001 (0.551) | <0.001 (0.375) | |
| Tb.Th [µm] | P (ηp2) | <0.001 | <0.001 | <0.001 | n.s. | 0.003 | 0.007 | <0.001 (0.664) | <0.001 (0.633) | n.s. | <0.001 (0.625) | <0.001 (0.486) | |
| Cort. Th [µm] | P (ηp2) | 0.001 | <0.001 | <0.001 | n.s. | <0.001 | 0.007 | <0.001 (0.363) | n.s. | n.s. | <0.001 (0.199) | n.s. | |
| Ob.S/BS [%] | P (ηp2) | n.s. | 0.003 | n.s. | n.s. | n.s. | n.s. | 0.006 (0.097) | 0.001 (0.420) | n.s. | <0.001 (0.189) | n.s. | |
| OS/BS [%] | P (ηp2) | <0.001 | <0.001 | n.s. | n.s. | n.s. | n.s. | <0.001 (0.274) | <0.001 (0.763) | n.s. | <0.001 (0.188) | 0.003 (0.107) | |
| MS/BS [X] (area 1) | P (ηp2) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | <0.001 (0.679) | n.s. | n.a. | n.a. | |
| MAR [X] (area 1) | P (ηp2) | n.s. | <0.001 | <0.001 | n.a. | n.a. | n.a. | <0.001 (0.310) | 0.001 (0.194) | 0.047 (0.071) | n.a. | n.a. | |
| BFR/BS [X] (area 1) | P (ηp2) | n.s. | 0.003 | 0.002 | n.a. | n.a. | n.a. | 0.002 (0.298) | <0.001 (0.602) | n.s. | n.a. | n.a. | |
| ES/BS [%] | P (ηp2) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.001 (0.472) | n.s. | n.s. | n.s. | |
| Group | Parameter | Injection Channel | Remote Area | |||||
|---|---|---|---|---|---|---|---|---|
| Control (C) | HA | HA/BMP | Control (C) | HA | HA/BMP | |||
| BMP-2 3 months | 1 µg | Ob.S/BS [%] | 0.91 ± 0.35 | 1.94 ± 0.65 * | 2.00 ± 0.37 # | 1.02 ± 0.37 | 1.14 ± 0.16 | 1.21 ± 0.09 |
| OS/BS [%] | 8.44 ± 6.80 | 12.79 ± 4.18 | 14.95 ± 6.71 | 8.44 ± 6.80 | 14.87 ± 6.86 | 23.32 ± 13.39 | ||
| MS/BS [x-fold] | 1.00 ± 0.00 | 0.64 ± 0.20 | 0.91 ± 0.26 | n. a. | n. a. | n. a. | ||
| MAR [x-fold] | 1.00 ± 0.00 | 0.55 ± 0.23 | 1.18 ± 0.19 + | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 0.50 ± 0.25 * | 1.09 ± 0.27 + | n. a. | n. a. | n. a. | ||
| 10 µg | Ob.S/BS [%] | 0.87 ± 0.29 | 1.19 ± 0.22 | 1.05 ± 0.12 | 0.87 ± 0.29 | 1.40 ± 0.31 | 1.40 ± 0.30 | |
| OS/BS [%] | 1.94 ± 1.20 | 9.99 ± 3.60 | 19.45 ± 7.07 # | 1.94 ± 1.20 | 0.23 ± 0.21 | 3.10 ± 2.78 | ||
| MS/BS [x-fold] | 1.00 ± 0.00 | 0.72 ± 0.31 | 1.22 ± 0.34 | n. a. | n. a. | n. a. | ||
| MAR [x-fold] | 1.00 ± 0.00 | 0.37 ± 0.21 | 1.10 ± 0.20 | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 0.37 ± 0.31 | 1.28 ± 0.28 | n. a. | n. a. | n. a. | ||
| GDF-5 3 months | 5 µg | Ob.S/BS [%] | 0.90 ± 0.10 | 1.53 ± 0.27 * | 1.42 ± 0.20 # | 0.90 ± 0.10 | 0.84 ± 0.21 | 1.22 ± 0.19 + |
| OS/BS [%] | 10.45 ± 4.36 | 21.62 ± 3.02 | 34.28 ± 4.59 #+ | 10.45 ± 3.98 | 17.04 ± 4.45 | 14.46 ± 3.68 | ||
| MS/BS [x-fold] | 1.00 ± 0.00 | 1.01 ± 0.57 | 1.16 ± 0.08 | n. a. | n. a. | n. a. | ||
| MAR [x-fold] | 1.00 ± 0.00 | 0.00 ± 0.00 * | 0.65 ± 0.34 | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 0.00 ± 0.00 * | 0.44 ± 0.26 | n. a. | n. a. | n. a. | ||
| 50 µg | Ob.S/BS [%] | 1.18 ± 0.09 | 1.41 ± 0.18 | 1.31 ± 0.26 | 1.18 ± 0.09 | 1.30 ± 0.26 | 1.39 ± 0.09 | |
| OS/BS [%] | 2.78 ± 1.80 | 30.60 ± 7.31 * | 26.36 ± 9.44 # | 2.78 ± 1.80 | 6.06 ± 1.84 | 13.96 ± 4.41 # | ||
| MS/BS [x-fold] | 1.00 ± 0.00 | 1.76 ± 0.27 * | 2.24 ± 0.54 | n. a. | n. a. | n. a. | ||
| MAR [x-fold] | 1.00 ± 0.00 | 8.00 ± 8.00 | 8.00 ± 8.00 | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 8.00 ± 8.00 | 8.00 ± 8.00 # | n. a. | n. a. | n. a. | ||
| BMP-2 | 1 µg 9 mths. | Ob.S/BS [%] | 1.77 ± 0.49 | 3.10 ± 0.47 | 4.01 ± 1.10 #+ | 2.86 ± 0.54 | 2.94 ± 0.48 | 3.84 ± 0.48 & |
| OS/BS [%] | 0.00 ± 0.00 | 19.18 ± 8.96 | 21.18 ± 8.85 | 0.00 ± 0.00 | 1.92 ± 1.11 | 1.79 ± 1.79 | ||
| 1 µg 6 mths. | MS/BS [x-fold] | 1.00 ± 0.00 | 0.88 ± 0.28 | 0.93 ± 0.27 | n. a. | n. a. | n. a. | |
| MAR [x-fold] | 1.00 ± 0.00 | 0.76 ± 0.26 | 0.95 ± 0.34 | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 0.65 ± 0.23 | 0.73 ± 0.39 | n. a. | n. a. | n. a. | ||
| 10 µg 9 mths. | Ob.S/BS [%] | 1.41 ± 0.25 | 1.53 ± 0.29 | 2.11 ± 0.61 | 1.62 ± 0.34 | 1.66 ± 0.22 | 1.85 ± 0.17 | |
| OS/BS [%] | 0.00 ± 0.00 | 3.20 ± 3.20 | 3.94 ± 2.48 | 0.00 ± 0.00 | 0.12 ± 0.12 | 1.80 ± 0.52 # | ||
| 10 µg 6 mths. | MS/BS [x-fold] | 1.00 ± 0.00 | 0.91 ± 0.15 | 1.35 ± 0.24 | n. a. | n. a. | n. a. | |
| MAR [x-fold] | 1.00 ± 0.00 | 0.59 ± 0.04 * | 1.14 ± 0.14 + | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 0.56 ± 0.11 * | 1.61 ± 0.46 + | n. a. | n. a. | n. a. | ||
| GDF-5 | 5 µg 9 mths. | Ob.S/BS [%] | 3.00 ± 0.70 | 2.99 ± 0.76 | 4.53 ± 1.32 & | 1.46 ± 0.15 | 1.53 ± 0.25 | 2.06 ± 0.40 |
| OS/BS [%] | 0.38 ± 0.38 | 8.03 ± 3.09 * | 7.93 ± 3.11 # | 0.38 ± 0.38 | 0.50 ± 0.50 | 1.08 ± 0.72 | ||
| 5 µg 6 mths. | MS/BS [x-fold] | 1.00 ± 0.00 | 0.87 ± 0.15 | 1.03 ± 0.10 | n. a. | n. a. | n. a. | |
| MAR [x-fold] | 1.00 ± 0.00 | 1.08 ± 0.22 && | 2.03 ± 0.13 #+&& | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 0.80 ± 0.21 && | 1.74 ± 0.20 +&& | n. a. | n. a. | n. a. | ||
| 50 µg 9 mths. | Ob.S/BS [%] | 2.56 ± 0.64 | 4.36 ± 1.59 | 4.49 ± 1.44 | 1.67 ± 0.38 | 1.91 ± 0.38 | 2.83 ± 0.52 # | |
| OS/BS [%] | 0.07 ± 0.07 | 3.81 ± 2.48 | 9.47 ± 4.22 # | 0.07 ± 0.07 | 1.76 ± 1.18 | 1.91 ± 1.22 | ||
| 50 µg 6 mths. | MS/BS [x-fold] | 1.00 ± 0.00 | 1.09 ± 0.21 | 0.97 ± 0.16 | n. a. | n. a. | n. a. | |
| MAR [x-fold] | 1.00 ± 0.00 | 1.20 ± 0.11 * | 1.84 ± 0.34 # | n. a. | n. a. | n. a. | ||
| BFR/BS [x-fold] | 1.00 ± 0.00 | 1.17 ± 0.28 | 1.42 ± 0.22 | n. a. | n. a. | n. a. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasenbein, I.; Sachse, A.; Hortschansky, P.; Schmuck, K.D.; Horbert, V.; Anders, C.; Lehmann, T.; Huber, R.; Maslaris, A.; Layher, F.; et al. Single Application of Low-Dose, Hydroxyapatite-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation and Biomechanical Stabilization of a Bone Defect in a Senile Sheep Lumbar Osteopenia Model. Biomedicines 2022, 10, 513. https://doi.org/10.3390/biomedicines10020513
Hasenbein I, Sachse A, Hortschansky P, Schmuck KD, Horbert V, Anders C, Lehmann T, Huber R, Maslaris A, Layher F, et al. Single Application of Low-Dose, Hydroxyapatite-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation and Biomechanical Stabilization of a Bone Defect in a Senile Sheep Lumbar Osteopenia Model. Biomedicines. 2022; 10(2):513. https://doi.org/10.3390/biomedicines10020513
Chicago/Turabian StyleHasenbein, Ines, André Sachse, Peter Hortschansky, Klaus D. Schmuck, Victoria Horbert, Christoph Anders, Thomas Lehmann, René Huber, Alexander Maslaris, Frank Layher, and et al. 2022. "Single Application of Low-Dose, Hydroxyapatite-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation and Biomechanical Stabilization of a Bone Defect in a Senile Sheep Lumbar Osteopenia Model" Biomedicines 10, no. 2: 513. https://doi.org/10.3390/biomedicines10020513
APA StyleHasenbein, I., Sachse, A., Hortschansky, P., Schmuck, K. D., Horbert, V., Anders, C., Lehmann, T., Huber, R., Maslaris, A., Layher, F., Braun, C., Roth, A., Plöger, F., & Kinne, R. W. (2022). Single Application of Low-Dose, Hydroxyapatite-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation and Biomechanical Stabilization of a Bone Defect in a Senile Sheep Lumbar Osteopenia Model. Biomedicines, 10(2), 513. https://doi.org/10.3390/biomedicines10020513






