Adipose-Derived Stem Cell Exosomes as a Novel Anti-Inflammatory Agent and the Current Therapeutic Targets for Rheumatoid Arthritis
Abstract
1. Introduction
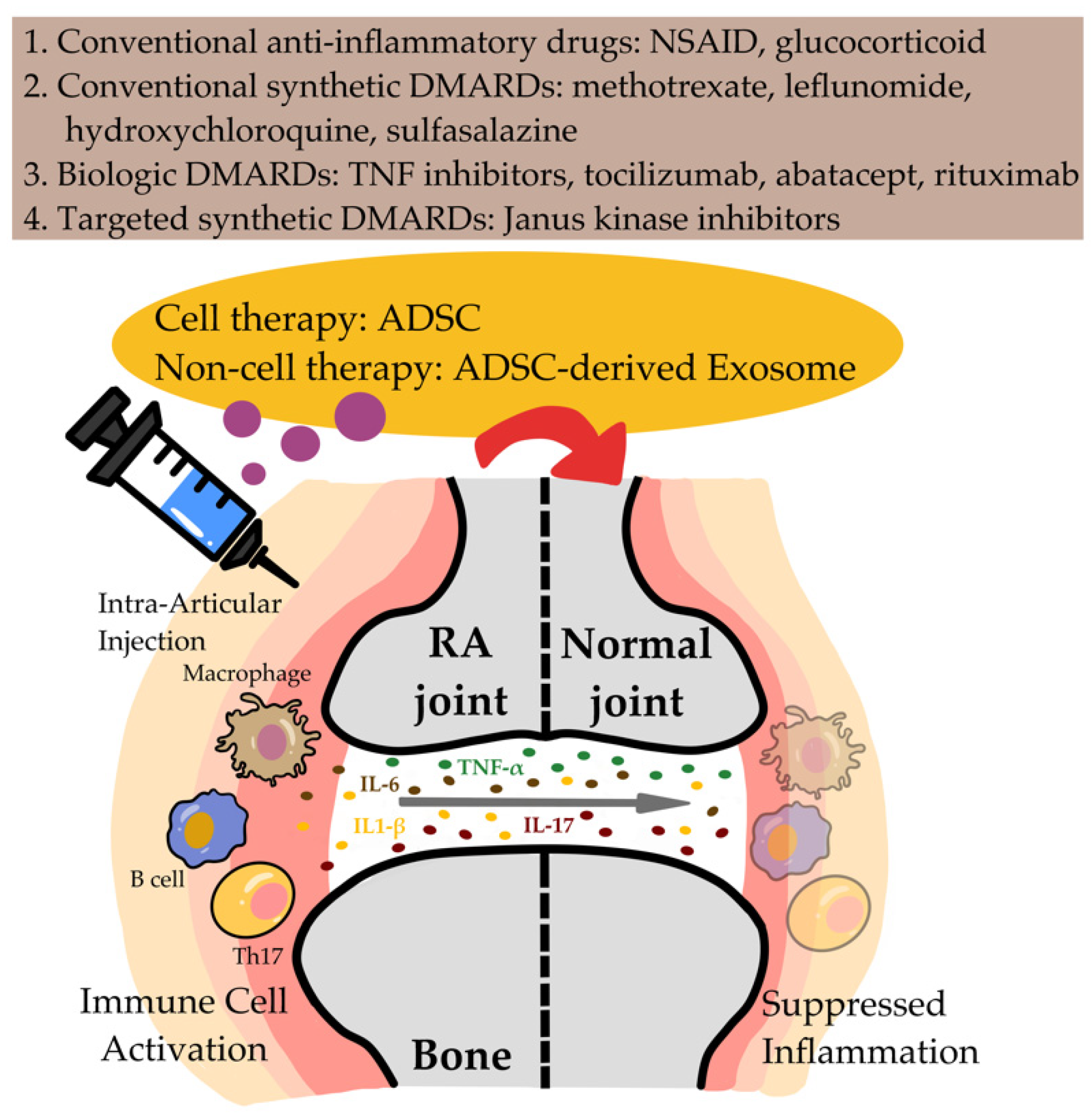
2. Current Therapy for RA
2.1. NSAIDs and Glucocorticoids
2.2. csDMARDs
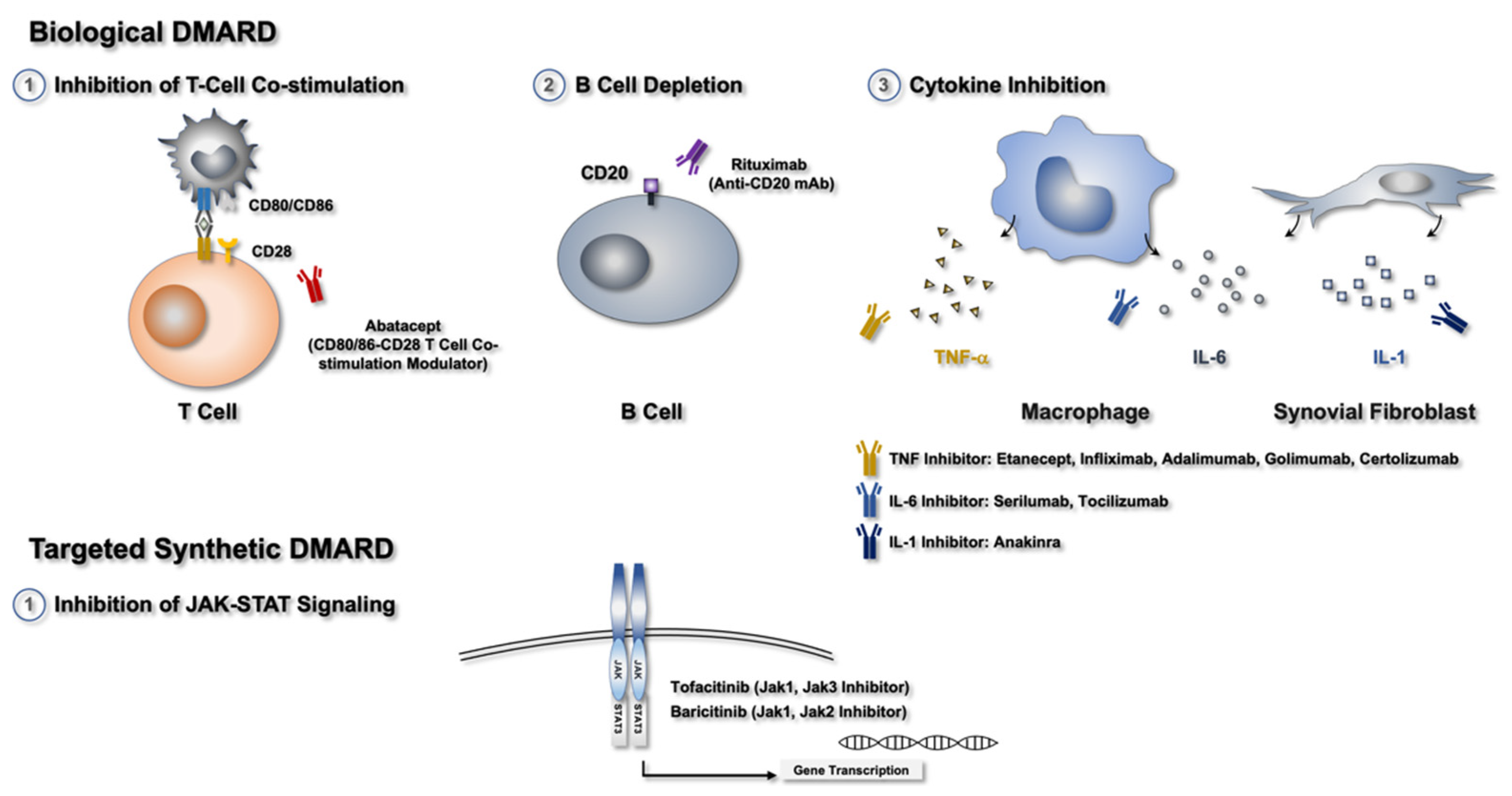
2.3. bDMARDs
2.4. Small-Molecule Compounds or tsDMARDs
3. Generation of ADSCs and Their Therapeutic Application in RA
3.1. Clinical Studies of ADSCs in Patients with RA
3.2. Preclinical Studies on ADSCs in Animal Models
4. Suppression of Joint Inflammation by MSC-Exos
4.1. Characteristics of MSC-Exos
4.2. ADSC-Exo Isolation and Identification
4.3. MSCs and MSC-Exos in Animal Models
4.4. Biomaterial and Clinical Applications of ADSC-Exos
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chemin, K.; Gerstner, C.; Malmstrom, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons from Rheumatoid Arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.A.; Wendholt, D.; Strietholt, S.; Frank, S.; Pundt, N.; Korb-Pap, A.; Joosten, L.A.; van den Berg, W.B.; Kollias, G.; Eckes, B.; et al. The loss of alpha2beta1 integrin suppresses joint inflammation and cartilage destruction in mouse models of rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Bird, P.; Peterfy, C.; Countryman, P.; Griffiths, H.; Barrett, R.; Youssef, P.; Joshua, F.; Hall, S. AC-CUTE: An Open-Label Study to Evaluate Progression of Structural Joint Damage and Inflammation in Subjects with Moderate to Severe Rheumatoid Arthritis. Int. J. Rheumatol. 2018, 8721753. [Google Scholar] [CrossRef]
- Poole, J.A.; Thiele, G.M.; Janike, K.; Nelson, A.J.; Duryee, M.J.; Rentfro, K.; England, B.R.; Romberger, D.J.; Carrington, J.M.; Wang, D.; et al. Combined Collagen-Induced Arthritis and Organic Dust-Induced Airway Inflammation to Model Inflammatory Lung Disease in Rheumatoid Arthritis. J. Bone Miner. Res. 2019, 34, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Greisen, S.R.; Kragstrup, T.W.; Thomsen, J.S.; Hansen, A.S.; Krishnamurthy, A.; Hørslev-Petersen, K.; Hetland, M.L.; Stengaard-Pedersen, K.; Østergaard, M.; Ørnbjerg, L.M.; et al. Programmed death ligand 2—A link between inflammation and bone loss in rheumatoid arthritis. J. Transl. Autoimmun. 2020, 3, 100028. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014, 506, 376–381. [Google Scholar] [CrossRef]
- Flogel, U.; Burghoff, S.; van Lent, P.L.; Temme, S.; Galbarz, L.; Ding, Z.; El-Tayeb, A.; Huels, S.; Bonner, F.; Borg, N.; et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012, 4, 146ra108. [Google Scholar] [CrossRef]
- Patlan, M.; Paez, A.; Masso, F.; Amezcua-Guerra, L.M. Relative increase of Th17 phenotype in senescent CD4+CD28null T cells from peripheral blood of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2021, 39, 925–926. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Prochnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 572858. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Palasiewicz, K.; Volkov, S.; Volin, M.V.; Arami, S.; Chang, H.J.; Zanotti, B.; Sweiss, N.; Shahrara, S. CCL21/CCR7 signaling in macrophages promotes joint inflammation and Th17-mediated osteoclast formation in rheumatoid arthritis. Cell. Mol. Life Sci. 2020, 77, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kwok, S.K.; Lim, M.A.; Kim, E.K.; Ryu, J.G.; Kim, S.M.; Oh, H.J.; Ju, J.H.; Park, S.H.; Kim, H.Y.; et al. STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.A.; Colas, R.A.; Souza, P.R.; Hands, R.; Lewis, M.J.; Bessant, C.; Pitzalis, C.; Dalli, J. Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis. Nat. Commun. 2020, 11, 5420. [Google Scholar] [CrossRef]
- Khadem Azarian, S.; Jafarnezhad-Ansariha, F.; Nazeri, S.; Azizi, G.; Aghazadeh, Z.; Hosseinzadeh, E.; Mirshafiey, A. Effects of guluronic acid, as a new NSAID with immunomodulatory properties on IL-17, RORgammat, IL-4 and GATA-3 gene expression in rheumatoid arthritis patients. Immunopharmacol. Immunotoxicol. 2020, 42, 22–27. [Google Scholar] [CrossRef]
- Movahedi, M.; Costello, R.; Lunt, M.; Pye, S.R.; Sergeant, J.C.; Dixon, W.G. Oral glucocorticoid therapy and all-cause and cause-specific mortality in patients with rheumatoid arthritis: A retrospective cohort study. Eur. J. Epidemiol. 2016, 31, 1045–1055. [Google Scholar] [CrossRef]
- Lauper, K.; Mongin, D.; Alpizar-Rodriguez, D.; Codreanu, C.; Iannone, F.; Kristianslund, E.K.; Kvien, T.K.; Pavelka, K.; Pombo-Suarez, M.; Santos, M.J.; et al. Drug retention of biological DMARD in rheumatoid arthritis patients: The role of baseline characteristics and disease evolution. Rheumatology 2019, 58, 2221–2229. [Google Scholar] [CrossRef]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519–531. [Google Scholar] [CrossRef]
- Tweehuysen, L.; den Broeder, A.A.; Schraa, K.; Netea, M.G.; van den Hoogen, F.H.J.; Joosten, L.A.B. Predictive value of ex-vivo drug-inhibited cytokine production for clinical response to biologic DMARD therapy in rheumatoid arthritis. Clin. Exp. Rheumatol. 2019, 37, 367–372. [Google Scholar]
- Wilke, T.; Mueller, S.; Lee, S.C.; Majer, I.; Heisen, M. Drug survival of second biological DMARD therapy in patients with rheumatoid arthritis: A retrospective non-interventional cohort analysis. BMC Musculoskelet. Disord. 2017, 18, 332. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.G.; Lee, J.; Hong, S.M.; Kwok, S.K.; Cho, M.L.; Park, S.H. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology 2020, 59, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Okano, T.; Orita, K.; Mamoto, K.; Sobajima, S.; Iwaguro, H.; Nakamura, H. Local transplantation of adipose-derived stem cells has a significant therapeutic effect in a mouse model of rheumatoid arthritis. Sci. Rep. 2020, 10, 3076. [Google Scholar] [CrossRef]
- Lopez-Santalla, M.; Bueren, J.A.; Garin, M.I. Mesenchymal stem/stromal cell-based therapy for the treatment of rheumatoid arthritis: An update on preclinical studies. EBioMedicine 2021, 69, 103427. [Google Scholar] [CrossRef]
- Mallinson, D.J.; Dunbar, D.R.; Ridha, S.; Sutton, E.R.; De la Rosa, O.; Dalemans, W.; Lombardo, E. Identification of Potential Plasma microRNA Stratification Biomarkers for Response to Allogeneic Adipose-Derived Mesenchymal Stem Cells in Rheumatoid Arthritis. Stem. Cells Transl. Med. 2017, 6, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Skalska, U.; Kontny, E. Adipose-derived mesenchymal stem cells from infrapatellar fat pad of patients with rheumatoid arthritis and osteoarthritis have comparable immunomodulatory properties. Autoimmunity 2016, 49, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Debreova, M.; Culenova, M.; Smolinska, V.; Nicodemou, A.; Csobonyeiova, M.; Danisovic, L. Rheumatoid arthritis: From synovium biology to cell-based therapy. Cytotherapy 2022, 24, 365–375. [Google Scholar] [CrossRef]
- Crossfield, S.S.R.; Buch, M.H.; Baxter, P.; Kingsbury, S.R.; Pujades-Rodriguez, M.; Conaghan, P.G. Changes in the pharmacological management of rheumatoid arthritis over two decades. Rheumatology 2021, 60, 4141–4151. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Rampakakis, E.; Movahedi, M.; Cesta, A.; Li, X.; Sampalis, J.S.; Bombardier, C. Predictors of patient decision to discontinue anti-rheumatic medication in patients with rheumatoid arthritis: Results from the Ontario best practices research initiative. Clin. Rheumatol. 2017, 36, 2421–2430. [Google Scholar] [CrossRef]
- Rosales Rosado, Z.; Font Urgelles, J.; Hernandez Rodriguez, I.; Leon Mateos, L.; Abasolo Alcazar, L.; Jover Jover, J.A. Clinical management and discontinuation of treatment in patients with recent onset rheumatoid arthritis in a rheumatology consultation. Reumatol. Clin. (Engl. Ed.) 2022, 18, 77–83. [Google Scholar] [CrossRef]
- Fletcher, A.; Lassere, M.; March, L.; Hill, C.; Barrett, C.; Carroll, G.; Buchbinder, R. Patterns of biologic and targeted-synthetic disease-modifying antirheumatic drug use in rheumatoid arthritis in Australia. Rheumatology 2022. [Google Scholar] [CrossRef]
- Tanaka, Y. Recent progress in treatments of rheumatoid arthritis: An overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology 2021, 60, vi12–vi20. [Google Scholar] [CrossRef]
- Nakayamada, S.; Kubo, S.; Iwata, S.; Tanaka, Y. Chemical JAK inhibitors for the treatment of rheumatoid arthritis. Expert Opin. Pharmacother. 2016, 17, 2215–2225. [Google Scholar] [CrossRef]
- Chen, Y.F.; Jobanputra, P.; Barton, P.; Bryan, S.; Fry-Smith, A.; Harris, G.; Taylor, R.S. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: A systematic review and economic evaluation. Health Technol. Assess. 2008, 12, 1–278. [Google Scholar] [CrossRef]
- Bickham, K.; Kivitz, A.J.; Mehta, A.; Frontera, N.; Shah, S.; Stryszak, P.; Popmihajlov, Z.; Peloso, P.M. Evaluation of two doses of etoricoxib, a COX-2 selective non-steroidal anti-inflammatory drug (NSAID), in the treatment of Rheumatoid Arthritis in a double-blind, randomized controlled trial. BMC Musculoskelet. Disord. 2016, 17, 331. [Google Scholar] [CrossRef]
- Moller, B.; Pruijm, M.; Adler, S.; Scherer, A.; Villiger, P.M.; Finckh, A.; Swiss Clinical Quality Management in Rheumatic Diseases Foundation, C.H.Z.S. Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann. Rheum. Dis. 2015, 74, 718–723. [Google Scholar] [CrossRef]
- Sultana, F.; Rasool, M. A novel therapeutic approach targeting rheumatoid arthritis by combined administration of morin, a dietary flavanol and non-steroidal anti-inflammatory drug indomethacin with reference to pro-inflammatory cytokines, inflammatory enzymes, RANKL and transcription factors. Chem. Biol. Interact. 2015, 230, 58–70. [Google Scholar] [CrossRef]
- Tacheci, I.; Bradna, P.; Douda, T.; Bastecka, D.; Kopacova, M.; Rejchrt, S.; Bures, J. NSAID-Induced Enteropathy in Rheumatoid Arthritis Patients with Chronic Occult Gastrointestinal Bleeding: A Prospective Capsule Endoscopy Study. Gastroenterol. Res. Pract. 2013, 2013, 268382. [Google Scholar] [CrossRef]
- Crilly, M.A.; Mangoni, A.A. Non-steroidal anti-inflammatory drug (NSAID) related inhibition of aldosterone glucuronidation and arterial dysfunction in patients with rheumatoid arthritis: A cross-sectional clinical study. BMJ Open 2011, 1, e000076. [Google Scholar] [CrossRef]
- Pazmino, S.; Boonen, A.; De Cock, D.; Stouten, V.; Joly, J.; Bertrand, D.; Westhovens, R.; Verschueren, P. Short-term glucocorticoids reduce risk of chronic NSAID and analgesic use in early methotrexate-treated rheumatoid arthritis patients with favourable prognosis: Subanalysis of the CareRA randomised controlled trial. RMD Open 2021, 7, e001615. [Google Scholar] [CrossRef]
- Strehl, C.; van der Goes, M.C.; Bijlsma, J.W.; Jacobs, J.W.; Buttgereit, F. Glucocorticoid-targeted therapies for the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs 2017, 26, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W. Disease control with glucocorticoid therapy in rheumatoid arthritis. Rheumatology 2012, 51 (Suppl. S4), iv9–iv13. [Google Scholar] [CrossRef]
- Palmowski, A.; Nielsen, S.M.; Buttgereit, T.; Palmowski, Y.; Boers, M.; Christensen, R.; Buttgereit, F. Glucocorticoid-trials in rheumatoid arthritis mostly study representative real-world patients: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020, 50, 1400–1405. [Google Scholar] [CrossRef]
- Kvien, T.K.; Greenwald, M.; Peloso, P.M.; Wang, H.; Mehta, A.; Gammaitoni, A. Do COX-2 inhibitors provide additional pain relief and anti-inflammatory effects in patients with rheumatoid arthritis who are on biological disease-modifying anti-rheumatic drugs and/or corticosteroids? Post-hoc analyses from a randomized clinical trial with etoricoxib. BMC Musculoskelet. Disord. 2015, 16, 26. [Google Scholar] [CrossRef]
- Stouten, V.; Westhovens, R.; Pazmino, S.; De Cock, D.; Van der Elst, K.; Joly, J.; Verschueren, P.; Care, R.A.s.g. Effectiveness of different combinations of DMARDs and glucocorticoid bridging in early rheumatoid arthritis: Two-year results of CareRA. Rheumatology 2019, 58, 2284–2294. [Google Scholar] [CrossRef]
- Luis, M.; Freitas, J.; Costa, F.; Buttgereit, F.; Boers, M.; Jap, D.S.; Santiago, T. An updated review of glucocorticoid-related adverse events in patients with rheumatoid arthritis. Expert Opin. Drug Saf. 2019, 18, 581–590. [Google Scholar] [CrossRef]
- Gossye, V.; Elewaut, D.; Bougarne, N.; Bracke, D.; Van Calenbergh, S.; Haegeman, G.; De Bosscher, K. Differential mechanism of NF-kappaB inhibition by two glucocorticoid receptor modulators in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009, 60, 3241–3250. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res 2018, 6, 15. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Dimitrova, P.; Kalden, J.R.; Schulze-Koops, H. Leflunomide: An immunosuppressive drug with multiple effects on T cell function. Mod. Rheumatol. 2002, 12, 195–200. [Google Scholar] [CrossRef]
- Meier, F.M.; Frerix, M.; Hermann, W.; Muller-Ladner, U. Current immunotherapy in rheumatoid arthritis. Immunotherapy 2013, 5, 955–974. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Martinez, J.M.; Dawson, J.R.D.; Flores, J.; Gabriel, M.; Garcia, G.; Guevara, A.; Murray, K.; Pacifici, N.; Vargas, M.V.; et al. The Therapeutic Landscape of Rheumatoid Arthritis: Current State and Future Directions. Front. Pharmacol. 2021, 12, 680043. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, N.B.; Kerstens, P.J.; Huizinga, T.W.; Dijkmans, B.A.; Allaart, C.F. Recent advances in the management of rheumatoid arthritis. BMJ 2010, 341, c6942. [Google Scholar] [CrossRef]
- Bai, L.L.; Chen, H.; Zhou, P.; Yu, J. Identification of Tumor Necrosis Factor-Alpha (TNF-alpha) Inhibitor in Rheumatoid Arthritis Using Network Pharmacology and Molecular Docking. Front. Pharmacol. 2021, 12, 690118. [Google Scholar] [CrossRef]
- Carvajal Alegria, G.; Cornec, D.; Saraux, A.; Devauchelle-Pensec, V.; Jamin, C.; Hillion, S.; Pers, J.O.; Pochard, P. Abatacept Promotes Regulatory B Cell Functions, Enhancing Their Ability to Reduce the Th1 Response in Rheumatoid Arthritis Patients through the Production of IL-10 and TGF-beta. J. Immunol. 2021, 207, 470–482. [Google Scholar] [CrossRef]
- Cacciapaglia, F.; Renna, D.; Fornaro, M.; Venerito, V.; Lopalco, G.; Iannone, F. Safety and effectiveness in switching from reference to biosimilar rituximab in rheumatoid arthritis patients: Real world experience from a single Italian rheumatology centre. Clin. Exp. Rheumatol. 2021, 39, 1147–1148. [Google Scholar] [CrossRef]
- Saki, A.; Rajaei, E.; Rahim, F. Safety and efficacy of tocilizumab for rheumatoid arthritis: A systematic review and meta-analysis of clinical trial studies. Reumatologia 2021, 59, 169–179. [Google Scholar] [CrossRef]
- Holdsworth, E.A.; Donaghy, B.; Fox, K.M.; Desai, P.; Collier, D.H.; Furst, D.E. Biologic and Targeted Synthetic DMARD Utilization in the United States: Adelphi Real World Disease Specific Programme for Rheumatoid Arthritis. Rheumatol. Ther. 2021, 8, 1637–1649. [Google Scholar] [CrossRef]
- de Launay, D.; van de Sande, M.G.; de Hair, M.J.; Grabiec, A.M.; van de Sande, G.P.; Lehmann, K.A.; Wijbrandts, C.A.; van Baarsen, L.G.; Gerlag, D.M.; Tak, P.P.; et al. Selective involvement of ERK and JNK mitogen-activated protein kinases in early rheumatoid arthritis (1987 ACR criteria compared to 2010 ACR/EULAR criteria): A prospective study aimed at identification of diagnostic and prognostic biomarkers as well as therapeutic targets. Ann. Rheum. Dis. 2012, 71, 415–423. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Dai, E.; Du, H.; Wang, L. Mechanism of action of celastrol against rheumatoid arthritis: A network pharmacology analysis. Int. Immunopharmacol. 2019, 74, 105725. [Google Scholar] [CrossRef]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016, 68, 1–25. [Google Scholar] [CrossRef]
- Senolt, L.; Vencovsky, J.; Pavelka, K.; Ospelt, C.; Gay, S. Prospective new biological therapies for rheumatoid arthritis. Autoimmun. Rev. 2009, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Hernan, M.G.; Miranda-Carus, M.E.; Martin-Mola, E. New drugs beyond biologics in rheumatoid arthritis: The kinase inhibitors. Rheumatology 2011, 50, 1542–1550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 1592. [Google Scholar] [CrossRef]
- Haikal, S.M.; Abdeltawab, N.F.; Rashed, L.A.; Abd El-Galil, T.I.; Elmalt, H.A.; Amin, M.A. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019, 8, 823. [Google Scholar] [CrossRef]
- El-Jawhari, J.J.; El-Sherbiny, Y.; McGonagle, D.; Jones, E. Multipotent Mesenchymal Stromal Cells in Rheumatoid Arthritis and Systemic Lupus Erythematosus; From a Leading Role in Pathogenesis to Potential Therapeutic Saviors? Front. Immunol. 2021, 12, 643170. [Google Scholar] [CrossRef]
- Ansboro, S.; Roelofs, A.J.; De Bari, C. Mesenchymal stem cells for the management of rheumatoid arthritis: Immune modulation, repair or both? Curr. Opin. Rheumatol. 2017, 29, 201–207. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef]
- Tanaka, Y. Human mesenchymal stem cells as a tool for joint repair in rheumatoid arthritis. Clin. Exp. Rheumatol. 2015, 33, S58–S62. [Google Scholar]
- Pedrosa, M.; Gomes, J.; Laranjeira, P.; Duarte, C.; Pedreiro, S.; Antunes, B.; Ribeiro, T.; Santos, F.; Martinho, A.; Fardilha, M.; et al. Immunomodulatory effect of human bone marrow-derived mesenchymal stromal/stem cells on peripheral blood T cells from rheumatoid arthritis patients. J. Tissue Eng. Regen. Med. 2020, 14, 16–28. [Google Scholar] [CrossRef]
- Shalev-Malul, G.; Soler, D.C.; Ting, A.E.; Lehman, N.A.; Barnboym, E.; McCormick, T.S.; Anthony, D.D.; Lazarus, H.M.; Caplan, A.I.; Breitman, M.; et al. Development of a Functional Biomarker for Use in Cell-Based Therapy Studies in Seropositive Rheumatoid Arthritis. Stem Cells Transl. Med. 2016, 5, 628–631. [Google Scholar] [CrossRef][Green Version]
- Dallos, T.; Krivosikova, M.; Chorazy-Massalska, M.; Warnawin, E.; Zanova, E.; Rudnicka, W.; Radzikowska, A.; Maslinski, W. BAFF from bone marrow-derived mesenchymal stromal cells of rheumatoid arthritis patients improves their B-cell viability-supporting properties. Folia Biol. 2009, 55, 166–176. [Google Scholar]
- Skalska, U.; Prochorec-Sobieszek, M.; Kontny, E. Osteoblastic potential of infrapatellar fat pad-derived mesenchymal stem cells from rheumatoid arthritis and osteoarthritis patients. Int. J. Rheum. Dis. 2016, 19, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Skalska, U.; Kuca-Warnawin, E.; Kornatka, A.; Janicka, I.; Musialowicz, U.; Burakowski, T.; Kontny, E. Articular and subcutaneous adipose tissues of rheumatoid arthritis patients represent equal sources of immunoregulatory mesenchymal stem cells. Autoimmunity 2017, 50, 441–450. [Google Scholar] [CrossRef]
- Alvaro-Gracia, J.M.; Jover, J.A.; Garcia-Vicuna, R.; Carreno, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martinez-Taboada, V.M.; Taylor, P.; Martin-Martin, C.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef]
- Ra, J.C.; Kang, S.K.; Shin, I.S.; Park, H.G.; Joo, S.A.; Kim, J.G.; Kang, B.C.; Lee, Y.S.; Nakama, K.; Piao, M.; et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J. Transl. Med. 2011, 9, 181. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, G.; Yang, K.; Xiang, W.; Li, J.; Chen, H. Efficacy and Safety of Mesenchymal Stem Cell Transplantation in the Treatment of Autoimmune Diseases (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Inflammatory Bowel Disease, Multiple Sclerosis, and Ankylosing Spondylitis): A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Stem Cells Int. 2022, 2022, 9463314. [Google Scholar] [CrossRef]
- Kim, K.C.; Lee, I.H.; Choi, J.H.; Oh, M.R.; Ahn, M.J.; Kim, S.Y. Autologous stem cell transplantation in the treatment of refractory rheumatoid arthritis. J. Korean Med. Sci. 2002, 17, 129–132. [Google Scholar] [CrossRef]
- Sayegh, S.; El Atat, O.; Diallo, K.; Rauwel, B.; Degboe, Y.; Cavaignac, E.; Constantin, A.; Cantagrel, A.; Trak-Smayra, V.; Alaaeddine, N.; et al. Rheumatoid Synovial Fluids Regulate the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells Through a TNF/NF-kappaB-Dependent Mechanism. Front. Immunol. 2019, 10, 1482. [Google Scholar] [CrossRef]
- Baharlou, R.; Rashidi, N.; Ahmadi-Vasmehjani, A.; Khoubyari, M.; Sheikh, M.; Erfanian, S. Immunomodulatory Effects of Human Adipose Tissue-derived Mesenchymal Stem Cells on T Cell Subsets in Patients with Rheumatoid Arthritis. Iran. J. Allergy Asthma Immunol. 2019, 18, 114–119. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Buscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, R.; Almeida, H.V.; Kelly, D.J.; O’Brien, F.J.; Kearney, C.J. Infrapatellar Fat Pad Stem Cells: From Developmental Biology to Cell Therapy. Stem Cells Int. 2017, 2017, 6843727. [Google Scholar] [CrossRef]
- Liao, H.J.; Chang, C.H.; Huang, C.F.; Chen, H.T. Potential of Using Infrapatellar-Fat-Pad-Derived Mesenchymal Stem Cells for Therapy in Degenerative Arthritis: Chondrogenesis, Exosomes, and Transcription Regulation. Biomolecules 2022, 12, 386. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Jia, C.; He, Y.; Deng, C. Therapeutic applications of adipose cell-free derivatives: A review. Stem Cell Res. Ther. 2020, 11, 312. [Google Scholar] [CrossRef]
- Rochette, L.; Mazini, L.; Malka, G.; Zeller, M.; Cottin, Y.; Vergely, C. The Crosstalk of Adipose-Derived Stem Cells (ADSC), Oxidative Stress, and Inflammation in Protective and Adaptive Responses. Int. J. Mol. Sci. 2020, 21, 9262. [Google Scholar] [CrossRef]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef]
- You, D.G.; Lim, G.T.; Kwon, S.; Um, W.; Oh, B.H.; Song, S.H.; Lee, J.; Jo, D.G.; Cho, Y.W.; Park, J.H. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci. Adv. 2021, 7, eabe0083. [Google Scholar] [CrossRef]
- Tsujimaru, K.; Takanashi, M.; Sudo, K.; Ishikawa, A.; Mineo, S.; Ueda, S.; Kumagai, K.; Kuroda, M. Extracellular microvesicles that originated adipose tissue derived mesenchymal stem cells have the potential ability to improve rheumatoid arthritis on mice. Regen. Ther. 2020, 15, 305–311. [Google Scholar] [CrossRef]
- Tavasolian, F.; Hosseini, A.Z.; Soudi, S.; Naderi, M. miRNA-146a Improves Immunomodulatory Effects of MSC-derived Exosomes in Rheumatoid Arthritis. Curr. Gene Ther. 2020, 20, 297–312. [Google Scholar] [CrossRef]
- Huang, F.; Chen, M.; Chen, W.; Gu, J.; Yuan, J.; Xue, Y.; Dang, J.; Su, W.; Wang, J.; Zadeh, H.H.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Inhibit Xeno-Graft-versus-Host Disease via CD39-CD73-Adenosine and IDO Signals. Front. Immunol. 2017, 8, 68. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; Chen, Y.; Deng, Y.; Zeng, D.; Liu, Y.; Huang, F.; Wang, J.; Liu, Y.; Bellanti, J.A.; et al. CD39 Produced from Human GMSCs Regulates the Balance of Osteoclasts and Osteoblasts through the Wnt/beta-Catenin Pathway in Osteoporosis. Mol. Ther. 2020, 28, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Schey, K.L.; Luther, J.M.; Rose, K.L. Proteomics characterization of exosome cargo. Methods 2015, 87, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noel, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, R.; Liu, T.; Yang, L.; Yin, G.; Xie, Q. Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Vickers, K.C.; Remaley, A.T. Lipid-based carriers of microRNAs and intercellular communication. Curr. Opin. Lipidol. 2012, 23, 91–97. [Google Scholar] [CrossRef]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Lopez-Santalla, M.; Fernandez-Perez, R.; Garin, M.I. Mesenchymal Stem/Stromal Cells for Rheumatoid Arthritis Treatment: An Update on Clinical Applications. Cells 2020, 9, 1852. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Q.; Zhang, Y.; Bian, Q.; Hong, Y.; Shen, Z.; Xu, H.; Rui, K.; Yin, K.; Wang, S. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Experimental Colitis via Modulating Th1/Th17 and Treg Cell Responses. Front. Immunol. 2020, 11, 598322. [Google Scholar] [CrossRef]
- Tian, X.; Wei, W.; Cao, Y.; Ao, T.; Huang, F.; Javed, R.; Wang, X.; Fan, J.; Zhang, Y.; Liu, Y.; et al. Gingival mesenchymal stem cell-derived exosomes are immunosuppressive in preventing collagen-induced arthritis. J. Cell. Mol. Med. 2022, 26, 693–708. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Xia, Y.; Yan, F.; Lu, Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J. Immunol. 2018, 201, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Fang, Y.X.; Liu, Y.; Meng, F.R.; Wu, X.; Zhang, C.W.; Zhang, Y.; Liu, Y.Q.; Liu, D. MicroRNA-21 from bone marrow mesenchymal stem cell-derived extracellular vesicles targets TET1 to suppress KLF4 and alleviate rheumatoid arthritis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211007369. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Kan, L. Mesenchymal Stem Cell-Originated Exosomal Circular RNA circFBXW7 Attenuates Cell Proliferation, Migration and Inflammation of Fibroblast-Like Synoviocytes by Targeting miR-216a-3p/HDAC4 in Rheumatoid Arthritis. J. Inflamm. Res. 2021, 14, 6157–6171. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, Y.; Ma, C.; Guan, C.; Ma, X.; Meng, S. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-kappaB pathway. J. Orthop. Surg. Res. 2021, 16, 116. [Google Scholar] [CrossRef]
- Meng, Q.; Qiu, B. Exosomal MicroRNA-320a Derived From Mesenchymal Stem Cells Regulates Rheumatoid Arthritis Fibroblast-Like Synoviocyte Activation by Suppressing CXCL9 Expression. Front. Physiol. 2020, 11, 441. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, L.; Iok In, I.; Chen, Y.; Jia, N.; Zhu, W. Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192-5p delays inflammatory response in rheumatoid arthritis. Int. Immunopharmacol. 2020, 78, 105985. [Google Scholar] [CrossRef]
- Meng, H.Y.; Chen, L.Q.; Chen, L.H. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet. Disord. 2020, 21, 150. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Wang, X.; Cheng, W.; Hu, X.; Wang, Y.; Luo, B.; Huang, W.; Gu, J. miR-34a in extracellular vesicles from bone marrow mesenchymal stem cells reduces rheumatoid arthritis inflammation via the cyclin I/ATM/ATR/p53 axis. J. Cell. Mol. Med. 2021, 25, 1896–1910. [Google Scholar] [CrossRef]
- Yan, X.; Cen, Y.; Wang, Q. Mesenchymal stem cells alleviate experimental rheumatoid arthritis through microRNA-regulated IkappaB expression. Sci. Rep. 2016, 6, 28915. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Holcar, M.; Kanduser, M.; Lenassi, M. Blood Nanoparticles—Influence on Extracellular Vesicle Isolation and Characterization. Front. Pharmacol. 2021, 12, 773844. [Google Scholar] [CrossRef] [PubMed]
| ADSC Administration in Patients With RA | ||||
|---|---|---|---|---|
| Cell Source | Treatment Conditions | Outcome | Number of Patients | Reference |
| Clinical Study | ||||
| Allogeneic subcutaneous adipose tissue | (3–12) × 106 cells/kg, i.v. | Treatment was generally well-tolerated, without dose-related toxicity in the dose range and time | 46 (RA) | [75] |
| Autologous subcutaneous adipose tissue | (1.5–3.5) × 108 ADSCs/kg, s.c. | Pain VAS and KWOMAC decreased, and walking improved | 3 (RA) | [76] |
| Allogeneic subcutaneous adipose tissue | Expanded allogeneic ADSCs in refractory RA, i.v. | Three miRNAs, namely, miR-26b-5p, miR-487b-3p, and miR-495-3p, were significantly upregulated in the responder group (reduced MRI score) compared to the nonresponder group | 14 (RA) | [25] |
| In Vitro Study | ||||
|---|---|---|---|---|
| Cell Source | Treatment Conditions | Outcome | Number of Patients | Reference |
| ADSC | ||||
| Subcutaneous adipose tissue | ADSCs first treated with SF and ADSC proliferation followed by gene expression of immunomodulatory factors | Conditioning ADSCs with proinflammatory RASF enhanced their ability to induce Treg cells and inhibited the proinflammatory markers CD40 and CD80 in activated macrophages | 8 (RA) | [79] |
| Subcutaneous adipose tissue | ADSC–PBMCs cocultured with PMA treatment | ADSCs greatly upregulated Th2- and Treg-cell transcription factors (i.e., GATA3 and Foxp3) and downregulated Th1 and Th17 transcription factors (i.e., T-bet and RORγt) | 14 (RA) | [80] |
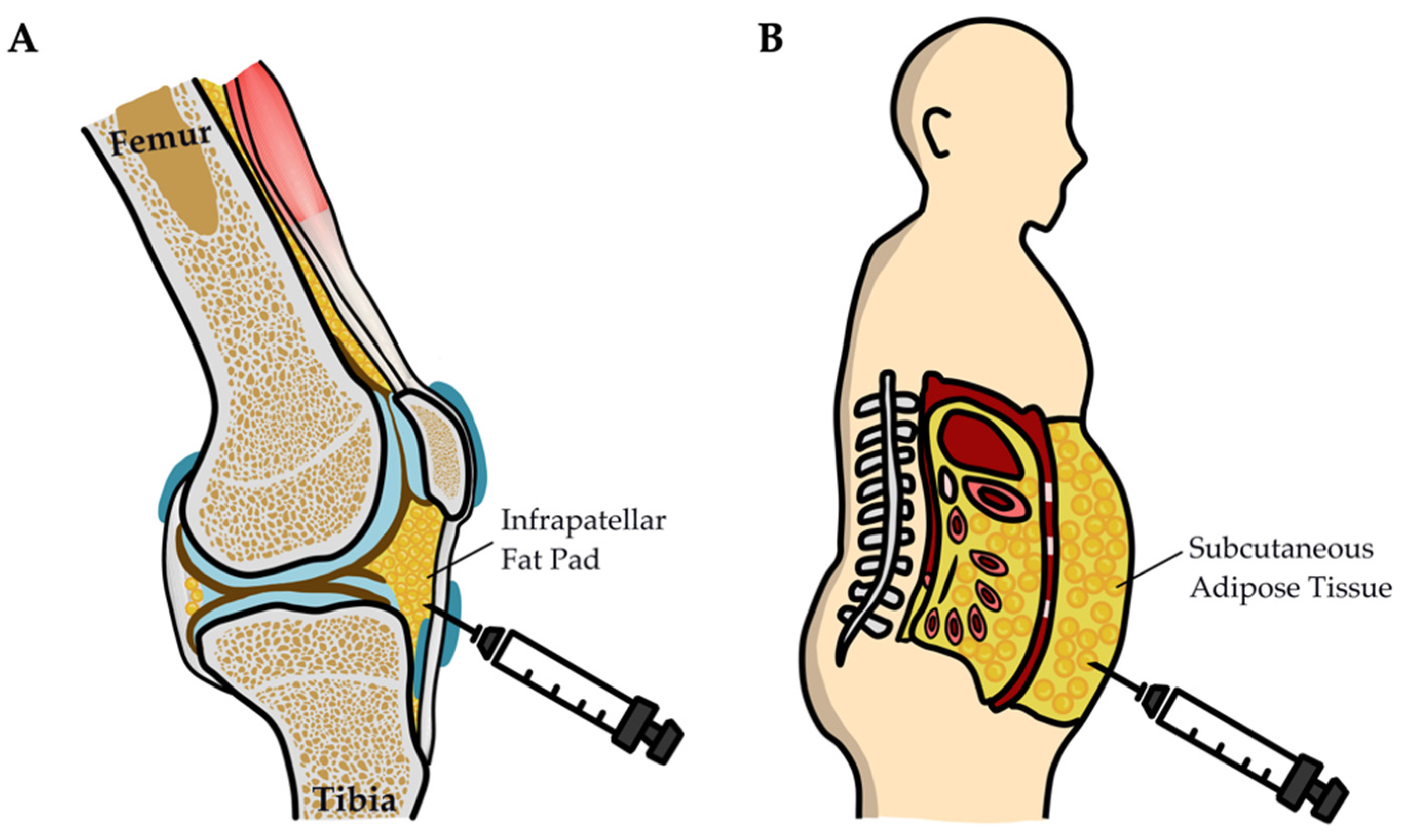
| Infrapatellar fat pad or subcutaneous adipose tissues | PBMCs stimulated with PHA cultured alone or in the presence of naïve or TNF/IFNγ-pretreated ASCs isolated from infrapatellar fat pads or subcutaneous adipose tissues | IPFP-MSCs and SC-MSCs obtained from patients with RA had similar immunomodulatory properties despite the different localization and distinct cytokine milieus of the tissues of origin | 8 (RA) | [74] |
| Infrapatellar fat pad | PBMCs from healthy donors cocultured with ADSCs from patients | The immunosuppressive properties of RA-ADSCs and OA-ADSCs were impaired | 29 (RA) 12 (OA) | [26] |
| Subcutaneous adipose tissue | ADSCs from healthy donor cultured with collagen-reactive RA human T cells | ADSCs stimulated the generation of FoxP3 protein-expressing Treg cells, with the capacity to suppress collagen-specific T-cell responses from patients with RA | 22 (RA, PBMC) | [81] |
| Preclinical Animal Study | ||||
|---|---|---|---|---|
| Cell Source | Treatment Conditions | Outcome | Animal Model/Species | Reference |
| ADSC | ||||
| Autologous subcutaneous adipose tissue | 1.5 × 104 ADSCs/knee, intra-articularly | Localized injection of ADSCs and spheroids reduced intra-articular inflammation and regenerated damaged cartilage in a mouse model of RA | Laminarin-induced arthritis/ SKG mice | [23] |
| ADSC-Exos | ||||
| Human ADSC cell line | 107–108 dibenzocyclooctyne (DBCO)-conjugated dextran sulfate (DS)-conjugated ADSC-Exos, i.v. | DS-Exos systemically administered to mice with collagen-induced arthritis effectively accumulated in the inflamed joints, inducing a cascade of anti-inflammatory activity via regulation of macrophage phenotypes | Collagen-induced arthritis/ DBA-1J mice | [88] |
| Subcutaneous adipose tissue | 5 mg EVs or 1 × 106 ADSCs, i.v. | ADSC-Exos alleviated RA via transfer of factors such as IL-1ra | Collagen-induced arthritis/ BALB/c mice | [89] |
| Subcutaneous adipose tissue | Exos extracted from normal MSCs with overexpressed miR-146a and miR-155 | Treatment with MSC-Exos and miR-146a/miR-155-transduced MSC-Exos significantly altered CIA mice’s Treg-cell levels and suppressed inflammation | Collagen-induced arthritis/ DBA-1J mice | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-H.; Wu, C.-S.; Chiou, S.-H.; Chang, C.-H.; Liao, H.-J. Adipose-Derived Stem Cell Exosomes as a Novel Anti-Inflammatory Agent and the Current Therapeutic Targets for Rheumatoid Arthritis. Biomedicines 2022, 10, 1725. https://doi.org/10.3390/biomedicines10071725
Chang T-H, Wu C-S, Chiou S-H, Chang C-H, Liao H-J. Adipose-Derived Stem Cell Exosomes as a Novel Anti-Inflammatory Agent and the Current Therapeutic Targets for Rheumatoid Arthritis. Biomedicines. 2022; 10(7):1725. https://doi.org/10.3390/biomedicines10071725
Chicago/Turabian StyleChang, Ting-Hui, Chien-Sheng Wu, Shih-Hwa Chiou, Chih-Hung Chang, and Hsiu-Jung Liao. 2022. "Adipose-Derived Stem Cell Exosomes as a Novel Anti-Inflammatory Agent and the Current Therapeutic Targets for Rheumatoid Arthritis" Biomedicines 10, no. 7: 1725. https://doi.org/10.3390/biomedicines10071725
APA StyleChang, T.-H., Wu, C.-S., Chiou, S.-H., Chang, C.-H., & Liao, H.-J. (2022). Adipose-Derived Stem Cell Exosomes as a Novel Anti-Inflammatory Agent and the Current Therapeutic Targets for Rheumatoid Arthritis. Biomedicines, 10(7), 1725. https://doi.org/10.3390/biomedicines10071725







