Investigation of Blood Coagulation Using Impedance Spectroscopy: Toward Innovative Biomarkers to Assess Fibrinogenesis and Clot Retraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blood-Clot Preparation
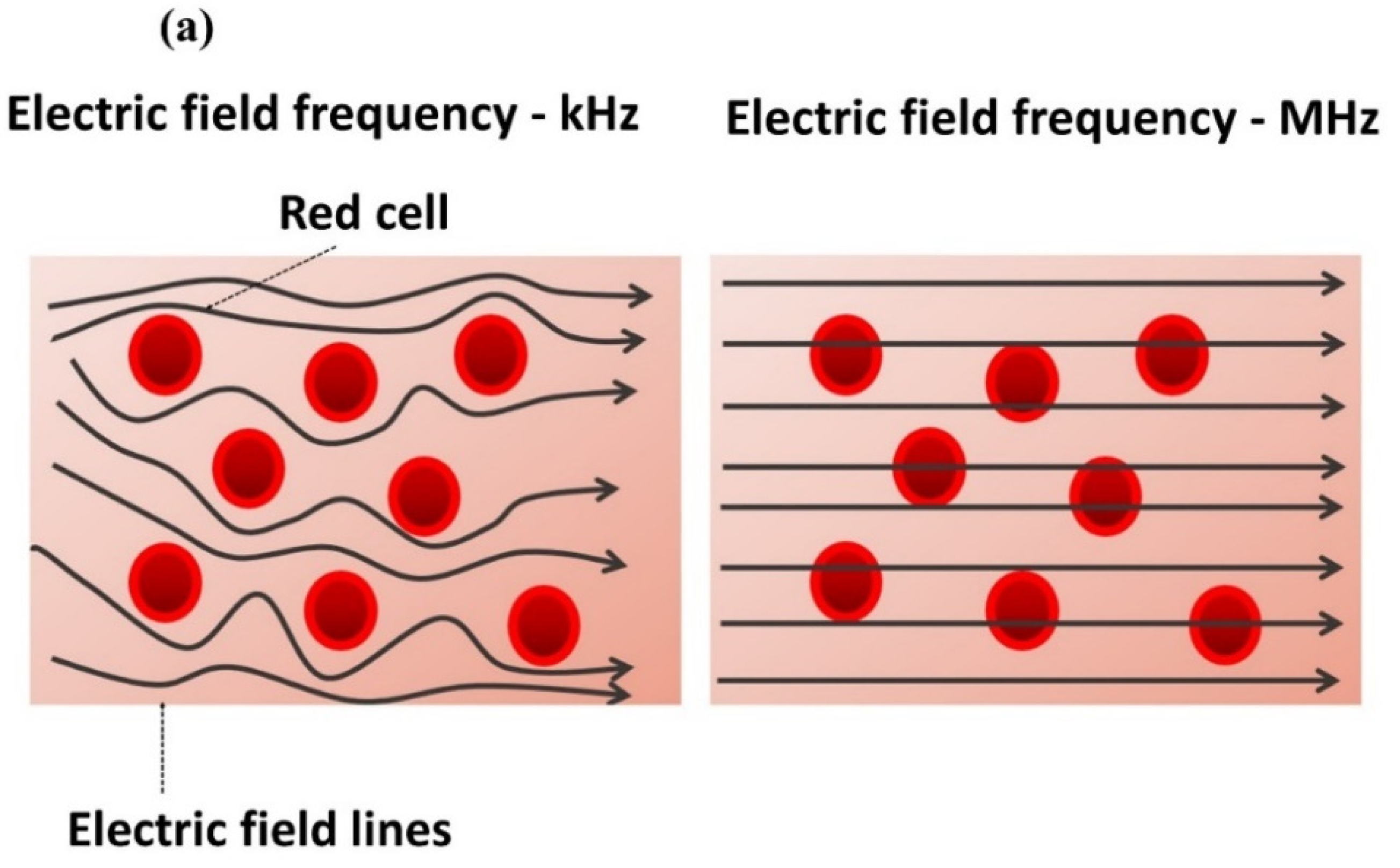
2.3. Impedance-Magnitude Spectroscopy
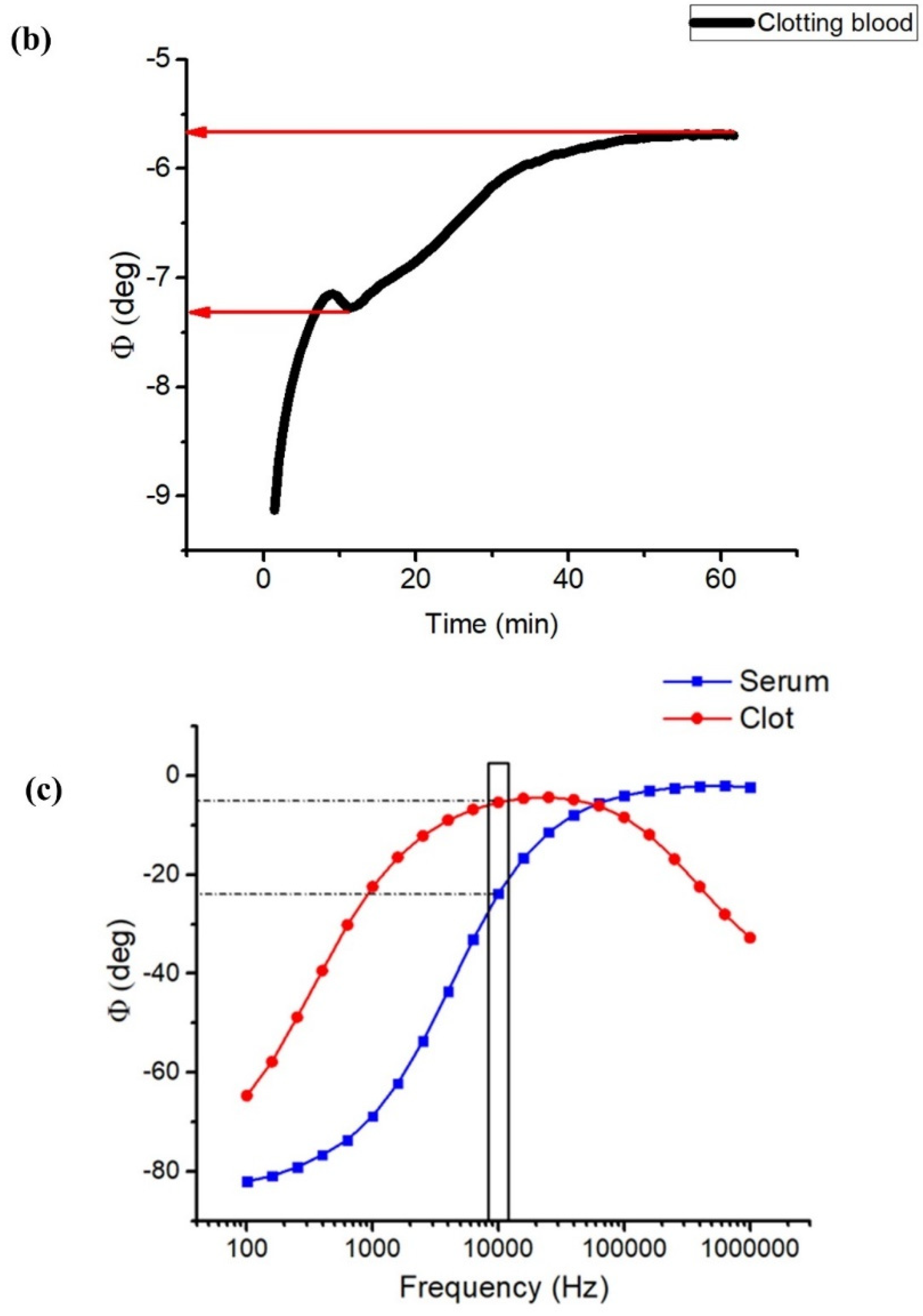
2.4. Impedance Phase Angle
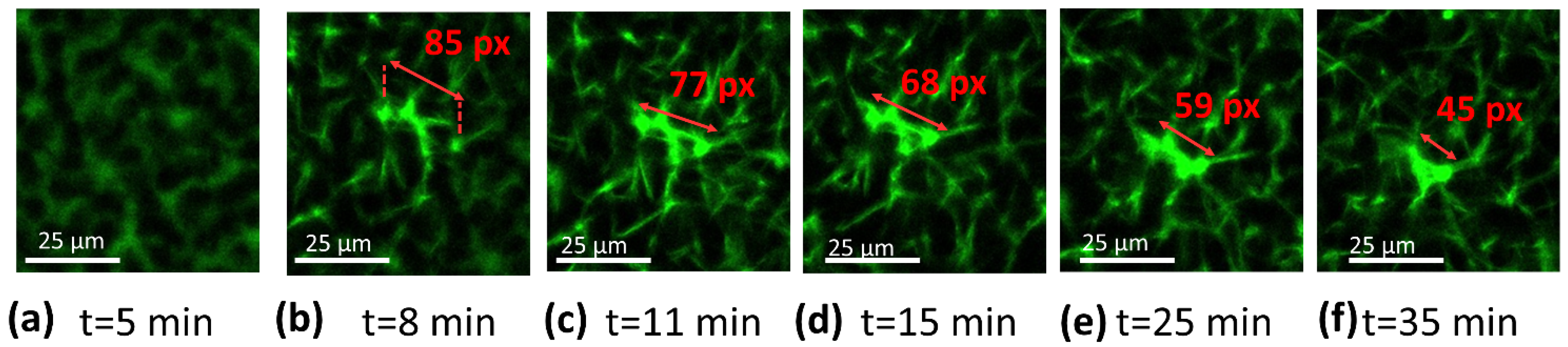
2.5. Confocal Microscopy
2.6. ROTEM® Characterization
3. Results and Discussion
3.1. Impedance Spectroscopy
3.2. Confocal Microscopy
3.3. ROTEM® Characterization
- Whatever the technique of measurement, recalcified blood samples with TF led to smaller values of CT;
- For all samples with or without TF, both the impedance and ROTEM® techniques led to similar results in terms of central tendency (mean and median values), dispersion (SD and IQR) and limit values (max and min);
- In any case, the data distributions were almost symmetrical, as the medians (horizonal lines in the whisker box) were close to the mean values (circles); thus, the skewness should be near zero;
- None of the observations showed outliers or extremes values (i.e., no values fell below Q1 − 1.5 IQR or were above Q3 + 1.5 IQR), meaning that the highest and lowest occurring values were within this limit interval;
- Finner analysis regarding the data dispersion via QCD:
- ✓
- The impedance technique gave rise to a somewhat higher dispersion of CT than the ROTEM® one;
- ✓
- The measurement of the samples without TF resulted in lower dispersion, regardless of which technique was chosen;
- ✓
- For all the cases, the QCD value was relatively low, confirming the good repeatability of the data.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, R.; Usama, S.; Babiker, H. Physiology, Coagulation Pathways. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482253/ (accessed on 1 July 2022).
- Dahlbäck, B. Blood coagulation. Lancet 2000, 355, 1627–1632. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Gailani, D.; Renné, T. The intrinsic pathway of coagulation: A target for treating thromboembolic disease? J. Thromb. Haemost. 2007, 5, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.L.; Alwis, I.; Maclean, J.A.A.; Priyananda, P.; Hawkett, B.; Schoenwaelder, S.M.; Jackson, S.P. Endogenous fibrinolysis facilitates clot retraction in vivo. Blood 2017, 130, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Tutwiler, V.; Wang, H.; Litvinov, R.I.; Weisel, J.W.; Shenoy, V.B. Interplay of Platelet Contractility and Elasticity of Fibrin/Erythrocytes in Blood Clot Retraction. Biophys. J. 2017, 112, 714–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, X.; McCracken, B.; Shao, Y.; Ward, K.; Fu, J. A Miniaturized Hemoretractometer for Blood Clot Retraction Testing. Small 2016, 12, 3926–3934. [Google Scholar] [CrossRef] [Green Version]
- Ganda, K.M. Primary and Secondary Hemostasis: Normal Mechanisms, Disease States, and Coagulation Tests: Assessment, Analysis, and Associated Dental Management Guidelines. In Dentist’s Guide to Medical Conditions, Medications, and Complications; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 229–242. [Google Scholar]
- Gale, A.J. Continuing Education Course #2: Current Understanding of Hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Hiller, E. Basic Principles of Hemostasis. In Modern Hematology; Humana Press: Totowa, NJ, USA, 2000; pp. 327–345. [Google Scholar]
- Rasche, H. Haemostasis and thrombosis: An overview. Eur. Heart J. Suppl. 2001, 3, Q3–Q7. [Google Scholar] [CrossRef]
- Labarre, D. Improving blood-compatibility of polymeric surfaces. Trends Biomater. Artif. Organs 2001, 15, 1–3. [Google Scholar]
- Sperling, C.; Maitz, M.F.; Grasso, S.; Werner, C.; Kanse, S.M. A Positively Charged Surface Triggers Coagulation Activation Through Factor VII Activating Protease (FSAP). ACS Appl. Mater. Interfaces 2017, 9, 40107–40116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Sales, K.M.; Hamilton, G.; Seifalian, A.M. Addressing thrombogenicity in vascular graft construction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.R.; Tucker, E.I.; Latour, R.A. Blood Coagulation and Blood–Material Interactions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Braune, S.; Latour, R.A.; Reinthaler, M.; Landmesser, U.; Lendlein, A.; Jung, F. In Vitro Thrombogenicity Testing of Biomaterials. Adv. Healthc. Mater. 2019, 8, 1900527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüscher, T.F.; Steffel, J.; Eberli, F.R.; Joner, M.; Nakazawa, G.; Tanner, F.C.; Virmani, R. Drug-Eluting Stent and Coronary Thrombosis. Circulation 2007, 115, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Mcmichael, M.A.; Smith, S.A. Viscoelastic coagulation testing: Technology, applications, and limitations. Vet. Clin. Pathol. 2011, 40, 140–153. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, M.; van Heerde, W.L. Global haemostasis assays, from bench to bedside. Thromb. Res. 2012, 129, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Dargaud, Y.; Luddington, R.; Gray, E.; Lecompte, T.; Siegemund, T.; Baglin, T.; Hogwood, J.; Regnault, V.; Siegemund, A.; Negrier, C. Standardisation of thrombin generation test—Which reference plasma for TGT?: An international multicentre study. Thromb. Res. 2010, 125, 353–356. [Google Scholar] [CrossRef]
- da Luz, L.T.; Nascimento, B.; Rizoli, S. Thrombelastography (TEG®): Practical considerations on its clinical use in trauma resuscitation. Scand. J. Trauma. Resusc. Emerg. Med. 2013, 21, 29. [Google Scholar] [CrossRef] [Green Version]
- Amgalan, A.; Allen, T.; Othman, M.; Ahmadzia, H.K. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 1813–1838. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Depka, V. Possibilities and limitations of thromboelastometry/thromboelastography. Hamostaseologie 2006, 26, S21–S29. [Google Scholar] [CrossRef]
- Bonanos, N.; Steele, B.C.H.; Butler, E.P.; Macdonald, J.R.; Johnson, W.B.; Worrell, W.L.; Niklasson, G.A.; Malmgren, S.; Strømme, M.; Sundaram, S.K.; et al. Applications of Impedance Spectroscopy. In Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 175–478. [Google Scholar]
- Hajra, S.; Mohanta, K.; Sahu, M.; Purohit, V.; Choudhary, R.N.P. Studies of structural, dielectric and impedance spectroscopy of fused silica ceramics fabricated through colloidal processing. Appl. Phys. A 2019, 125, 369. [Google Scholar] [CrossRef]
- Andreasen, N.; Crandall, H.; Brimhall, O.; Miller, B.; Perez-Tamayo, J.; Martinsen, O.G.; Kauwe, S.K.; Sanchez, B. Skin Electrical Resistance as a Diagnostic and Therapeutic Biomarker of Breast Cancer Measuring Lymphatic Regions. IEEE Access 2021, 9, 152322–152332. [Google Scholar] [CrossRef] [PubMed]
- Dallé, E.; Mabandla, M.V.; Daniels, W.M.U. Dielectric Constant and Conductivity of Blood Plasma: Possible Novel Biomarkers for Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2020, 2020, 5756382. [Google Scholar] [CrossRef] [PubMed]
- Parupudi, T.; Garner, A.; Sundararajan, R. Electrical Impedance measurement as biomarker for brain tumors. In Proceedings of the Electrostatics Joint Conference, Boston, MA, USA, 18–20 June 2018. [Google Scholar]
- National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker (accessed on 20 June 2022).
- Zhang, Z.; Huang, X.; Liu, K.; Lan, T.; Wang, Z.; Zhu, Z. Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis. Biosensors 2021, 11, 470. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of Impedance Spectroscopy. In Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–20. [Google Scholar]
- Denessen, E.J.S.; Van Den Kerkhof, D.L.; Jeurissen, M.L.J.; Wetzels, R.J.H.; Verhezen, P.W.M.; Henskens, Y.M. Determining the optimal storage time and temperature for performing platelet function assays and global hemostasis assays. Platelets 2022, 33, 416–424. [Google Scholar] [CrossRef]
- Ichikawa, J.; Kouta, M.; Oogushi, M.; Komori, M. Effects of room temperature and cold storage on the metabolic and haemostatic properties of whole blood for acute normovolaemic haemodilution. PLoS ONE 2022, 17, e0267980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T. Capacitive Sensing: Water Level Application. Bachelor’s Thesis, Helsinki Metropolia University of Applied Sciences, Helsinki, Finland, 2016. [Google Scholar]
- Amini, M.; Hisdal, J.; Kalvøy, H. Applications of bioimpedance measurement techniques in tissue engineering. J. Electr. Bioimpedance 2018, 9, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Wallner, B.; Schenk, B.; Hermann, M.; Paal, P.; Falk, M.; Strapazzon, G.; Martini, W.Z.; Brugger, H.; Fries, D. Hypothermia-Associated Coagulopathy: A Comparison of Viscoelastic Monitoring, Platelet Function, and Real Time Live Confocal Microscopy at Low Blood Temperatures, an in vitro Experimental Study. Front. Physiol. 2020, 11, 843. [Google Scholar] [CrossRef]
- Kim, O.V.; Litvinov, R.I.; Alber, M.S.; Weisel, J.W. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat. Commun. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Vrigkou, E.; Tsantes, A.; Konstantonis, D.; Rapti, E.; Maratou, E.; Pappas, A.; Halvatsiotis, P.; Tsangaris, I. Platelet, Fibrinolytic and Other Coagulation Abnormalities in Newly-Diagnosed Patients with Chronic Thromboembolic Pulmonary Hypertension. Diagnostics 2022, 12, 1238. [Google Scholar] [CrossRef]
- Sucker, C.; Tharra, K.; Litmathe, J.; Scharf, R.; Zotz, R. Rotation thromboelastography (ROTEM) parameters are influenced by age, gender, and oral contraception. Perfusion 2011, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Ramström, S. Clotting time analysis of citrated blood samples is strongly affected by the tube used for blood sampling. Blood Coagul. Fibrinolysis 2005, 16, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Variable: CT | Impedance | ROTEM® | ||
|---|---|---|---|---|
| With TF | Without TF | With TF | Without TF | |
| Mean value (min) | 2.96 | 8.76 | 3.08 | 8.71 |
| SD (min) | 0.65 | 1.38 | 0.68 | 1.23 |
| Min value (min) | 2.15 | 6.10 | 2.13 | 6.46 |
| Max value (min) | 3.60 | 10.90 | 4.15 | 10.75 |
| Q1 (min) | 2.50 | 8.30 | 2.73 | 8.13 |
| Q2 (min) | 2.99 | 8.80 | 3.04 | 8.76 |
| Q3 (min) | 3.60 | 9.80 | 3.41 | 9.50 |
| IQR (min) | 1.10 | 1.50 | 0.68 | 1.36 |
| QCD (%) | 18.0 | 8.3 | 11.1 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrogio, G.; Zahhaf, O.; Le, M.-Q.; Gouriou, Y.; Josset, L.; Pialoux, V.; Lermusiaux, P.; Capsal, J.-F.; Cottinet, P.-J.; Schiava, N.D. Investigation of Blood Coagulation Using Impedance Spectroscopy: Toward Innovative Biomarkers to Assess Fibrinogenesis and Clot Retraction. Biomedicines 2022, 10, 1833. https://doi.org/10.3390/biomedicines10081833
D’Ambrogio G, Zahhaf O, Le M-Q, Gouriou Y, Josset L, Pialoux V, Lermusiaux P, Capsal J-F, Cottinet P-J, Schiava ND. Investigation of Blood Coagulation Using Impedance Spectroscopy: Toward Innovative Biomarkers to Assess Fibrinogenesis and Clot Retraction. Biomedicines. 2022; 10(8):1833. https://doi.org/10.3390/biomedicines10081833
Chicago/Turabian StyleD’Ambrogio, Giulia, Omar Zahhaf, Minh-Quyen Le, Yves Gouriou, Laurie Josset, Vincent Pialoux, Patrick Lermusiaux, Jean-Fabien Capsal, Pierre-Jean Cottinet, and Nellie Della Schiava. 2022. "Investigation of Blood Coagulation Using Impedance Spectroscopy: Toward Innovative Biomarkers to Assess Fibrinogenesis and Clot Retraction" Biomedicines 10, no. 8: 1833. https://doi.org/10.3390/biomedicines10081833
APA StyleD’Ambrogio, G., Zahhaf, O., Le, M.-Q., Gouriou, Y., Josset, L., Pialoux, V., Lermusiaux, P., Capsal, J.-F., Cottinet, P.-J., & Schiava, N. D. (2022). Investigation of Blood Coagulation Using Impedance Spectroscopy: Toward Innovative Biomarkers to Assess Fibrinogenesis and Clot Retraction. Biomedicines, 10(8), 1833. https://doi.org/10.3390/biomedicines10081833







