Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease
Abstract
:1. Introduction
2. Discovery of Mature CD4+CD8+ Double-Positive T Cells in the Periphery
3. Potential Origin of Mature CD4+CD8+ Double-Positive T Cells
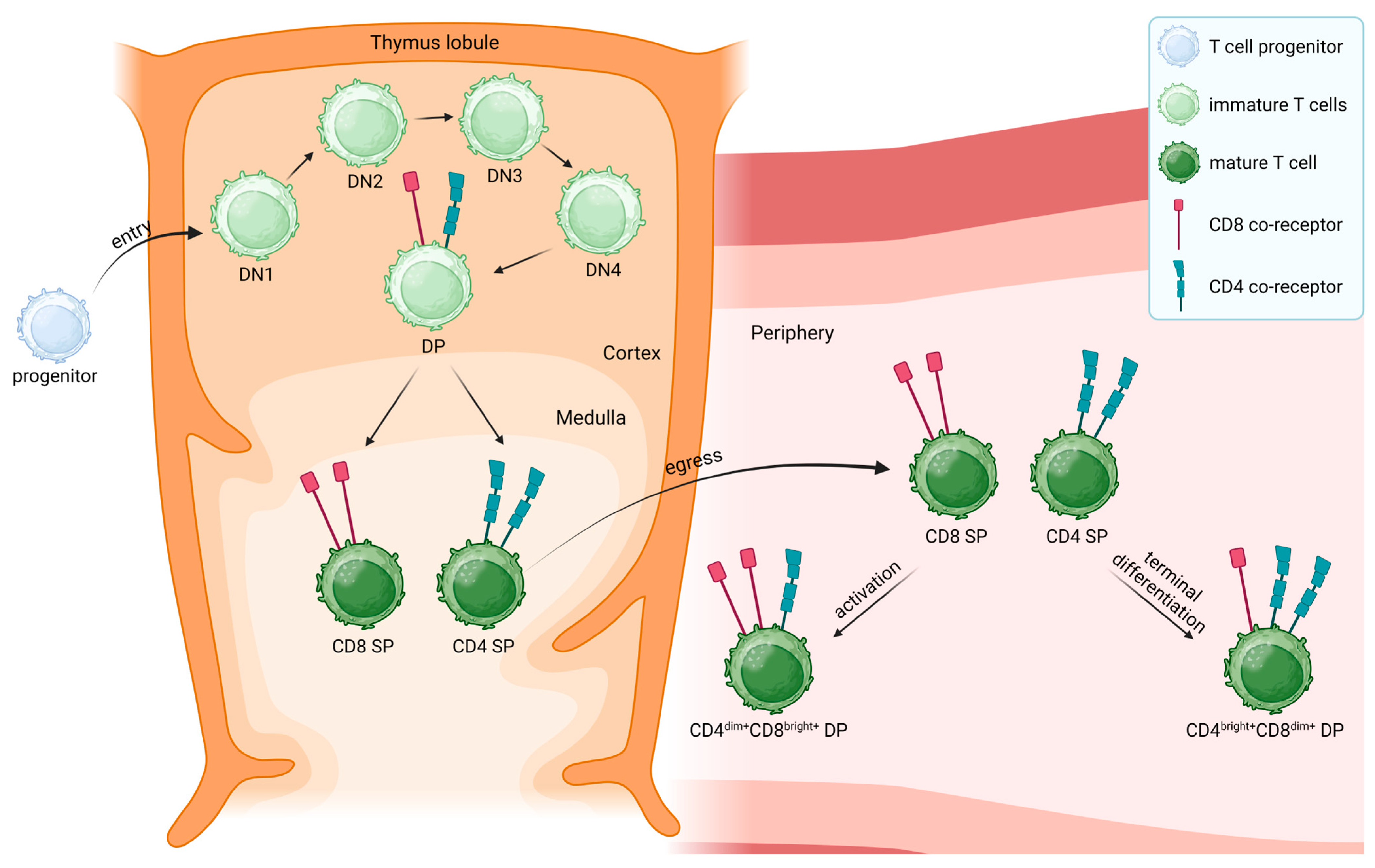
4. CD4+ and CD8+ Single-Positive T Cells
5. Peripheral CD4+CD8+ Double-Positive T Cells
6. Peripheral CD4+CD8+ Double-Positive T Cells in Disease
- CD4+CD8+ DP T cells were found to be increased in patients with viral infections such as HIV and COVID-19, indicating a potential role of this minor T cell population in the clearance of viruses [32,58,59,60,61]. In patients infected with hepatitis C, increased numbers of CD4bright+CD8dim+ DP T cells were documented [62]. High percentages of DP T cells were also found in patients suffering from chronic Chagas disease caused by the parasite Trypanosoma cruzii [63]. In some cases, the DP T cell population is suggested as a marker to assess the severity of a disease, as is the case for hemorrhagic fever with renal syndrome (HFRS) [64]. In patients suffering from Dengue viral infection, the frequency of DP T cells is significantly increased in individuals at risk of developing plasma leakage and can be therefore used as a marker for the disease progression [65].
- DP T cells are also increased in different autoimmune diseases such as multiple sclerosis (MS) and rheumatoid arthritis (RA) and in patients suffering from Sjögren’s syndrome [66,67,68,69,70]. The presence of DP T cells is also proposed as a marker for severity in several inflammatory diseases. In systemic lupus erythematosus (SLE), peripheral DP cells are associated with the risk of developing renal impairment [71]. In addition, the expansion of DP T cells in RA is associated with joint damage and frequent escalation of therapy [70]. Another example of DP T cell presence in inflammation is the development of graft versus host disease (GVHD), which has been recently associated with the appearance of a DP T cell population that was originally not present in the graft [72].
- In the context of cancer, CD4+CD8+ DP T cells were found to infiltrate cutaneous T cell lymphomas and were increased in nodular lymphocyte predominant Hodgkin lymphoma, breast cancer, and hepatocellular carcinoma [73,74,75,76,77]. Other studies have identified DP T cells in human melanoma, which originate from TCR-stimulated CD4+ or CD8+ SP cells [78] and may potentiate antitumor responses via helper functions of CD4dim+CD8bright+ DP cells [79].
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ellmeier, W.; Sawada, S.; Littman, D.R. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu. Rev. Immunol. 1999, 17, 523–554. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.; Farber, D.L. Human T cell development, localization, and function throughout life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-cell development and the CD4–CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Meryk, A.; Pangrazzi, L.; Hagen, M.; Hatzmann, F.; Jenewein, B.; Jakic, B.; Hermann-Kleiter, N.; Baier, G.; Jylhävä, J.; Hurme, M.; et al. Fcμ receptor as a Costimulatory Molecule for T Cells. Cell Rep. 2019, 26, 2681–2691.e5. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Henning, A.N.; Roychoudhuri, R.; Restifo, N.P. Epigenetic control of CD8+ T cell differentiation. Nat. Rev. Immunol. 2018, 18, 340–356. [Google Scholar] [CrossRef]
- Overgaard, N.H.; Jung, J.-W.; Steptoe, R.J.; Wells, J.W. CD4+/CD8+ double-positive T cells: More than just a developmental stage? J. Leukoc. Biol. 2015, 97, 31–38. [Google Scholar] [CrossRef]
- Blue, M.L.; Daley, J.F.; Levine, H.; Schlossman, S.F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J. Immunol. 1985, 134, 2281–2286. [Google Scholar] [CrossRef]
- Luhtala, M.; Lassila, O.; Toivanen, P.; Vainio, O. A novel peripheral CD4+CD8+ T cell population: Inheritance of CD8α expression on CD4+ T cells. Eur. J. Immunol. 1997, 27, 189–193. [Google Scholar] [CrossRef]
- Kenny, E.; Mason, D.; Pombo, A.; Ramírez, F. Phenotypic analysis of peripheral CD4+ CD8+ T cells in the rat. Immunology 2000, 101, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Akari, H.; Terao, K.; Murayama, Y.; Nam, K.H.; Yoshikawa, Y. Peripheral blood CD4+CD8+ lymphocytes in cynomolgus monkeys are of resting memory T lineage. Int. Immunol. 1997, 9, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, F.A.; Husmann, R.J. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology 1996, 87, 500–512. [Google Scholar] [PubMed]
- Harada, Y.; Sujino, T.; Miyamoto, K.; Nomura, E.; Yoshimatsu, Y.; Tanemoto, S.; Umeda, S.; Ono, K.; Mikami, Y.; Nakamoto, N.; et al. Intracellular metabolic adaptation of intraepithelial CD4+CD8αα+ T lymphocytes. iScience 2022, 25, 104021. [Google Scholar] [CrossRef]
- Gonzalez-Mancera, M.S.; Bolaños, N.I.; Salamanca, M.; Orjuela, G.A.; Rodriguez, A.N.; Gonzalez, J.M. Percentages of CD4+CD8+ Double-positive T Lymphocytes in the Peripheral Blood of Adults from a Blood Bank in Bogotá, Colombia. Turk. J. Haematol. 2020, 37, 36–41. [Google Scholar] [CrossRef]
- Ghia, P.; Prato, G.; Stella, S.; Scielzo, C.; Geuna, M.; Caligaris-Cappio, F. Age-dependent accumulation of monoclonal CD4+CD8+ double positive T lymphocytes in the peripheral blood of the elderly. Br. J. Haematol. 2007, 139, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, C.; Forti, E.; Radin, E.; Cibin, R.; Cossarizza, A. Cytofluorometric Identification of Two Populations of Double Positive (CD4+,CD8+) T Lymphocytes in Human Peripheral Blood. Biochem. Biophys. Res. Commun. 1993, 191, 601–609. [Google Scholar] [CrossRef]
- Nascimbeni, M.; Shin, E.-C.; Chiriboga, L.; Kleiner, D.E.; Rehermann, B. Peripheral CD4+CD8+ T cells are differentiated effector memory cells with antiviral functions. Blood 2004, 104, 478–486. [Google Scholar] [CrossRef]
- Abuzakouk, M.; Carton, J.; Feighery, C.; O’Donoghue, D.P.; Weir, D.G.; O’ Farrelly, C. CD4+ CD8+ and CD8alpha+ beta- T lymphocytes in human small intestinal lamina propria. Eur. J. Gastroenterol. Hepatol. 1998, 10, 325–329. [Google Scholar] [CrossRef]
- Norris, S.; Collins, C.; Doherty, D.G.; Smith, F.; McEntee, G.; Traynor, O.; Nolan, N.; Hegarty, J.; O’Farrelly, C. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J. Hepatol. 1998, 28, 84–90. [Google Scholar] [CrossRef]
- García-Dabrio, M.C.; Pujol-Moix, N.; Martinez-Perez, A.; Fontcuberta, J.; Souto, J.C.; Soria, J.M.; Nomdedéu, J.F. Influence of age, gender and lifestyle in lymphocyte subsets: Report from the Spanish Gait-2 Study. Acta Haematol. 2012, 127, 244–249. [Google Scholar] [CrossRef]
- Marini, A.; Avino, D.; De Donno, M.; Romano, F.; Morganti, R. Percentages and Absolute Numbers of CD4+CD8+ Double-positive T Lymphocytes in the Peripheral Blood of Normal Italian Subjects; Relationship with Age and Sex. Turk. J. Haematol. 2020, 37, 125–126. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. T Cells and MHC Proteins. In Molecular Biology of the Cell, 4th ed.; National Library of Medicine: Bethesda, MD, USA, 2002. [Google Scholar]
- Herbert, J.A.; Panagiotou, S. Immune Response to Viruses. Encycl. Infect. Immun. 2022, 1, 429–444. [Google Scholar] [CrossRef]
- Spits, H. Development of αβ T cells in the human thymus. Nat. Rev. Immunol. 2002, 2, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Adoro, S.; Park, J.-H. Lineage fate and intense debate: Myths, models and mechanisms of CD4/CD8 lineage choice. Nat. Rev. Immunol. 2008, 8, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual expression of Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat. Immunol. 2013, 14, 271–280. [Google Scholar] [CrossRef]
- Hagen, M.; Derudder, E. Inflammation and the Alteration of B-Cell Physiology in Aging. GER 2020, 66, 105–113. [Google Scholar] [CrossRef]
- Gui, J.; Mustachio, L.M.; Su, D.-M.; Craig, R.W. Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis. 2012, 3, 280–290. [Google Scholar] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, Y.B.; Landay, A.L.; Zack, J.A.; Kitchen, S.G.; Al-Harthi, L. Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology 2001, 103, 270–280. [Google Scholar] [CrossRef]
- Imlach, S.; McBreen, S.; Shirafuji, T.; Leen, C.; Bell, J.E.; Simmonds, P. Activated peripheral CD8 lymphocytes express CD4 in vivo and are targets for infection by human immunodeficiency virus type 1. J. Virol. 2001, 75, 11555–11564. [Google Scholar] [CrossRef]
- Weiss, L.; Roux, A.; Garcia, S.; Demouchy, C.; Haeffner-Cavaillon, N.; Kazatchkine, M.D.; Gougeon, M. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J. Infect. Dis. 1998, 178, 1158–1162. [Google Scholar] [CrossRef]
- Colombatti, A.; Doliana, R.; Schiappacassi, M.; Argentini, C.; Tonutti, E.; Feruglio, C.; Sala, P. Age-related persistent clonal expansions of CD28(-) cells: Phenotypic and molecular TCR analysis reveals both CD4(+) and CD4(+)CD8(+) cells with identical CDR3 sequences. Clin. Immunol. Immunopathol. 1998, 89, 61–70. [Google Scholar] [CrossRef]
- Das, G.; Augustine, M.M.; Das, J.; Bottomly, K.; Ray, P.; Ray, A. An important regulatory role for CD4+CD8 alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2003, 100, 5324–5329. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; Chen, W. Balancing acts: The role of TGF-β in the mucosal immune system. Trends Mol. Med. 2011, 17, 668–676. [Google Scholar] [CrossRef]
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes; Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Mizuki, M.; Tagawa, S.; Machii, T.; Shibano, M.; Tatsumi, E.; Tsubaki, K.; Tako, H.; Yokohama, A.; Satou, S.; Nojima, J.; et al. Phenotypical heterogeneity of CD4+CD8+ double-positive chronic T lymphoid leukemia. Leukemia 1998, 12, 499–504. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+CD4-CD8- (Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef]
- Broadley, I.; Pera, A.; Morrow, G.; Davies, K.A.; Kern, F. Expansions of Cytotoxic CD4+CD28− T Cells Drive Excess Cardiovascular Mortality in Rheumatoid Arthritis and Other Chronic Inflammatory Conditions and Are Triggered by CMV Infection. Front. Immunol. 2017, 8, 195. [Google Scholar] [CrossRef]
- Carrasco, J.; Godelaine, D.; Van Pel, A.; Boon, T.; Van der Bruggen, P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood 2006, 108, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Th1/Th2 cells. Inflamm. Bowel. Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Iliev, I.D.; Hohl, T.M. Immunity against fungi. JCI Insight 2017, 2, e93156. [Google Scholar] [CrossRef]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Özcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Samji, T.; Khanna, K.M. Understanding Memory CD8+ T cells. Immunol. Lett. 2017, 185, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhuijze, A.; Liston, A. Chapter Four—The Molecular Control of Regulatory T Cell Induction. Prog. Mol. Biol. Transl. Sci. 2015, 136, 69–97. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.-X.; Leonard, W.J. IL-2 Family Cytokines: New Insights into the Complex Roles of IL-2 as a Broad Regulator of T helper Cell Differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Reidla, J.; Arana, J.A.C.; Naismith, E.; Miggitsch, C.; Meryk, A.; Keller, M.; Krause, A.A.N.; Melzer, F.L.; Trieb, K.; et al. CD28 and CD57 define four populations with distinct phenotypic properties within human CD8(+) T cells. Eur. J. Immunol. 2020, 50, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Waschbisch, A.; Sammet, L.; Schröder, S.; Lee, D.H.; Barrantes-Freer, A.; Stadelmann, C.; Linker, R.A. Analysis of CD4+ CD8+ double-positive T cells in blood, cerebrospinal fluid and multiple sclerosis lesions. Clin. Exp. Immunol. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Hunter, M.C.; Teijeira, A.; Halin, C. T Cell Trafficking through Lymphatic Vessels. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Sujino, T.; London, M.; Hoytema van Konijnenburg, D.P.; Rendon, T.; Buch, T.; Silva, H.M.; Lafaille, J.J.; Reis, B.S.; Mucida, D. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Science 2016, 352, 1581–1586. [Google Scholar] [CrossRef]
- Marrero, Y.T.; Suárez, V.M.; Abraham, C.M.M.; Hernández, I.C.; Ramos, E.H.; Domínguez, G.D.; Pérez, Y.D.; Zamora, M.C.R.; Guerra, L.F.H. Immunophenotypic characterization of double positive T lymphocytes in Cuban older adults. Exp. Gerontol. 2021, 152, 111450. [Google Scholar] [CrossRef]
- Furukawa, S.; Sasai, K.; Matsubara, J.; Yabuta, K.; Hiramatsu, K.; Yamamoto, J.; Shirai, T.; Okumura, K. Increase in T cells expressing the gamma/delta receptor and CD4+CD8+ double-positive T cells in primary immunodeficiency complicated by human T-cell lymphotropic virus type I infection. Blood 1992, 80, 3253–3255. [Google Scholar] [CrossRef]
- Ribrag, V.; Salmon, D.; Picard, F.; Guesnu, M.; Sicard, D.; Dreyfus, F.; Li, I.I.; Friedman-Kien, A.E. Increase in double-positive CD4+CD8+ peripheral T-cell subsets in an HIV-infected patient. AIDS 1993, 7, 1530. [Google Scholar] [CrossRef]
- Zou, S.; Tan, Y.; Xiang, Y.; Liu, Y.; Zhu, Q.; Wu, S.; Guo, W.; Luo, M.; Shen, L.; Liang, K. The Role of CD4+CD8+ T Cells in HIV Infection With Tuberculosis. Front. Public Health 2022, 10, 895179. [Google Scholar] [CrossRef]
- Zahran, A.M.; Zahran, Z.A.M.; Mady, Y.H.; Mahran, E.E.M.O.; Rashad, A.; Makboul, A.; Nasif, K.A.; Abdelmaksoud, A.A.; El-Badawy, O. Differential alterations in peripheral lymphocyte subsets in COVID-19 patients: Upregulation of double-positive and double-negative T cells. Multidiscip. Respir. Med. 2021, 16, 758. [Google Scholar] [CrossRef]
- Nascimbeni, M.; Pol, S.; Saunier, B. Distinct CD4+CD8+ Double-Positive T Cells in the Blood and Liver of Patients during Chronic Hepatitis B and C. PLoS ONE 2011, 6, e20145. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Bolaños, N.I.; Cuellar, A.; Guzman, F.; Uribe, A.M.; Bedoya, A.; Olaya, N.; Cucunubá, Z.M.; Roa, N.; Rosas, F.; et al. Increased CD4+/CD8+ double-positive T cells in chronic Chagasic patients. PLoS Negl. Trop. Dis. 2011, 5, e1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.D.; Sun, J.J.; Yu, T.B.; Liu, H.L. Predictive value of CD4+CD8+ double positive T cells for the severity of hemorrhagic fever with renal syndrome. Clin. Biochem. 2023, 120, 110643. [Google Scholar] [CrossRef]
- Yu, E.D.; Wang, H.; da Silva Antunes, R.; Tian, Y.; Tippalagama, R.; Alahakoon, S.U.; Premawansa, G.; Wijewickrama, A.; Premawansa, S.; De Silva, A.D.; et al. A Population of CD4+CD8+ Double-Positive T Cells Associated with Risk of Plasma Leakage in Dengue Viral Infection. Viruses 2022, 14, 90. [Google Scholar] [CrossRef]
- Ferraccioli, G.F.; Tonutti, E.; Casatta, L.; Pegoraro, I.; De Vita, S.; Sala, P.; Ravaioli, T.; Bartoli, E. CD4 cytopenia and occasional expansion of CD4+CD8+lymphocytes in Sjögren’s syndrome. Clin. Exp. Rheumatol. 1996, 14, 125–130. [Google Scholar] [PubMed]
- Wang, S.; Shen, H.; Bai, B.; Wu, J.; Wang, J. Increased CD4+CD8+ Double-Positive T Cell in Patients with Primary Sjögren’s Syndrome Correlated with Disease Activity. J. Immunol. Res. 2021, 2021, 6658324. [Google Scholar] [CrossRef]
- Ziaber, J.; Stopczyk, D.; Tchórzewski, H.; Chmielewski, H.; Baj, Z.; Paśnik, J. Increased percentage of “double phenotype” form T lymphocytes in blood of patients with multiple sclerosis. Neurol. Neurochir. Pol. 2000, 34, 1137–1143. [Google Scholar]
- Quandt, D.; Rothe, K.; Scholz, R.; Baerwald, C.W.; Wagner, U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS ONE 2014, 9, e93293. [Google Scholar] [CrossRef]
- Nguyen, P.; Melzer, M.; Beck, F.; Krasselt, M.; Seifert, O.; Pierer, M.; Rothe, K.; Wagner, U. Expansion of CD4+CD8+ double-positive T cells in rheumatoid arthritis patients is associated with erosive disease. Rheumatology 2022, 61, 1282–1287. [Google Scholar] [CrossRef]
- Chang, K.; Na, W.; Liu, C.; Xu, H.; Liu, Y.; Wang, Y.; Jiang, Z. Peripheral CD4 +CD8 + double positive T cells: A potential marker to evaluate renal impairment susceptibility during systemic lupus erythematosus. J. Biomed. Res. 2022, 37, 59–68. [Google Scholar] [CrossRef]
- Hess, N.J.; Turicek, D.P.; Riendeau, J.; McIlwain, S.J.; Guzman, E.C.; Nadiminti, K.; Hudson, A.; Callander, N.S.; Skala, M.C.; Gumperz, J.E.; et al. Inflammatory CD4/CD8 double-positive human T cells arise from reactive CD8 T cells and are sufficient to mediate GVHD pathology. Sci. Adv. 2023, 9, eadf0567. [Google Scholar] [CrossRef]
- Bagot, M.; Echchakir, H.; Mami-Chouaib, F.; Delfau-Larue, M.-H.; Charue, D.; Bernheim, A.; Chouaib, S.; Boumsell, L.; Bensussan, A. Isolation of tumor-specific cytotoxic CD4+ and CD4+CD8dim+ T-cell clones infiltrating a cutaneous T-cell lymphoma. Blood 1998, 91, 4331–4341. [Google Scholar] [CrossRef]
- Rahemtullah, A.; Harris, N.L.; Dorn, M.E.; Preffer, F.I.; Hasserjian, R.P. Beyond the lymphocyte predominant cell: CD4+CD8+ T-cells in nodular lymphocyte predominant Hodgkin lymphoma. Leuk. Lymphoma 2008, 49, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Wizniak, J.; Shang, C.; Lai, R. Flow Cytometric Detection of the Double-Positive (CD4+CD8+)/PD-1bright T-Cell Subset Is Useful in Diagnosing Nodular Lymphocyte-Predominant Hodgkin Lymphoma. Arch Pathol. Lab. Med. 2022, 146, 718–726. [Google Scholar] [CrossRef]
- Desfrançois, J.; Derré, L.; Corvaisier, M.; Le Mével, B.; Catros, V.; Jotereau, F.; Gervois, N. Increased frequency of nonconventional double positive CD4CD8 alphabeta T cells in human breast pleural effusions. Int. J. Cancer 2009, 125, 374–380. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, D.; Qiu, X.; Luo, G.; Wu, T.; Yang, S.; Li, Z.; Zhu, Y.; Wang, S.; Wu, R.; et al. Trajectory and Functional Analysis of PD-1high CD4+CD8+ T Cells in Hepatocellular Carcinoma by Single-Cell Cytometry and Transcriptome Sequencing. Adv. Sci. 2020, 7, 2000224. [Google Scholar] [CrossRef]
- Schad, S.E.; Chow, A.; Mangarin, L.; Pan, H.; Zhang, J.; Ceglia, N.; Caushi, J.X.; Malandro, N.; Zappasodi, R.; Gigoux, M.; et al. Tumor-induced double positive T cells display distinct lineage commitment mechanisms and functions. J. Exp. Med. 2022, 219, e20212169. [Google Scholar] [CrossRef] [PubMed]
- PParrot, T.; Oger, R.; Benlalam, H.; de la Blétière, D.R.; Jouand, N.; Coutolleau, A.; Preisser, L.; Khammari, A.; Dréno, B.; Guardiola, P.; et al. CD40L confers helper functions to human intra-melanoma class-I-restricted CD4+CD8+ double positive T cells. Oncoimmunology 2016, 5, e1250991. [Google Scholar] [CrossRef]
- Rentenaar, R.J.; Wever, P.C.; van Diepen, F.N.; Schellekens, P.T.; Wertheim, P.M.; Berge, I.J. CD4dullCD8bright double-positive T-lymphocytes have a phenotype of granzyme Bpos CD8pos memory T-lymphocytes. Nephrol. Dial. Transpl. 1999, 14, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.K.; Vajpayee, M.; Mojumdar, K.; Singh, R.; Singh, A. Study of CD4+CD8+ double positive T-lymphocyte phenotype and function in Indian patients infected with HIV-1. J. Med. Virol. 2012, 84, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Caraballo Cortés, K.; Osuch, S.; Perlejewski, K.; Pawełczyk, A.; Kaźmierczak, J.; Janiak, M.; Jabłońska, J.; Nazzal, K.; Stelmaszczyk-Emmel, A.; Berak, H.; et al. Expression of programmed cell death protein 1 and T-cell immunoglobulin- and mucin-domain-containing molecule-3 on peripheral blood CD4+CD8+ double positive T cells in patients with chronic hepatitis C virus infection and in subjects who spontaneously cleared the virus. J. Viral. Hepat. 2019, 26, 942–950. [Google Scholar] [CrossRef]
- Durand, C.M.; Buckheit, R.W.; Salgado, M.; Pohlmeyer, C.W.; Walker-Sperling, V.E.; Hegarty, R.W.; Ambinder, R.F.; Blankson, J.N. A Human Immunodeficiency Virus Controller With a Large Population of CD4(+)CD8(+) Double-Positive T Cells. Open Forum Infect. Dis. 2015, 2, ofv039. [Google Scholar] [CrossRef] [PubMed]
- Frahm, M.A.; Picking, R.A.; Kuruc, J.D.; McGee, K.S.; Gay, C.L.; Eron, J.J.; Hicks, C.B.; Tomaras, G.D.; Ferrari, G. CD4+CD8+ T-cells Represent a Significant Portion of the Anti-HIV T-cell Response to Acute HIV Infection. J. Immunol. 2012, 188, 4289–4296. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Fu, X.; Li, L. CD4+ Cytotoxic T Lymphocytes in Cancer Immunity and Immunotherapy. Adv. Biol. 2023, 7, e2200169. [Google Scholar] [CrossRef] [PubMed]
- Bohner, P.; Chevalier, M.F.; Cesson, V.; Rodrigues-Dias, S.C.; Dartiguenave, F.; Burruni, R.; Tawadros, T.; Valerio, M.; Lucca, I.; Nardelli-Haefliger, D.; et al. Double Positive CD4+CD8+ T Cells Are Enriched in Urological Cancers and Favor T Helper-2 Polarization. Front. Immunol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Murayama, K.; Ikegami, I.; Kamekura, R.; Sakamoto, H.; Yanagi, M.; Kamiya, S.; Sato, T.; Sato, A.; Shigehara, K.; Yamamoto, M.; et al. CD4+CD8+ T follicular helper cells regulate humoral immunity in chronic inflammatory lesions. Front. Immunol. 2022, 13, 941385. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, M.; Pangrazzi, L.; Rocamora-Reverte, L.; Weinberger, B. Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease. Biomedicines 2023, 11, 2702. https://doi.org/10.3390/biomedicines11102702
Hagen M, Pangrazzi L, Rocamora-Reverte L, Weinberger B. Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease. Biomedicines. 2023; 11(10):2702. https://doi.org/10.3390/biomedicines11102702
Chicago/Turabian StyleHagen, Magdalena, Luca Pangrazzi, Lourdes Rocamora-Reverte, and Birgit Weinberger. 2023. "Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease" Biomedicines 11, no. 10: 2702. https://doi.org/10.3390/biomedicines11102702
APA StyleHagen, M., Pangrazzi, L., Rocamora-Reverte, L., & Weinberger, B. (2023). Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease. Biomedicines, 11(10), 2702. https://doi.org/10.3390/biomedicines11102702





