The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review
Abstract
:1. Introduction
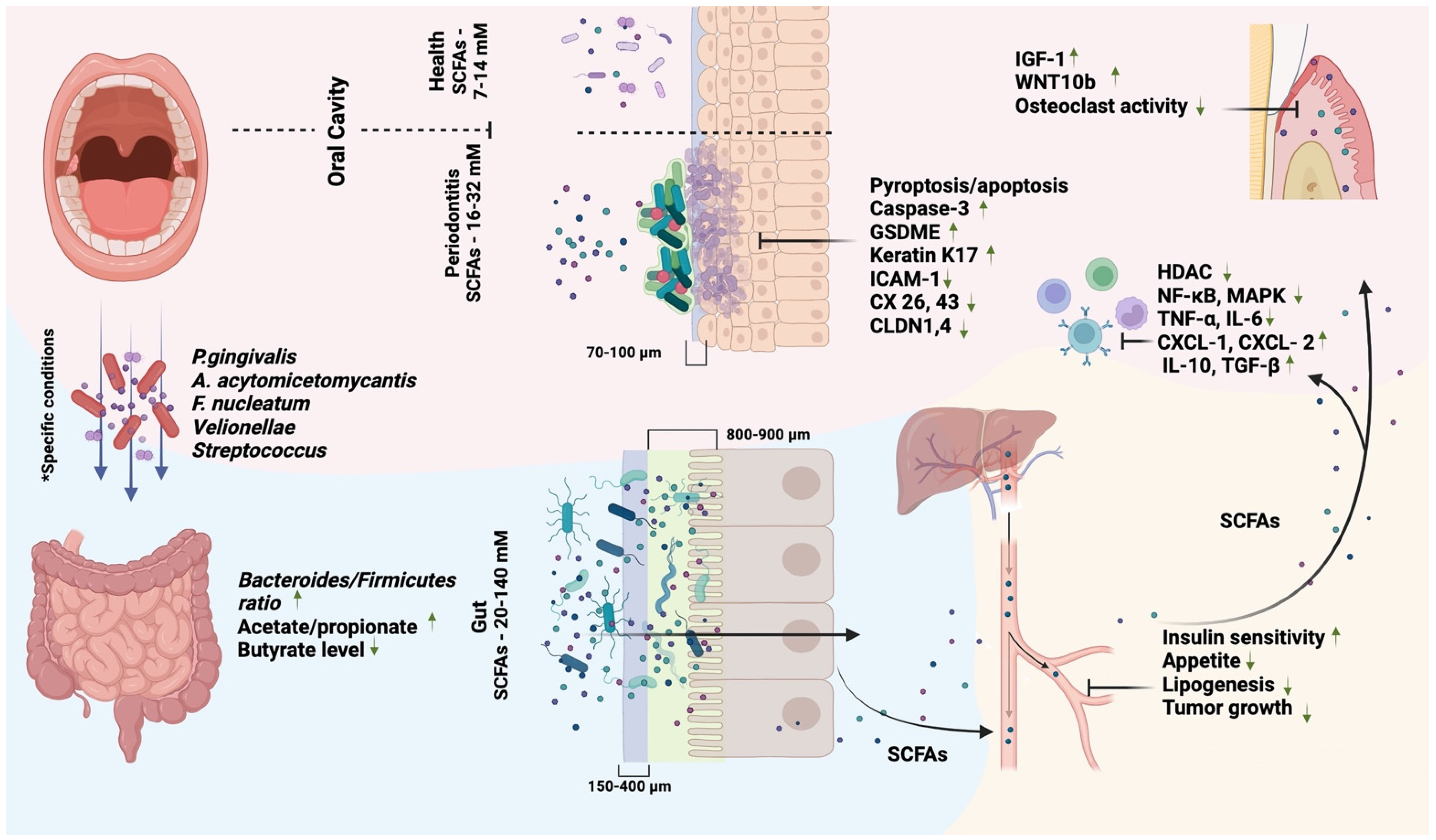
2. SCFAs and Oral Health
3. SCFAs and Oral Diseases
3.1. SCFAs and Dental Caries
| Disease | Authors | Year | SCFAs | Population | Brief Results |
|---|---|---|---|---|---|
| Periodontitis | K Hatanaka et al. [78] | 2022 | C2 and C3–C6 | 10 healthy participants and 10 participants with mild and severe periodontal disease | No significant difference in saliva was observed between healthy participants and patients. However, C3–C6 significantly differed between mild and severe periodontal disease. |
| JC Provenzano et al. [79] | 2014 | All | 18 adult patients (ages ranging from 20 to 39 years) with asymptomatic apical periodontitis | Both propionate and butyrate were found in most of the root canal tissue samples examined. Before treatment, the predominant bacteria were F. nucleatum and members of the Actinobacteria phylum, and after treatment, Streptococcus. | |
| OJ Park et al. [80] | 2023 | Butyrate | THP-1 cell line and six-week-old female Sprague Dawley rats | Butyrate combined with lipoteichoic acid significantly increased caspase-1 activation and IL-1β secretion both in vitro and in vivo in a rat model of apical periodontitis. | |
| AD Rudin et al. [81] | 2021 | All | Primary human neutrophils | P. gingivalis from both lab strains and clinical specimens has been shown to produce significant amounts of SCFAs. A similar mixture of SCFAs induces Ca2+ signaling and chemotaxis in human neutrophils through activation of FFAR2. | |
| L. Qiqiang et al. [82] | 2012 | All | 37 individuals (21 patients with chronic periodontitis and 16 periodontally healthy controls) | Periodontal treatment decreased the concentration of lactic, propionic, butyric, and isovaleric acids in the gingival crevicular fluid to the level of a healthy control group. The formic acid concentration increased. A rebound effect was observed for all SCFAs within 2–6 months. | |
| R Lu et al. [48] | 2014 | All | 40 individuals (20 with generalized aggressive periodontitis, 20 healthy controls) | Formic acid concentration increased significantly, and acetic, propionic, and butyric acid concentrations decreased after conservative treatment of periodontitis. Sites containing P gingivalis, T denticola, P intermedia, or F. nucleatum showed a similar correlation. | |
| N Murakami et al. [83] | 2022 | Butyrate | Male C57BL/6 mice | Butyrate modulates periodontal mechanical nociception via FFAR3 signaling in P. gingivalis-induced periodontitis. | |
| ME Cueno et al. [84] | 2018 | Butyrate | 10-week-old male Wistar rats | Administration of butyrate (5 mM) resulted in increased NADPH-related oxidative stress and inflammation, presumably mediated by MMP-9, in a rat model of periodontitis. | |
| S Ji et al. [85] | 2023 | All | 16 individuals (8 with periodontitis, 8 healthy controls) | A total of 570 human proteins associated with inflammation, cell death, and metabolism were found to be differentially expressed in periodontitis. Microbial proteins associated with butyrate metabolism were upregulated in the periodontitis group. | |
| R Lu et al. [86] | 2013 | All | 34 individuals (20 with generalized aggressive periodontitis, 14 healthy controls) | Patients with periodontitis had significantly higher concentrations of succinic, acetic, propionic, butyric, and isovaleric acids and a higher -abundance of both P. gingivalis and T. denticola. The level of SCFAs correlated positively with the number of these bacteria. | |
| HS Na et al. [51] | 2021 | All | 112 individuals (79 with periodontitis, 33 healthy controls) | SCFAs correlated with the abundance of the following bacterial species in the periodontitis cohort: T. denticola, Treponema socranskii, Filifactor alocis, T. forsythia, P. gingivalis, Porphyromonas endodontalis, Prevotella dentalis, and F. nucleatum. | |
| AD Rudin et al. [87] | 2021 | Acetate and Butyrate | Primary human neutrophils | Predominantly acetate and butyrate, which are released in large amounts as end products of F. nucleatum metabolism, induce human neutrophil chemotaxis and cytosolic Ca 2+ signals via the FFAR2 receptor. | |
| Oral cancer | Z Nouri et al. [88] | 2023 | All | 309 adult cancer patients and 745 healthy controls | Leuconostoc, Streptococcus, Abiotrophia, and Prevotella were decreased in the cancer group, while Haemophilus and Neisseria were increased. Total SCFA and FFAR2 expression levels were higher in the control group, while TNFAIP8, IL6, and STAT3 levels were higher in the cancer group. |
| Y Miyazaki et al. [89] | 2010 | Butyrate | Ca9-22, HSC-2, -3, and -4 cells | Butyrate induced the expression of podoplanin in HSC-2 and -3 cells and vimentin in Ca9-22 cells. Cell migration was stimulated at low concentrations of butyrate, especially in HSC-3 cells, while it was inhibited in HSC-2 and -4 cells. | |
| HIV | K Imai et al. [90] | 2009 | Butyrate | ACH-2 and U1 cells | P. gingivalis produces high levels of butyric acid, which acts as an HDAC inhibitor and induces histone acetylation, leading to the induction of RNA polymerase II and the reactivation of HIV-1. |
| Epstein–Barr virus | K Imai et al. [91] | 2012 | Butyrate | Daudi cell line | The butyric acid of P. gingivalis stimulates the expression of ZEBRA and inhibits HDAC, which results in increased histone acetylation, increased activity of the BZLF1 gene transcription, and the induction of EBV reactivation. |
| KSHV | X Yu et al. [92] | 2014 | All | BCBL1 cells | SCFAs dose-dependently and synergistically stimulate lytic expression of KSHV genes. By transactivating viral chromatin, SCFAs inhibit HDAC, suppress the expression of SIRT1, EZH2, and SUV39H1, increase acetylation, and decrease histone trimethylation. |
| Dental caries | J Wu et al. [93] | 2022 | Acetate | Biofilm in vitro | Lactobacillus casei in a multi-species biofilm has a competitive advantage over S. mutans due to acetate production. |
| T Park et al. [94] | 2021 | All | Biofilm in vitro | A mix of SCFAs inhibits S. gordonii biofilm formation by downregulating comD and comE mRNAs, which are regulators of the CSP pathway. |
3.2. SCFAs and Periodontitis
3.3. SCFAs and Oral Cancer
3.4. SCFAs and Viral Disease
4. SCFAs and the Oral–Gut Axis
4.1. Oral Bacteria and SCFAs Production in the Gut
4.2. Oral and Systemic Effects of Gut Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tidjani Alou, M.; Naud, S.; Khelaifia, S.; Bonnet, M.; Lagier, J.-C.; Raoult, D. State of the Art in the Culture of the Human Microbiota: New Interests and Strategies. Clin. Microbiol. Rev. 2020, 34, e00129-19. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chiș, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- HOMD. Human Oral Microbiome Database. Available online: https://www.homd.org/ (accessed on 31 August 2023).
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef] [PubMed]
- Read, E.; Curtis, M.A.; Neves, J.F. The Role of Oral Bacteria in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 731–742. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The Oral Microbiome in the Pathophysiology of Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 386–403. [Google Scholar] [CrossRef]
- Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Amador, G.; Denis, F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 11157. [Google Scholar] [CrossRef]
- Qin, H.; Li, G.; Xu, X.; Zhang, C.; Zhong, W.; Xu, S.; Yin, Y.; Song, J. The Role of Oral Microbiome in Periodontitis under Diabetes Mellitus. J. Oral Microbiol. 2022, 14, 2078031. [Google Scholar] [CrossRef]
- Chu, X.J.; Cao, N.W.; Zhou, H.Y.; Meng, X.; Guo, B.; Zhang, H.Y.; Li, B.Z. Oral and Gut Microbiome in Rheumatoid Arthritis Patients: A Systematic Review. Rheumatology 2021, 60, 1054–1066. Available online: https://academic.oup.com/rheumatology/article/60/3/1054/6101642 (accessed on 31 August 2023). [CrossRef] [PubMed]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral Microflora and Pregnancy: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef]
- Mukherjee, C.; Moyer, C.O.; Steinkamp, H.M.; Hashmi, S.B.; Beall, C.J.; Guo, X.; Ni, A.; Leys, E.J.; Griffen, A.L. Acquisition of Oral Microbiota Is Driven by Environment, Not Host Genetics. Microbiome 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, Y.-H.; Zhao, Y.-Q.; Feng, Y.; Yan, F.; Gao, Z.-R.; Ye, Q.; Chen, Y.; Liu, Q.; Tan, L.; et al. Gender Variations in the Oral Microbiomes of Elderly Patients with Initial Periodontitis. J. Immunol. Res. 2021, 2021, 7403042. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Peña, N.; Kawar, N.; Zhang, A.; Callahan, N.; Robles, S.J.; Griebel, A.; Adami, G.R. Old Age and Other Factors Associated with Salivary Microbiome Variation. BMC Oral Health 2021, 21, 490. [Google Scholar] [CrossRef]
- Wu, J.; Peters, B.A.; Dominianni, C.; Zhang, Y.; Pei, Z.; Yang, L.; Ma, Y.; Purdue, M.P.; Jacobs, E.J.; Gapstur, S.M.; et al. Cigarette Smoking and the Oral Microbiome in a Large Study of American Adults. ISME J. 2016, 10, 2435–2446. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Renson, A.; Jones, H.E.; Beghini, F.; Segata, N.; Zolnik, C.P.; Usyk, M.; Moody, T.U.; Thorpe, L.; Burk, R.; Waldron, L.; et al. Sociodemographic Variation in the Oral Microbiome. Ann. Epidemiol. 2019, 35, 73–80.e2. [Google Scholar] [CrossRef]
- Cheng, X.; He, F.; Si, M.; Sun, P.; Chen, Q. Effects of Antibiotic Use on Saliva Antibody Content and Oral Microbiota in Sprague Dawley Rats. Front. Cell. Infect. Microbiol. 2022, 12, 721691. [Google Scholar] [CrossRef]
- Kumar, J.; Teoh, S.L.; Das, S.; Mahakknaukrauh, P. Oxidative Stress in Oral Diseases: Understanding Its Relation with Other Systemic Diseases. Front. Physiol. 2017, 8, 693. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. CCS 2022, 20, 64. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, C.; Chen, Y.; He, X.; Gao, F.; Xue, D. Quantitative Increase in Short-Chain Fatty Acids, Especially Butyrate Protects Kidney from Ischemia/Reperfusion Injury. J. Investig. Med. 2022, 70, 29–35. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.F.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.D.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. JASN 2015, 26, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Altimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and Medium-Chain Fatty Acids Exhibit Antimicrobial Activity for Oral Microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Lyu, W.; Yang, H.; Li, N.; Lu, L.; Yang, C.; Jin, P.; Xiao, Y. Molecular Characterization, Developmental Expression, and Modulation of Occludin by Early Intervention with Clostridium butyricum in Muscovy Ducks. Poult. Sci. 2021, 100, 101271. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; Abbar, F.E.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s Role in Human Health and the Current Progress towards Its Clinical Application to Treat Gastrointestinal Disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of Inflammatory Microglia by Dietary Fiber and Short-Chain Fatty Acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids—A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The Short Chain Fatty Acids, Butyrate and Propionate, Have Differential Effects on the Motility of the Guinea Pig Colon. Neurogastroenterol. Motil. 2014, 26, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Perry, E.K.; Tan, M.-W. Bacterial biofilms in the human body: Prevalence and impacts on health and disease. Front. Cell. Infect. Microbiol. 2023, 13, 1237164. [Google Scholar] [CrossRef]
- Ng, H.M.; Kin, L.X.; Dashper, S.G.; Slakeski, N.; Butler, C.A.; Reynolds, E.C. Bacterial Interactions in Pathogenic Subgingival Plaque. Microb. Pathog. 2016, 94, 60–69. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Rossi, E.; Paroni, M.; Landini, P. Biofilm and Motility in Response to Environmental and Host-related Signals in Gram Negative Opportunistic Pathogens. J. Appl. Microbiol. 2018, 125, 1587–1602. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Giordano-Kelhoffer, B.; Lorca, C.; March Llanes, J.; Rábano, A.; del Ser, T.; Serra, A.; Gallart-Palau, X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines 2022, 10, 1803. [Google Scholar] [CrossRef]
- Seidel, C.L.; Gerlach, R.G.; Wiedemann, P.; Weider, M.; Rodrian, G.; Hader, M.; Frey, B.; Gaipl, U.S.; Bozec, A.; Cieplik, F.; et al. Defining Metaniches in the Oral Cavity According to Their Microbial Composition and Cytokine Profile. Int. J. Mol. Sci. 2020, 21, 8218. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Cimermancic, P.; Schulze, C.J.; Wieland Brown, L.C.; Martin, J.; Mitreva, M.; Clardy, J.; Linington, R.G.; Fischbach, M.A. A Systematic Analysis of Biosynthetic Gene Clusters in the Human Microbiome Reveals a Common Family of Antibiotics. Cell 2014, 158, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N. Oral Microbiome Metabolism: From “Who Are They?” to “What Are They Doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, M.; Liu, Y.; Luo, B.; Cui, J.; Huang, L.; Chen, K.; Liu, Y. The Oral Microbiota and Cardiometabolic Health: A Comprehensive Review and Emerging Insights. Front. Immunol. 2022, 13, 1010368. [Google Scholar] [CrossRef] [PubMed]
- Ndeh, D.; Gilbert, H.J. Biochemistry of Complex Glycan Depolymerisation by the Human Gut Microbiota. FEMS Microbiol. Rev. 2018, 42, 146–164. [Google Scholar] [CrossRef]
- Norimatsu, Y.; Kawashima, J.; Takano-Yamamoto, T.; Takahashi, N. Nitrogenous Compounds Stimulate Glucose-Derived Acid Production by Oral Streptococcus and Actinomyces. Microbiol. Immunol. 2015, 59, 501–506. [Google Scholar] [CrossRef]
- Lu, R.; Meng, H.; Gao, X.; Xu, L.; Feng, X. Effect of Non-Surgical Periodontal Treatment on Short Chain Fatty Acid Levels in Gingival Crevicular Fluid of Patients with Generalized Aggressive Periodontitis. J. Periodontal Res. 2014, 49, 574–583. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic Balancing of Intestinal Short-Chain Fatty Acids: The Crucial Role of Bacterial Metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Tennert, C.; Reinmuth, A.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An Oral Health Optimized Diet Reduces the Load of Potential Cariogenic and Periodontal Bacterial Species in the Supragingival Oral Plaque: A Randomized Controlled Pilot Study. MicrobiologyOpen 2020, 9, e1056. [Google Scholar] [CrossRef]
- Na, H.S.; Kim, S.; Kim, S.; Yu, Y.; Kim, S.Y.; Kim, H.-J.; Lee, J.Y.; Lee, J.-H.; Chung, J. Molecular Subgroup of Periodontitis Revealed by Integrated Analysis of the Microbiome and Metabolome in a Cross-Sectional Observational Study. J. Oral Microbiol. 2021, 13, 1902707. [Google Scholar] [CrossRef]
- Ochiai, K.; Kurita-Ochiai, T. Effects of Butyric Acid on the Periodontal Tissue. Jpn. Dent. Sci. Rev. 2009, 45, 75–82. [Google Scholar] [CrossRef]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut Bacteria Identified in Colorectal Cancer Patients Promote Tumourigenesis via Butyrate Secretion. Nat. Commun. 2021, 12, 5674. [Google Scholar] [CrossRef] [PubMed]
- Mirković, B.; Murray, M.A.; Lavelle, G.M.; Molloy, K.; Azim, A.A.; Gunaratnam, C.; Healy, F.; Slattery, D.; McNally, P.; Hatch, J.; et al. The Role of Short-Chain Fatty Acids, Produced by Anaerobic Bacteria, in the Cystic Fibrosis Airway. Am. J. Respir. Crit. Care Med. 2015, 192, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Ochiai, K.; Suzuki, N.; Otsuka, K. Butyrate, a Bacterial Metabolite, Induces Apoptosis and Autophagic Cell Death in Gingival Epithelial Cells. J. Periodontal Res. 2010, 45, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Magrin, G.L.; Strauss, F.J.; Benfatti, C.A.M.; Maia, L.C.; Gruber, R. Effects of Short-Chain Fatty Acids on Human Oral Epithelial Cells and the Potential Impact on Periodontal Disease: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 4895. [Google Scholar] [CrossRef]
- Guan, X.; Li, W.; Meng, H. A Double-Edged Sword: Role of Butyrate in the Oral Cavity and the Gut. Mol. Oral Microbiol. 2021, 36, 121–131. [Google Scholar] [CrossRef]
- Zhang, J.; Kashket, S. Cytotoxic Effects of Short-Chain Carboxylic Acids on Human Gingival Epithelial Cells. Oral Microbiol. Immunol. 1997, 12, 345–349. [Google Scholar] [CrossRef]
- Shirasugi, M.; Nishioka, K.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Normal Human Gingival Fibroblasts Undergo Cytostasis and Apoptosis after Long-Term Exposure to Butyric Acid. Biochem. Biophys. Res. Commun. 2017, 482, 1122–1128. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Meng, H.; Yu, J.; Lu, H.; Li, W.; Lu, R.; Zhao, Y.; Li, Q.; Su, L. Butyrate Rather than LPS Subverts Gingival Epithelial Homeostasis by Downregulation of Intercellular Junctions and Triggering Pyroptosis. J. Clin. Periodontol. 2019, 46, 894–907. [Google Scholar] [CrossRef]
- Pöllänen, M.T.; Salonen, J.I. Effect of Short Chain Fatty Acids on Human Gingival Epithelial Cell Keratins in Vitro. Eur. J. Oral Sci. 2000, 108, 523–529. [Google Scholar] [CrossRef]
- Magrin, G.L.; Di Summa, F.; Strauss, F.-J.; Panahipour, L.; Mildner, M.; Magalhães Benfatti, C.A.; Gruber, R. Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; Semlali, A.; Jacques, É.; Alanazi, M.; Zakrzewski, A.; Chmielewski, W.; Rouabhia, M. Long-Term Exposure of Human Gingival Fibroblasts to Cigarette Smoke Condensate Reduces Cell Growth by Modulating Bax, Caspase-3 and P53 Expression. J. Periodontal Res. 2015, 50, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.P.; Cervantes, L.C.C.; Panahipour, L.; Souza, F.Á.; Gruber, R. Proof-of-Principle Study Suggesting Potential Anti-Inflammatory Activity of Butyrate and Propionate in Periodontal Cells. Int. J. Mol. Sci. 2022, 23, 11006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Guo, W.; Li, H.; Lei, L. Periodontitis-Level Butyrate-Induced Ferroptosis in Periodontal Ligament Fibroblasts by Activation of Ferritinophagy. Cell Death Discov. 2020, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Whibley, N.; MacCallum, D.M.; Vickers, M.A.; Zafreen, S.; Waldmann, H.; Hori, S.; Gaffen, S.L.; Gow, N.A.R.; Barker, R.N.; Hall, A.M. Expansion of Foxp3+ T-Cell Populations by Candida albicans Enhances Both Th17-Cell Responses and Fungal Dissemination after Intravenous Challenge. Eur. J. Immunol. 2014, 44, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial Interactions in Dental Biofilm Development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 227. [Google Scholar]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- May, K.S.; den Hartigh, L.J. Gut Microbial-Derived Short Chain Fatty Acids: Impact on Adipose Tissue Physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of Immune Cell Function by Short-Chain Fatty Acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Ney, L.-M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short Chain Fatty Acids: Key Regulators of the Local and Systemic Immune Response in Inflammatory Diseases and Infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.-L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e6. [Google Scholar] [CrossRef] [PubMed]
- Schlatterer, K.; Peschel, A.; Kretschmer, D. Short-Chain Fatty Acid and FFAR2 Activation—A New Option for Treating Infections? Front. Cell. Infect. Microbiol. 2021, 11, 785833. [Google Scholar] [CrossRef]
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial Interactions: Impact on Pathogenesis and Human Disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, K.; Shirahase, Y.; Yoshida, T.; Kono, M.; Toya, N.; Sakasegawa, S.; Konishi, K.; Yamamoto, T.; Ochiai, K.; Takashiba, S. Enzymatic Measurement of Short-Chain Fatty Acids and Application in Periodontal Disease Diagnosis. PLoS ONE 2022, 17, e0268671. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, J.C.; Rôças, I.N.; Tavares, L.F.D.; Neves, B.C.; Siqueira, J.F. Short-Chain Fatty Acids in Infected Root Canals of Teeth with Apical Periodontitis before and after Treatment. J. Endod. 2015, 41, 831–835. [Google Scholar] [CrossRef]
- Park, O.-J.; Ha, Y.-E.; Sim, J.-R.; Lee, D.; Lee, E.-H.; Kim, S.-Y.; Yun, C.-H.; Han, S.H. Butyrate Potentiates Enterococcus faecalis Lipoteichoic Acid-Induced Inflammasome Activation via Histone Deacetylase Inhibition. Cell Death Discov. 2023, 9, 107. [Google Scholar] [CrossRef]
- Dahlstrand Rudin, A.; Khamzeh, A.; Venkatakrishnan, V.; Persson, T.; Gabl, M.; Savolainen, O.; Forsman, H.; Dahlgren, C.; Christenson, K.; Bylund, J. Porphyromonas gingivalis Produce Neutrophil Specific Chemoattractants Including Short Chain Fatty Acids. Front. Cell. Infect. Microbiol. 2021, 10, 620681. [Google Scholar] [CrossRef]
- Qiqiang, L.; Huanxin, M.; Xuejun, G. Longitudinal Study of Volatile Fatty Acids in the Gingival Crevicular Fluid of Patients with Periodontitis before and after Nonsurgical Therapy. J. Periodontal Res. 2012, 47, 740–749. [Google Scholar] [CrossRef]
- Murakami, N.; Yoshikawa, K.; Tsukada, K.; Kamio, N.; Hayashi, Y.; Hitomi, S.; Kimura, Y.; Shibuta, I.; Osada, A.; Sato, S. Butyric Acid Modulates Periodontal Nociception in Porphyromonas Gingivalis-Induced Periodontitis. J. Oral Sci. 2022, 64, 91–94. Available online: https://www.jstage.jst.go.jp/article/josnusd/64/1/64_21-0483/_article/-char/ja/ (accessed on 31 August 2023). [CrossRef] [PubMed]
- Cueno, M.E.; Ochiai, K. Gingival Periodontal Disease (PD) Level-Butyric Acid Affects the Systemic Blood and Brain Organ: Insights Into the Systemic Inflammation of Periodontal Disease. Front. Immunol. 2018, 9, 1158. [Google Scholar] [CrossRef]
- Ji, S.; Kook, J.-K.; Park, S.-N.; Lim, Y.K.; Choi, G.H.; Jung, J.-S. Characteristics of the Salivary Microbiota in Periodontal Diseases and Potential Roles of Individual Bacterial Species To Predict the Severity of Periodontal Disease. Microbiol. Spectr. 2023, 11, e0432722. Available online: https://journals.asm.org/doi/full/10.1128/spectrum.04327-22 (accessed on 31 August 2023). [CrossRef]
- Lu, R.; Feng, L.; Gao, X.; Meng, H.; Feng, X. Relationship between volatile fatty acids and Porphyromonas gingivalis and Treponema denticola in gingival crevicular fluids of patients with aggressive periodontitis. Beijing Da Xue Xue Bao 2013, 45, 12–16. [Google Scholar] [PubMed]
- Dahlstrand Rudin, A.; Khamzeh, A.; Venkatakrishnan, V.; Basic, A.; Christenson, K.; Bylund, J. Short Chain Fatty Acids Released by Fusobacterium nucleatum Are Neutrophil Chemoattractants Acting via Free Fatty Acid Receptor 2 (FFAR2). Cell. Microbiol. 2021, 23, e13348. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Choi, S.W.; Choi, I.J.; Ryu, K.W.; Woo, S.M.; Park, S.-J.; Lee, W.J.; Choi, W.; Jung, Y.-S.; Myung, S.-K.; et al. Exploring Connections between Oral Microbiota, Short-Chain Fatty Acids, and Specific Cancer Types: A Study of Oral Cancer, Head and Neck Cancer, Pancreatic Cancer, and Gastric Cancer. Cancers 2023, 15, 2898. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Kikuchi, K.; González-Alva, P.; Inoue, H.; Noguchi, Y.; Tsuchiya, H.; Hayashi, J.; Shin, K.; Ochiai, K.; Kusama, K. Association of Butyric Acid Produced by Periodontopathic Bacteria with Progression of Oral Cancer. J. Cancer Sci. Ther. 2010, 2, 26–32. [Google Scholar] [CrossRef]
- Imai, K.; Ochiai, K.; Okamoto, T. Reactivation of Latent HIV-1 Infection by the Periodontopathic Bacterium Porphyromonas gingivalis Involves Histone Modification1. J. Immunol. 2009, 182, 3688–3695. [Google Scholar] [CrossRef]
- Imai, K.; Inoue, H.; Tamura, M.; Cueno, M.E.; Inoue, H.; Takeichi, O.; Kusama, K.; Saito, I.; Ochiai, K. The Periodontal Pathogen Porphyromonas gingivalis Induces the Epstein–Barr Virus Lytic Switch Transactivator ZEBRA by Histone Modification. Biochimie 2012, 94, 839–846. [Google Scholar] [CrossRef]
- Yu, X.; Shahir, A.-M.; Sha, J.; Feng, Z.; Eapen, B.; Nithianantham, S.; Das, B.; Karn, J.; Weinberg, A.; Bissada, N.F.; et al. Short-Chain Fatty Acids from Periodontal Pathogens Suppress Histone Deacetylases, EZH2, and SUV39H1 To Promote Kaposi’s Sarcoma-Associated Herpesvirus Replication. J. Virol. 2014, 88, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, X.; Yang, Q.; Zhang, Y.; Wang, C.; Huang, R. Inhibition of Streptococcus mutans Biofilm Formation by the Joint Action of Oxyresveratrol and Lactobacillus casei. Appl. Environ. Microbiol. 2022, 88, e02436-21. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Im, J.; Kim, A.R.; Lee, D.; Jeong, S.; Yun, C.-H.; Han, S.H. Short-Chain Fatty Acids Inhibit the Biofilm Formation of Streptococcus Gordonii through Negative Regulation of Competence-Stimulating Peptide Signaling Pathway. J. Microbiol. 2021, 59, 1142–1149. [Google Scholar] [CrossRef]
- Nyvad, B.; Takahashi, N. Integrated Hypothesis of Dental Caries and Periodontal Diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [PubMed]
- Mashima, I.; Nakazawa, F. Interaction between Streptococcus Spp. and Veillonella tobetsuensis in the Early Stages of Oral Biofilm Formation. J. Bacteriol. 2015, 197, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Bernstein, Y.; Findler, M. Periodontal Disease and Its Prevention, by Traditional and New Avenues. Exp. Ther. Med. 2020, 19, 1504. [Google Scholar] [CrossRef]
- Siddiqui, R.; Badran, Z.; Boghossian, A.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Increasing Importance of the Oral Microbiome in Periodontal Health and Disease. Future Sci. OA 2023, 9, FSO856. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red Complex: Polymicrobial Conglomerate in Oral Flora: A Review. J. Fam. Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef]
- Suzuki, I.; Shimizu, T.; Senpuku, H. Short Chain Fatty Acids Induced the Type 1 and Type 2 Fimbrillin-Dependent and Fimbrillin-Independent Initial Attachment and Colonization of Actinomyces oris Monoculture but Not Coculture with Streptococci. BMC Microbiol. 2020, 20, 329. [Google Scholar] [CrossRef]
- Llama-Palacios, A.; Potupa, O.; Sánchez, M.C.; Figuero, E.; Herrera, D.; Sanz, M. Proteomic Analysis of Fusobacterium nucleatum Growth in Biofilm versus Planktonic State. Mol. Oral Microbiol. 2020, 35, 168–180. [Google Scholar] [CrossRef]
- Zhu, Y.; Dashper, S.G.; Chen, Y.-Y.; Crawford, S.; Slakeski, N.; Reynolds, E.C. Porphyromonas gingivalis and Treponema denticola Synergistic Polymicrobial Biofilm Development. PLoS ONE 2013, 8, e71727. [Google Scholar] [CrossRef]
- Irani, S. New Insights into Oral Cancer—Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Zhang, H.; Li, C.; Zhang, T.; Liu, H.; Zhu, S.; Chen, J. Tumor Microenvironment and Immunotherapy of Oral Cancer. Eur. J. Med. Res. 2022, 27, 198. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial Metabolite Butyrate Facilitates M2 Macrophage Polarization and Function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Wang, G.; Cao, B.; Yang, H.; Jin, L.; Cui, M.; Mao, Y. Syndecan-1 Suppresses Cell Growth and Migration via Blocking JAK1/STAT3 and Ras/Raf/MEK/ERK Pathways in Human Colorectal Carcinoma Cells. BMC Cancer 2019, 19, 1160. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and Its Associated Systemic Diseases: Epidemiologic Studies and Possible Mechanisms. J. Oral Microbiol. 2022, 15, 2145729. [Google Scholar] [CrossRef]
- Brennan, C.A.; Clay, S.L.; Lavoie, S.L.; Bae, S.; Lang, J.K.; Fonseca-Pereira, D.; Rosinski, K.G.; Ou, N.; Glickman, J.N.; Garrett, W.S. Fusobacterium nucleatum Drives a Pro-Inflammatory Intestinal Microenvironment through Metabolite Receptor-Dependent Modulation of IL-17 Expression. Gut Microbes 2021, 13, 1987780. [Google Scholar] [CrossRef]
- Johnson, N.W.; Anaya-Saavedra, G.; Webster-Cyriaque, J. Viruses and Oral Diseases in HIV-Infected Individuals on Long-Term Antiretroviral Therapy: What Are the Risks and What Are the Mechanisms? Oral Dis. 2020, 26, 80–90. [Google Scholar] [CrossRef]
- Coker, M.O.; Cairo, C.; Garzino-Demo, A. HIV-Associated Interactions Between Oral Microbiota and Mucosal Immune Cells: Knowledge Gaps and Future Directions. Front. Immunol. 2021, 12, 676669. [Google Scholar] [CrossRef] [PubMed]
- Coker, M.O.; Mongodin, E.F.; El-Kamary, S.S.; Akhigbe, P.; Obuekwe, O.; Omoigberale, A.; Langenberg, P.; Enwonwu, C.; Hittle, L.; Blattner, W.A.; et al. Immune Status, and Not HIV Infection or Exposure, Drives the Development of the Oral Microbiota. Sci. Rep. 2020, 10, 10830. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? eBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef]
- Gomez-Casado, C.; Sanchez-Solares, J.; Izquierdo, E.; Díaz-Perales, A.; Barber, D.; Escribese, M.M. Oral Mucosa as a Potential Site for Diagnosis and Treatment of Allergic and Autoimmune Diseases. Foods 2021, 10, 970. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Newberry, R.D. Intestinal Macromolecular Transport Supporting Adaptive Immunity. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 729–737. [Google Scholar] [CrossRef]
- Derrien, M.; van Passel, M.W.J.; van de Bovenkamp, J.H.B.; Schipper, R.; de Vos, W.; Dekker, J. Mucin-Bacterial Interactions in the Human Oral Cavity and Digestive Tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The Adherent Gastrointestinal Mucus Gel Layer: Thickness and Physical State in Vivo. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Cueno, M.E.; Ochiai, K. Re-Discovering Periodontal Butyric Acid: New Insights on an Old Metabolite. Microb. Pathog. 2016, 94, 48–53. [Google Scholar] [CrossRef]
- Kampmann, C.; Dicksved, J.; Engstrand, L.; Rautelin, H. Composition of Human Faecal Microbiota in Resistance to Campylobacter Infection. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 61.e1–61.e8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive Transmission of Microbes along the Gastrointestinal Tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Ahmed, T.; Brüssow, H. Hunger and Microbiology: Is a Low Gastric Acid-induced Bacterial Overgrowth in the Small Intestine a Contributor to Malnutrition in Developing Countries? Microb. Biotechnol. 2017, 10, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Torralba, M.G.; Moncera, K.J.; DiLello, L.; Petrini, J.; Nelson, K.E.; Pieper, R. Gastro-Intestinal and Oral Microbiome Signatures Associated with Healthy Aging. GeroScience 2019, 41, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ni, Z.; Zhao, K.; Li, X.; Gao, X.; Kang, Y.; Yu, Z.; Qin, Y.; Zhao, J.; Peng, W.; et al. The Association between Oral and Gut Microbiota in Male Patients with Alcohol Dependence. Front. Microbiol. 2023, 14, 1203678. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Li, M.; Campbell, T.B.; Flores, S.C.; Linderman, D.; Gebert, M.J.; Knight, R.; Fontenot, A.P.; Palmer, B.E. Alterations in the Gut Microbiota Associated with HIV-1 Infection. Cell Host Microbe 2013, 14, 329–339. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.F.; Brown, E.M.; Temple, E.R.; Onyekaba, M.A.; Mohamed, A.M.T.; Duncan, K.; Schirmer, M.; Walker, R.L.; Mayassi, T.; Pierce, K.A.; et al. Inflammation-Associated Nitrate Facilitates Ectopic Colonization of Oral Bacterium Veillonella parvula in the Intestine. Nat. Microbiol. 2022, 7, 1673–1685. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, R.; Zhou, C.; Yu, H.; Luo, W.; Zhu, J.; Liu, J.; Zhang, Z.; Xie, N.; Peng, X.; et al. ANGPTL4-Mediated Promotion of Glycolysis Facilitates the Colonization of Fusobacterium nucleatum in Colorectal Cancer. Cancer Res. 2021, 81, 6157–6170. [Google Scholar] [CrossRef]
- Kitamura, K.; Shionoya, H.; Suzuki, S.; Fukai, R.; Uda, S.; Abe, C.; Takemori, H.; Nishimura, K.; Baba, H.; Katayama, K.; et al. Oral and Intestinal Bacterial Substances Associated with Disease Activities in Patients with Rheumatoid Arthritis: A Cross-Sectional Clinical Study. J. Immunol. Res. 2022, 2022, e6839356. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Cheng, L.; Zeng, B.; Yu, J.; Peng, X.; Zhao, J.; Li, W.; Ren, B.; Li, M.; et al. Oral Bacteria Colonize and Compete with Gut Microbiota in Gnotobiotic Mice. Int. J. Oral Sci. 2019, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haraga, H.; Sato, T.; Watanabe, K.; Hamada, N.; Tani-Ishii, N. Effect of the Progression of Fusobacterium nucleatum–Induced Apical Periodontitis on the Gut Microbiota. J. Endod. 2022, 48, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral Pathobiont Induces Systemic Inflammation and Metabolic Changes Associated with Alteration of Gut Microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0134234 (accessed on 6 September 2023). [CrossRef]
- Lourenςo, T.G.B.; Spencer, S.J.; Alm, E.J.; Colombo, A.P.V. Defining the Gut Microbiota in Individuals with Periodontal Diseases: An Exploratory Study. J. Oral Microbiol. 2018, 10, 1487741. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Roles of Oral Microbiota and Oral-Gut Microbial Transmission in Hypertension. J. Adv. Res. 2023, 43, 147–161. [Google Scholar] [CrossRef]
- Komazaki, R.; Katagiri, S.; Takahashi, H.; Maekawa, S.; Shiba, T.; Takeuchi, Y.; Kitajima, Y.; Ohtsu, A.; Udagawa, S.; Sasaki, N.; et al. Periodontal Pathogenic Bacteria, Aggregatibacter Actinomycetemcomitans Affect Non-Alcoholic Fatty Liver Disease by Altering Gut Microbiota and Glucose Metabolism. Sci. Rep. 2017, 7, 13950. [Google Scholar] [CrossRef]
- Kunath, B.J.; Hickl, O.; Queirós, P.; Martin-Gallausiaux, C.; Lebrun, L.A.; Halder, R.; Laczny, C.C.; Schmidt, T.S.B.; Hayward, M.R.; Becher, D.; et al. Alterations of Oral Microbiota and Impact on the Gut Microbiome in Type 1 Diabetes Mellitus Revealed by Integrated Multi-Omic Analyses. Microbiome 2022, 10, 243. [Google Scholar] [CrossRef]
- Fei, N.; Zhao, L. An Opportunistic Pathogen Isolated from the Gut of an Obese Human Causes Obesity in Germfree Mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ogawa, Y.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Oral Bacteria Affect the Gut Microbiome and Intestinal Immunity. Pathog. Dis. 2020, 78, ftaa024. [Google Scholar] [CrossRef]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The Oral Microbiota in Colorectal Cancer Is Distinctive and Predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.-Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e7. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut Microbiota Induce IGF-1 and Promote Bone Formation and Growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-Chain Fatty Acids Regulate Systemic Bone Mass and Protect from Pathological Bone Loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, L.; Qian, J.; Wang, M.; Li, L.; Huang, Y.; Zhang, Q.; Li, Y.; Yan, F. Salivary Microbiota of Periodontitis Aggravates Bone Loss in Ovariectomized Rats. Front. Cell. Infect. Microbiol. 2022, 12, 983608. [Google Scholar] [CrossRef]
- Stanisic, D.; Jeremic, N.; Majumder, S.; Pushpakumar, S.; George, A.; Singh, M.; Tyagi, S.C. High Fat Diet Dysbiotic Mechanism of Decreased Gingival Blood Flow. Front. Physiol. 2021, 12, 625780. [Google Scholar] [CrossRef]
- Luo, S.; Li, W.; Li, Q.; Zhang, M.; Wang, X.; Wu, S.; Li, Y. Causal Effects of Gut Microbiota on the Risk of Periodontitis: A Two-Sample Mendelian Randomization Study. Front. Cell. Infect. Microbiol. 2023, 13, 1160993. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Hatting, M.; Bott, A.; Dahlhausen, A.; Keller, D.; Trautwein, C.; Conrads, G. The Oral-Gut Axis: Salivary and Fecal Microbiome Dysbiosis in Patients with Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 1010853. [Google Scholar] [CrossRef]
| Species | SCFAs | ||||
|---|---|---|---|---|---|
| Acetic | Propanoic | Butyric | Isobutyric | Isovaleric | |
| P. gingivalis | ++ | + | +++ | + | ++ |
| P. asaccharolytica | + | +++ | ++ | + | ++ |
| P. intermedia | ++ | +++ | + | + | + |
| F. nucleatum | ++ | + | +++ | + | + |
| A. actinomycetemcomitans | +++ | - | - | - | - |
| V. parvula | ++ | +++ | - | - | - |
| S. sanguinis | ++ | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonov, G.E.; Varaeva, Y.R.; Livantsova, E.N.; Starodubova, A.V. The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines 2023, 11, 2749. https://doi.org/10.3390/biomedicines11102749
Leonov GE, Varaeva YR, Livantsova EN, Starodubova AV. The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines. 2023; 11(10):2749. https://doi.org/10.3390/biomedicines11102749
Chicago/Turabian StyleLeonov, Georgy E., Yurgita R. Varaeva, Elena N. Livantsova, and Antonina V. Starodubova. 2023. "The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review" Biomedicines 11, no. 10: 2749. https://doi.org/10.3390/biomedicines11102749
APA StyleLeonov, G. E., Varaeva, Y. R., Livantsova, E. N., & Starodubova, A. V. (2023). The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines, 11(10), 2749. https://doi.org/10.3390/biomedicines11102749





