Abstract
The successful development of nonviral delivery systems for nucleic acids has been reported extensively over the past years. Increasingly employed to improve the delivery efficiency and therapeutic efficacy of RNA are lipid nanoparticles (LNPs). Many of the various critical formulation parameters can affect the quality attributes and effectiveness of these nano-formulations. Therefore, the systematic drug development approach (QbD) and multivariate design and statistical analysis (DOE) can be very helpful and recommended for the optimization of the composition and production of RNA–LNPs. This review addresses the concepts and applications of QbD and/or DOE for the development of lipid nanoparticles for the delivery of different types of RNA, reporting examples published in the ten recent years presenting the latest trends and regulatory requirements as well as the modern mathematical and statistical design methods. As the topic explored in this review is a novel approach, the full QbD has been described in only a few papers, and a few refer only to some aspects of QbD. In contrast, the DOE approach has been used in most of the optimization works. Different approaches and innovations in DOE have been observed. Traditional statistical tests and modeling (ANOVA, regression analysis) are slowly being replaced by artificial intelligence and machine learning methods.
1. Introduction
In recent years, important developments in the pharmaceutical industry have occurred. Modern systems have been developed to ensure product quality in manufacturing information, quality management systems, and risk management [,,]. These tools allow drug manufacturers to detect, analyze, correct, and prevent problems while continuously improving the drug manufacturing process [,,]. Supporting this modernization in the rules used to manage drug manufacturing and product quality, in 2002, the FDA introduced the regulatory environment of the Current Good Manufacturing Practices (cGMP) [,].
After the publication of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use considerations (ICH) guidelines Q8 (R2), Q9, Q10, and Q11 [,,,], regulatory agencies (Food and Drug Administration—FDA and European Medicines Agency—EMA) approved the Quality by Design (QbD) approach. The ICH Q8 guideline recommended the integration of the “quality by design (QbD)” concept into the pharmaceutical industry [].
The development of safe and efficient new drugs is a long, difficult, and expensive process. Using the new QbD approach, which ensures that the quality is provided by manufacturing instead of by-product testing, decreases the costs of product optimization. QbD studies may provide some income to the drug manufacturers and benefits for patient safety—ensuring that they will obtain higher-quality drugs in a shorter time.
As defined by ICH Q8 [], QbD is a systematic drug development approach based on predefined goals, an understanding of the product and processes, and sound science and quality risk management.
In recent decades, research related to nanosystems has started a technological revolution in medicines, especially in RNA delivery. Some researchers apply QbD in the development of drug RNA-LNP systems [,,,,,,,,,,,,,,,,].
This review summarized the QbD concepts and applications to the development of pharmaceutical products containing RNA-LNPs. The second section describes the concepts of QbD, pharmaceutical QbD implementation, risk assessment, design of experiment (DoE), and process analytical technology (PAT). Afterward, the use of the QbD and DOE approach in the development of lipid nanoparticles is shown through examples of works published in the last 10 years.
2. Quality by Design Concept
Generally, the steps for implementing QbD in the development of pharmaceutical products are similar and include (Figure 1) the use of risk assessment to identify risk parameters, defining the main quality target product profile (QTTP), identifying parameters that influence the process performance, carrying out a DOE and definition of dependency of critical quality attributes (CQAs) to critical material attributes (CMAs) and critical process parameters (CPPs), defining a process design space that originates a final product with the desired QTPP, developing a risk control strategy to identify the causes of variability, and continuous monitoring and improving the manufacturing process [,,,,,].
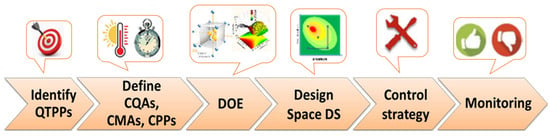
Figure 1.
Scheme of quality by design for a development pharmaceutical product.
2.1. Quality Target Product Profile (QTTP)
A quality target product profile (QTPP) consists of quantitative support for clinical safety and efficiency, and it makes a foundation to design and optimize a formulation or manufacturing process. The QTPP is a set of the attributes and characteristics of a product that determine its quality. According to the FDA, the QTPP is more focused on the chemical, manufacturing, and control stages of development [,].
The QTPP defines the quantitative characteristic of the drug and refers to specifications such as the dosage form, application way, packing, appearance, and diagnosis. Examples of QTPP are clinical use, administration route, therapeutic dosage, pharmaceutical dosage form, drug delivery system, packing container, factors affecting pharmacokinetic parameters, and quality criteria of the final product, such as stability during storage, sterility, and drug release. The QTPP only includes the product properties related to the patient. QTPP served as a guide for the optimization of formulation and process parameters to ensure that CQAs were within the desired range [,,,,,].
2.2. Critical Quality Attributes (CQA)
CQAs are physical, chemical, biological, or microbiological attributes/characteristics that should be controlled to ensure product quality []. CQAs are parameters that affect QTPP and are critical to product quality. These are generally related to the selection of correct amounts of excipients and drugs [,,,,,].
2.3. Critical Process Parameters (CPP)
CPPs are production parameters that affect CQAs and should be controlled. For example, parameters that interfere with the quality of the final product should be monitored during the production process. A parameter represents a measured or calculated characteristic of a system or process. These are parameters of the manufacturing system and are usually properties of materials or processes that affect production, such as temperature and composition.
2.4. Critical Material Attributes (CMAs)
CMAs are properties of materials that must reach adequate limits to guarantee the quality of excipients, drugs, and other materials used during the process.
It is important to understand the difference between CQAs (output data) and CMAs (input data) during development. For example, CQAs of an intermediate may become CMAs of an intermediate in the next production step. Moreover, based on the QbD approach, CMAs and CPPs may differ in the defined design space without significantly interfering with CQAs. Additionally, following the ICH guideline “Quality Risk Management” (ICH Q9), the identification of possible causes of process variability and effective risk analysis is of key importance during process optimization. This procedure can be performed in the initial or final steps, repeating or redefining it as needed. Thus, the quality of the final product is verified by the previous experience in the determination of CQAs, CPPs, and risk assessment [,,].
2.5. Risk Assessment
Risk assessment is used to identify what can go wrong, the likelihood of it going wrong, and the consequences. According to ICH Q9, risk control is related to procedures that should be adopted to reduce risks []. This guidance provides examples of commonly used risk management tools. At this stage, it is necessary to decide which risks can be accepted and which should be minimized. Analysis of variance (ANOVA) or multiple regression analysis is used for risk assessment based on experimental data. A mathematical equation for the relationship between variables can be derived using multiple regression analysis. While using ANOVA, the statistical significance of the influence of each factor and interaction effects is assessed []. In the final stage, the results should be monitored using a risk assessment, taking into account previous knowledge and experience. Identifying QTPP, CQA, and CPP requires prior experience and knowledge as various risk assessment tools are used, including risk filtering, Ishikawa diagram, and failure mode and effects analysis [,].
2.6. Design Space (DS)
The design space (DS) has an important place in the pharmaceutical industry. The design space defines the multivariate functional relationships between CQA and CPP and includes their relationships. These relationships can be found by applying risk assessment, design of experiments (DOE), and modeling.
The design space is specific to a unit operation or a single manufacturing process and defines operating process parameters (such as moisture ratio) that are known to affect product quality. DS can also be considered as a link between CQA and CPP []. It is a way to show the development of understanding of a process, and the benefits of creating a design space are obvious. However, one of the challenges for using DS effectively is the cost of creating it. The most important point in developing the design space is to demonstrate or determine that the unclassified parameters excluded from the DOE are non-critical process parameters and, therefore, do not interact. Therefore, DOE screening can be used to identify interactions between process parameters. In the absence of interaction, single-variable intervals can be directly added to the design space as non-critical parameters.
2.7. Control Strategy
A control strategy is a very important step to secure the process performance and product quality. It should be carefully planned based on product and process information []. In the QbD approach, a control strategy is performed during product development and allows a deeper understanding of the product and process. Control strategy options with QbD are easier for areas requiring more time and specialty knowledge by delivering more information than the standard approach. In this novelty approach to pharmaceutical quality, the finished product quality is built by identifying and controlling an optimal range of formulation and manufacturing variables. Finished products are finally tested to confirm the product quality [].
The development of an effective control strategy should be risk-based to ensure that product quality requirements are met. The development of the control strategy starts with the QTPP. The first studies are focused on the characterization of the active ingredient and important physical, chemical, biological, and microbiological properties of the formulation. The development of the process is also defined in this phase. For example, if the active ingredient has low solubility in water, the immediate-release tablet form must ensure sufficient dissolution of the drug. Toxicity studies performed during the early stages provide a baseline assessment of the impurity profile of the active ingredient. Understanding impurity generation and removal, at which stages elements of the control strategy will take place, and the development of acceptance criteria and methods to be included in the specifications will provide an important point of view. Although a control strategy should be developed using these steps, it can also be developed using the quality risk management principles defined in ICH Q9 [,].
During production, the continuous control strategy enables understanding and stabilization of the process. It is very important to modify the pharmaceutical quality system for supporting to the use of a QbD approach in the control strategy. In the commercial manufacturing process, it should be made possible to update, modify, or continuously improve the control strategy. When product information, documents, and operating procedures must be changed, the pharmaceutical quality system should provide the appropriate technical and administrative audits required to obtain the necessary reviews and approvals [].
3. QbD and DOE in the Development of the RNA-LNP System
QbD implementation in the development of pharmaceutical products containing RNA-LNPs is closely connected to the structure (Figure 2) and the main therapeutic goal of the nanosystem.
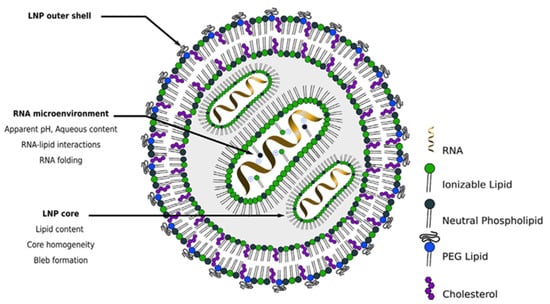
Figure 2.
Scheme of the hypothetical structure of an RNA-LNP system (adapted from []).
The definition of the main steps of the QbD approach is similar to different published research (see Table 1) and includes generally the following parameters []:

Table 1.
Optimization of RNA-LNP system information about optimizing variables and the tested range was described in the Section DOE Approach—Design Models, Variables, and Range.
- QTPP: safety and efficacy.
- CQAs: z-average, PDI, zeta potential, encapsulation efficiency, loading efficiency, stability, shelf life, storage conditions, and lipids contents.
- CMAs: N/P ratio, lipids type, non-toxicity, biodegradability.
- Critical process parameters (CPPs): temperature, microfluidics, filtration.
In the DOE, a wide range of different statistical approaches and modeling methods were used.
The following table shows examples of recent studies using the QbD and/or DOE approach to optimize formulations containing RNA-LNPs (Table 1). Particular information about optimized variables and their tested range applied in the DOE approach was described in Section DOE Approach—Design Models, Variables, and Range. More detailed results of these papers are shown in Supplementary Materials (Table S1).
DOE Approach—Design Models, Variables, and Range
In the first paper listed in Table 1, Cun J. et al. [] applied 2(5−1) fractional factorial design (FFD)—five input variables with a center point (each on the three levels): inner water phase and the oil phase volume ratio (0.1; 0.3; 0.5), poly(DL-lactide-glycolide acid) (PLGA) concentration (20.0; 40.0; 60.0 mg/mL), sonication time for the primary emulsification (30.0; 60.0; 90.0 s), siRNA load (35.9; 62.8; 89.7 µg), acetylated bovine serum albumin (Ac-BSA) content in the inner water phase (0.0; 200; 400 mg). Optimized variables were encapsulation efficiency (%EE) of the siRNA, obtained in the range of 2.01% to 51.18%, and particle size in the range of 218.3 nm (standard deviation, SD (n = 3) = 0.7 nm) to 257.9 nm (SD = 2.7 nm). Optimal values of significant input parameters are volume ratio 0.46, PLGA concentration 40.7 mg/mL, siRNA load 39 µg, acetylated bovine serum albumin (Ac-BSA) content in the inner water phase 400 µg for low siRNA load (770 ng/mg nanoparticles, SD = 33 ng/mg nanoparticles), volume ratio 0.40, PLGA concentration 42.4 mg/mL, siRNA load 90 µg, acetylated bovine serum albumin (Ac-BSA) content in the inner water phase 400 µg for high siRNA load (2192 ng/mg nanoparticles, SD = 115 ng/mg nanoparticles). The optimal values of %EE were equal to 64.35% (SD = 2.78%) for low siRNA load and 70.63% (SD = 5.75%) for high siRNA load. The optimal average particle size was equal to about 260 nm both for low and high siRNA load. The zeta potentials, additionally measured, were all negative in the range of −45.5 mV to −37.5 mV.
Chen D. et al. [] optimized siRNA-LNP in terms of the structure of used cationic lipids. The matrix of 70 different compounds of cationic lipids was tested—5 different hydrocarbon chain lengths (C8–C14) and 14 different amine headgroups. Optimized output parameters were: hydrodynamic diameter of particles (obtained in the range of 50 to 130 nm), total siRNA concentration, the percentage of particle-entrapped siRNA and luciferase silencing in vitro, and factor VII silencing in vivo (C57BL/6 mice). The lipids composition of LNPs was 2.0 mg/mL of cationic lipid, 0.28 mg/mL of DSPC, 0.52 mg/mL of cholesterol, and 0.13 mg/mL of mPEG2000-DMG. The concentration of polynucleotide was 0.4 mg/mL in 10 mM citrate buffer, pH 3.0. The dose of LNPs for in vivo factor VII delivery was 1.0 mg/kg (siRNA/body weight), and volume injection was less than 200 μL into the animal. Two main dependencies were identified from the LNPs test: siRNA delivery efficiencies in vitro and in vivo for different amine headgroups are a function of the hydrocarbon chain length, the potency of in vitro did not change significantly with hydrocarbon chain length, the in vivo potency of LNPs decreased with increasing hydrophobicity of the cationic lipids. In addition, based on this optimized approach, seven novel materials with in vivo gene silencing potencies of >90% at a dose of 1.0 mg/kg in mice were discovered.
In turn, Li et al. [] carried out optimization of lipid-like nanoparticles (LLNs) composed with different derivatives of N1,N3,N5-tris(2-aminoethyl)benzene-1,3,5-tri carboxamide (TT) for mRNA delivery. In the first step, the correlation analysis was performed to choose the most optimal type of TT (seven different compounds were tested). The output variables were: particle size (obtained in the range of 99 nm (SD = 2 nm) to 178 nm (SD = 1 nm), polydispersity index (PDI < 0.2), zeta potential (all nanoparticles were positively charged), entrapment efficiency of mRNA (15–82%), delivery efficiency, and cytotoxicity of TT2-TT8 LLNs-FLuc mRNA in Hep3B cells. At a dose of 1.2 μg/mL of luciferase mRNA, LLNs with TT3 as components characterized statistically significantly higher expression of the firefly luciferase compared to other TT LLNs. A significant positive correlation was observed between transfection efficiency and entrapment efficiency. No significant correlation was noticed between transfection efficiency and particle size, surface charge, and cell viability. In the next steps, the content of other ingredients of TT3-LLNs was optimized. Two separate orthogonal experimental designs, L16(44), were performed for different ranges of tested parameters. Four input variables on four levels were considered. The first design concerned the TT3 mole ratio (30; 40; 50; 60), dioleoyl phosphatidylethanolamine (DOPE) mole ratio (1.25; 2.5; 5.0; 10.0), cholesterol mole ratio (18.5; 28.5; 38.5; 48.5), DMG-PEG2000 mole ratio (0.75; 1.5; 3.0; 6.0), and for the second, the TT3 mole ratio (10; 20; 30; 40), DOPE mole ratio (10.0; 20.0; 30.0; 40.0), cholesterol (Chol) mole ratio (25; 30; 35; 40), DMG-PEG2000 mole ratio (0.0; 0.03; 0.15; 0.75). In both cases, the mRNA delivery efficiency expressed as a relative intensity of luminescence was used as an output variable. In the first optimization, the efficiency was obtained in the range of 20 to 4663, and in the second, obtained in the range of 1917 to 101,863, depending on the composition of TT3-LLNs. The optimal composition of the formulation was TT3/DOPE/Chol/DMG-PEG2000: 20/30/40/0, but the particle size of these LLNs, with a low ratio of DMG-PEG2000, increased dramatically 5 h after. Whereas for formulation TT3/DOPE/Chol/DMG-PEG2000: 20/30/40/0.75 were stable for a minimum of 2 weeks.
In the next paper, Kauffman et al. [] carried out the optimization of the composition of LNPs Erythropoietin(EPO)-mRNA-loaded with C12-200. Six input variables were considered: C12-200:mRNA weight ratio, phospholipid type and phospholipid, C12-200, cholesterol, and PEG molar contents. The output variables were encapsulation efficiency (%EE), particle size, polydispersity index (PDI), and EPO serum concentration. In the first step, library A was built with a definitive screening design (14 experiments). The most optimal phospholipid type was chosen as a matrix of qualitative variables: tail group (DS, DO) and head group (PC, PE). Quantitative variables were on the three levels: C12-200:mRNA weight ratio (2.5; 5.0; 7.5), C12-200 %mol (40; 50; 60), phospholipid %mol (4; 10; 16), PEG %mol (0.5; 1.5; 2.5), and cholesterol %mol was added to 100% (21.5–55.50). DSPC and DOPE were selected for further optimization. Within library B, Taguchi fractional factorial screening design (18 experiments) was performed with the following input variables: C12-200:mRNA weight ratio (7.5, 10.0, 12.5), C12-200 %mol (30, 35, 40), phospholipid %mol (16, 22, 28), PEG %mol (2.5, 3.0), and cholesterol %mol (from 28.5 to 51.5). The DOPE phospholipid was selected as the most optimal. In the final step (library C, 6 experiments), the C12-200:mRNA weight ratio was optimized in the range of 5.0; 7.5; 10.0; 15.0; 20.0; 25.0. The optimal composition of the formulation was 10:1 C12-200:mRNA weight ratio, 35% C12-200, 16% DOPE, 46.5% cholesterol, and 2.5% C14-PEG2000. The average efficacy with 15 µg total EPO mRNA injection in vivo was obtained at 7065 ± 513 ng/µL. The particle size of optimal LNP was 102 nm, PDI: 0.158, encapsulation efficiency: 43%, pKa: 6.9, and zeta potential: −5.0 mV.
Next, Thanki et al. [] tested the lipidoids as the lipid component of siRNA-loaded lipid-polymer hybrid nanoparticles (LPNs). They checked whether replacing the cationic lipids with lipidoids improved safety and was more efficacious (efficient gene silencing at lower doses). The initial experiments of one factor at a time were performed to identify criticality influencing the overall quality attributes of the LPNs: the lipidoid content and siRNA: lipidoid ratio. A 17-run design of an experiment with an I-optimal approach was performed to systematically assess the effect of lipidoid content % (in the range of 10–20) and weight ratio of lipidoid:siRNA (from 10:1 to 20:1) on physicochemical properties (hydrodynamic size, zeta potential, and siRNA encapsulation/loading) and the biological performance (in vitro gene silencing and cell viability). The response surface methodology was applied to the identification of optimal operating space (OOS). The optimal lipidoid-modified LPNs showed more than 50 times higher in vitro gene silencing at well-tolerated doses and about twice the increase in siRNA loading than the LPNs with cationic lipid dioleyltrimethylammonium propane (DOTAP). Another work by Thanki and co-authors [] described the optimization of LPNs composition prepared of cationic lipidoid (L5) and poly(DL-lactic-co-glycolic acid) (PLGA) or delivery of an antisense oligonucleotide (ASO) mediating splice correction of a luciferase gene transcript (Luc-ASO). Critical formulation variables and their levels were identified in one-factor-at-a-time (OFAT) experiments. Multilevel factorial design (25 experiments) was performed for critical, independent variables with three levels: L5 content (10–20% (w/w)), L5:Luc-ASO weight ratio (in the range of 10:1 to 30:1), and molecular weight of PLGA (10–50 kDa). The following output variables were considered: Z-average, PDI, zeta potential, encapsulation efficiency, loading, and biological responses (i.e., in vitro splice correction efficiency and cell viability). Quadratic and an I-optimal model were performed, and the obtained responses were subjected to model fitting using analysis of variance (ANOVA). The best model was selected on the basis of statistical analysis. The process validation was performed by preparing and testing the five formulations with composition within the design space (range of 14–17% (w/w) L5 content and L5:Luc-ASO ratios from 11:1 to 21:1).
In turn, Blakney et al. [] presented the optimization of self-amplifying mRNA (saRNA) lipid nanoparticle composition in human skin explants. Full factorial design was performed for five input variables: complexing lipid type (C12-200 (ionizable), dimethyldioctadecylammonium bromide [(DDA), cationic], 1,2-dioleoyl-3-trimethylammonium-propane [(DOTAP), cationic] and cephalin (zwitterionic)), total lipid to RNA ratio (w/w) (1:1, 4:1, 18:1, 90:1), lipid concentration (high (7.5 mg/mL), medium, low), particle concentration (high 109, medium 108, low 107 particles/mL), and cationic lipid to zwitterionic lipid ratio (0.1:1, 1:1, 10:1). The luciferase expression in human skin explants after 10 days was the output variable. Cephalin lipid had the strongest effect. Cephalin LNPs with a ratio of total lipids to RNA of 18:1 (w/w), low lipid concentration, and medium particle concentration yielded a 7-fold increase in luciferase expression over the original formulation (with C12-200 lipid).
In the next paper, Hashiba et al. [] described the optimization of liver-targeted mRNA-loaded LNPs prepared with pH-sensitive cationic lipids that had been previously designed for siRNA delivery. The first 34 × 22 definitive screening design (DSD, 18 formulations) was performed (library A). The input variables were qualitative factors: cationic lipid (CL) type (CL4H6, CL15H6) and phospholipid (PL) type (DSPC, DOPE) and quantitative factors: mRNA: lipid ratio (18.3–36.7), mol% of CL (40–60), mol% of PL (5–25), and mol% of PEG-DMG (0.5–2.5). The second step, a 34 × 21 Taguchi fractional factorial design (FFD, 18 formulations), was performed (Library B). The input variables were the qualitative factor phospholipid (PL) type (DSPC, ESM—egg sphingomyelin) and quantitative factors: mol% of CL (50–70), mol% of PL (5–15), and mol% of PEG-DMG (0.5–2.5). The cationic lipid (CL) type was fixed as a CL4H6, and the mRNA/lipid ratio was fixed as 18.3. Output variables were gene expression in the liver (Nluc expression was measured 24 h after the injection), physicochemical properties (LNP diameter and polydispersity index (PdI)), and liver-specificity (gene expression in off-target organs (spleen, lung, and kidney)). Detailed results are described in Supplementary Table S1.
The work of Lokras et al. [] presented an approach to optimization of the intracellular delivery of therapeutic anti-inflammatory TNF-a siRNA-loaded LNPs to activated macrophages. The formulation design space was defined based on the I-optimal design for three independent formulation batches and three technical replicates. Input variables were L5 content (15; 20; 25% (w/w)) and L5:TNF-a siRNA weight ratio (5.0:1; 7.5:1; 10.0:1; 15.0:1). Output variables were PDI, zeta potential (mV), encapsulation efficiency (%), TNF-a siRNA loading (μg siRNA/mg LPNs), and fold reduction in IC50 value for TNF-a gene silencing. The optimal operating space was defined: L5 content of 15%, L5:TNF-a siRNA weight ratio of 13.5:1, and 25%, 15.0:1 respectively.
The next paper, published by Zheng et al. [], deals with the optimization of lipid-like nanoparticles (LLNs) on the expression of luciferase mRNA in the liver containing three new cholesterol derivatives Chol-PEG400-self peptide (Chol-PEG400-SP), Chol-PEG400-Mannose (Chol-PEG400-Man), and Chol-PEG2000-W5R4K (Chol-PEG2000-WRK)) to achieve the liver-targeting delivery of mRNA. The central composite design (CCD) model (20 experiments), a multi-factor, five-level experimental design, which is formed by adding extreme points and center points based on a two-level factorial design, was investigated. Input variables were the molar ratio of the Chol-PEG400-SP (from 1.0 to 7.5%), Chol-PEG400-Man (from 1.0 to 10.0%), and Chol-PEG2000-WRK (from 1.0 to 7.5%). The output variable was in vivo Luc expression of livers measured by the IVIS (Balb/c mice i.v. injection)—bioluminescence.
Van de Berg and co-authors [], in turn, described the bioprocess model development of rapid RNA vaccine production against emerging infectious diseases. The model parameters were fitted to a subset of 51 experimental samples from a statistical DoE dataset obtained from lab-scale saRNA synthesis experiments using wild-type nucleotide triphosphate (NTP). Thirty-three samples correspond to the RNA yield at 0.04 M NTP and 1 × 10−8 M of T7 RNA Polymerase (T7RNAP) vs. 11 concentrations of Mg ranging from 0.025 to 0.125 M after 2, 4, and 6 h. Moreover, 12 samples correspond to the RNA yield at 0.04 M NTP and 0.075 M Mg for 1.25 × 10−9, 2.5 × 10−9, 5 × 10−9, and 1 × 10−8 M of T7RNAP after 2, 4, and 6 h. six samples correspond to the RNA yield after 2 h at 0.02, 0.04, and 0.08 M NTP at 0.075 and 0.14 M Mg. Optimal values of input variables were NTP concentration 40.8 mM (optimization range 0.01–0.06M), T7RNAP concentration 1.5 × 10−8 M (optimization range 0.5 × 10−8–1.5 × 10−8), Mg concentration 85 mM (optimization 0.01–0.09 M), and reaction time: after 2, 4, and 6 h. The optimal output variables were the yield in a bioreactor: 4.34 g × L−1, and the cost of T7RNAP and NTPs per g of RNA: 2740 USD × g−1.
In the paper by Fan et al. [], the optimization of LNPs loaded with antisense oligonucleotides (ASOs), formulated by an automated solvent-injection method using a robotic liquid handler (e.g., muscular dystrophy Duchenne’s) was performed. In the first step, optimization of the phase mixing process using a robotic TECAN liquid handler was carried out. Input variables were investigated as an injection speed: 0.1, 0.5, or 0.9 mL/s at 10 mixing repeats, and the ethanol-to-buffer injection at a speed of 0.5 or 0.9 mL/s followed by 10 or 20 mixing repeats. Output variables were particle size, polydispersity, and encapsulation efficiency (calculated based on free ASO-1 measured by OD260). The second step involved the high throughput screening (HTS) workflow for ASO-loaded LNP formulations (96 samples). Input variables were two levels of total lipid concentrations (1 mM; 2 mM), four levels of ASO loading controlled by N/P ratios (0.5; 1; 2; 5), and four levels of the PEGylated lipid (DSPE-PEG2000) content (0; 1.5; 3; 5 %mol of total 2 mM lipids). Output variables were particle size (obtained in the range of 45–145 nm), polydispersity %PD (10–50), and ASO encapsulation efficiency measured by absorbance at 260 nm. We observed that 5 mol% of DSPE-PEG2000 and an N/P ratio ≥ 1 would produce optimal LNP formulations with a homogeneous and stable particle size as well as high ASO loading. Other detailed results are summarized in Table S1.
Terada et al. [] described the DOE approach to find the preparation conditions and their relationship with the properties of LNP-siRNA. The design included three levels of the three-factor Box–Behnken design: low, center, and high, and three center samples (15 preparation). The influence of the input variables: lipid concentration (10, 20, 30 mM), flow rate ratio (FRR) (1, 3, 5 (v/v)), and total flow rate (TFR) (1, 2, 3 mL/min) on the output parameters: the particle size, polydispersity index (PDI), and siRNA entrapment, was tested. The charge ratio (N/P) in the prepared LNP-siRNA systems was fixed at 3. LNPs were prepared using hydrogenated soy phosphatidylcholine (HSPC), cholesterol (Chol), and 1,2-distearoyl-sn-glycero-3-phosphoethanol amine-N-[methoxy-(polyethylene glycol)-2000] (ammonium salt) (PEG-DSPE). In addition, 1,2-dioleoyl-3-dimethylammonium propane (DODAP) was used which has a reported apparent pKa of 6.5. The optimal lipid composition was DODAP/Chol/HSPC/PEG-DSPE (50/10/39/1 %mol).
The next authors, Bevers et al. [], optimized mRNA-LNP compositions to achieve high-magnitude tumor-specific CD8 T cell antitumor immunotherapy by engaging the splenic immune cells. A Roquemore hybrid design was used to select the formulations to be tested (11 different experimental conditions). Each condition was applied in triplicate (3 mice /treatment), and each lipid’s molar percentage takes five values. The experimental protocol was carried out for three different choices of PEG-lipid chemistry (DMG-PEG2000, DSG-PEG2000, and DSPE-PEG1000) and different lipid molar ratios (10, 20, and 30 mM). The highest %E7-specfic T cell response was investigated as an output variable. The optimal LNPs composition were: DMG-PEG2000: ionizable lipid 56.5%, DOPE 5.25%, cholesterol 37.75%, PEG-lipid 0.5%, and DSG-PEG2000: ionizable lipid 64.4%, DOPE 8%, cholesterol 27.1%, PEG-lipid 0.5%. More detailed relationships between variables are presented in Table S1.
Karl et al. [], in turn, proposed a holistic workflow for lipid nanoparticle (LNP) formulation optimization for in vivo gene delivery systems. They used designed mixture-process experiments and a self-validated ensemble model (SVEM) (23 runs, LNP batches were tested). The generally used input variables were listed and presented on the fishbone diagram, e.g., ionizable lipid type (H101, H102, H103), ionizable lipid molar content (10–60%), helper lipid type, helper lipid molar content (10–60%), PEG lipid type, PEG lipid molar content (10–50%), N/P ratio (6–14), buffer concentration, and flow rate (1–3 mL/min). Output variables were maximum potency, minimum particle size, minimum PDI (polydispersity index), maximum % encapsulation, and minimum side effects—such as body weight loss in in vivo studies. Exemplary optimal LNP formulation candidates were described in Table S1.
The paper by Schmidt et al. [] refers to a general approach to process automation and control strategy by QbD in total continuous mRNA manufacturing platforms (mRNA)-based vaccines. The work contains very detailed information about all aspects of LNP-mRNA formation, process parameters, and product composition based on risk analysis. The authors presented, e.g., Ishikawa diagrams to identify all possible sources of variability. Different multivariate optimization prediction was comparable: Ordinary least squares (OLS) regression, partial least squares (PLS) regression, and neural network (NN) regression. Full factorial design and one factor at a time (OFAT) analysis were also used. The optimal process and product parameters are listed in Table S1.
In the next paper, Toma et al. [] presented a general QbD approach for the development of miRNA nonviral vectors for genetic material delivery in cancer therapy. The DOE is defined generally as providing better results with a minimum number of experiments and evaluating CMAs and CPPs to obtain a product meeting the QTPP.
Young and co-authors [] described that optimization of LNP composition drives the delivery of mRNA to the placenta. In the DOE approach, a factorial design study was performed. The definitive screening design (DSD) was used to create a mini-library of 18 chemically unique LNPs (A1–A18). A combination of three-level continuous and two-level categorical factors to identify linear and quadratic effects was used: ionizable lipid type (C12-200, DLin-MC3-DMA), phospholipid type (DSPC, DOPE), ionizable lipid molar percentage (25–45%), phospholipid molar percentage (10–22%), DMPE-PEG molar percentage (1.5–3.5%), and cholesterol molar percentage (add up to 100%). Output variables were hydrodynamic diameter (obtained in the range of 72.2–171.5 nm), polydispersity index (0.120–0.317), mRNA encapsulation efficiency (35.6–83.2%), transfection efficiency, and apparent pKa (TNS assay) (5.298 to 7.111). Optimal LNP was formulation A10 with the following composition and characterization: 35% C12-200, 10% DOPE, 1.5% PEG, 53.5% cholesterol; 130.2 nm hydrodynamic diameter, 0.064 PDI, 56.5% EE, 6.607 pKa.
The next paper, by Nag et al. [], presented a novel approach to size regulation of LNPs using the combined effects of buffer exchanger pH and sonication time. Input variables were: pH of buffer exchanger (4.0–5.0), sonication time (0–100 s), buffer dialysis type (50 mM HEPES pH 6.7, 50 mM HEPES/50 mM sodium acetate pH 6.7, PBS pH 7.2, PBS pH 7.4), and % of dialysis buffer (75–100%). Optimized parameters were LNP size (nm) and polydispersity index (PDI). Optimal values of LNP diameter were 60–180 nm (±10 nm) and PDI ≤ 0.200.
DOE approach to the optimization of lipid nanoparticles for self-amplifying mRNA (saRNA) expression and cellular activation was presented by Ly et al. []. The first optimization was performed based on a definitive screening design (DSD). A 7 three-level matrix was built with two two-level qualitative factors and one three-level qualitative factor (iteration A, 26 formulations). Input variables were: N/P ratio (5; 10; 15), phospholipid type: DOPE; DSPC phospholipid content (10; 15; 20 mol %), ionizable lipid type (pKa) (DLin-MC3-DMA (6.4); ALC-0315 (6.09); SM-102 (6.75)), ionizable lipid content (30; 40; 50 %mol), DMG-PEG-2000 content (0; 1.25; 2.5 %mol) total flow rate during preparation (2; 9; 16 mL/min), ambient temperature during formulation (4; 20; 37 °C), aqueous-phase pH (3; 5; 7), and RNA type (mRNA; saRNA). Output variables were size, PDI, EE%, charge, % filled particles, and % full RNA transcripts. Apart from standard statistical methods such as variance analysis (ANOVA), linear regression, and Spearman’s correlation, Python’s scikit-learn module, statsmodels package, and the seaborn library were also used. The second optimization was performed based on a Box–Behnken design (BBD). Three three-level and 1 three-level quantitative factor (iteration B, 26 formulation) were tested. Input variables were phospholipid content (12.5; 15; 17.5 %mol), ionizable lipid type (pKa) (DLin-MC3-DMA (6.4); ALC-0315 (6.09); SM-102 (6.75)), ionizable lipid content (35; 40; 45 %mol), and aqueous-phase pH (4; 5; 6). The N/P ratio was fixed at 10, DOPE was used as a phospholipid, DMG-PEG-2000 content was fixed at 1.25 %mol, the total flow rate was fixed at 16 mL/min, and ambient temperature during formulation was fixed at 20 °C). Output variables were size, PDI, EE%, charge, % filled particles, % full RNA transcripts, protein expression, and cellular activation. Response surface modeling was carried out using second-order ordinary least square (OLS) regression based on the scikit-learn module and statsmodels package. A Box–Cox transformation of response variables was used to improve model accuracy. The optimization of simultaneous responses was realized by the desirability function, and optimal operating conditions were obtained using the SciPy 1.0 library and Broyden–Fletcher–Goldfarb–Shanno (BFGS) optimization algorithm.
The paper by Mendonca et al. [] presented a review of LNP delivery of different types of nucleic acids: siRNA, mRNA, and pDNA. Stricted DOE was not described, but a lot of important variables that affected the effectiveness of LNP formulations were listed (Table S1).
The last presented work by Bastogne et al. [] concerns the optimization of cationic nano-lipid for siRNA transfection. DOE was performed based on a D-optimal mixture design (36 formulations were tested). Input variables were DOTAP proportion (%) in the LNP content, the concentration of PEG surfactant (%), the lecithin proportion (%), and LNP size (small, medium, large). Output variables were safety attributes related to the LNP stability (unstable or stable states, 0 or 1), siRNA transfection rate (PC3-GFP) (min 30%), and PDI. Response surface equations, a class of polynomial models, are used to describe the links between the input and output variables. Bayesian estimation method, the posterior predictive check, and the leave-one-out cross-correlation were used to evaluate the model prediction.
4. Discussion
Optimization of lipid-based RNA formulations is essential to achieve reproducible quality of pharmaceutical products in terms of efficacy and safety; therefore, the application of QbD and DOE appears to be a very useful tool for the robust development of this complex RNA-LNP system. The performance of LNPs is strongly influenced by the chemical structure of each component, the interactions between them, and the physicochemical properties of the final formulation.
The most important characteristics of RNA-LNPs are zeta potential, size and particle size distribution, shape, morphology, encapsulation efficiency, cellular uptake, and transfection efficiency. The chemical structure of lipids, lipid concentration, and lipid molar ratio were the most significant factors regulating formulation average and gene expression [,,,,]. Cationic lipids for gene transfer have become a major research tool for the transfer of genetic material into cells, and there is great potential for progress in this direction [].
Particle size was retained as one parameter of QTPP as it is widely known to affect pharmacokinetics, tissue distribution, tissue extravasation, uptake and/or accumulation in clearance organs []. The particle size of formulation LNPs depends most on the composition of lipids. In general, the presence of PEG improved particle stability and reduced size but hindered delivery efficiency [,,,]. Particle size and PDI decrease with increasing RNA: ionizable lipid ratio due to higher RNA concentrations and concomitantly reduced amount of ionizable lipid [,].
Another parameter characteristic of RNA-LNP is the zeta potential, which also depends on the lipid type and concentration. In general, QTPP for zeta potential was set to >0 mV because nanoparticles with positive zeta potential enhanced interactions with the plasma membrane. Positively charged nanoparticles can encapsulate negatively charged genetic material by electrostatic interaction []. Depending on the composition and especially the modifications of the end groups (acid, amine, or esters), LNP particles can have a negative [,,] or a positive charge [,,]. Zeta potential increased proportionally as a function of ionizable lipid content and RNA: ionizable lipid ratio [,,]. Zeta potential showed a significant correlation with transfection efficiency [,,].
Another important CQA is entrapment efficiency, measured as an encapsulation efficiency. Encapsulation efficiency is more dependent on the LPN preparation process rather than independent variables [,]. No statistically significant differences in encapsulation efficiency were observed with particle size and PDI [] but increased with higher RNA: ionizable lipid ratio [,,,].
All of the above particle properties contribute to biological efficacy but are not always the same in vitro and in vivo studies. For example, the efficacy of lipoplex increased with increasing hydrocarbon chain length, whereas the in vitro efficacy of LNP did not change significantly []. In vivo, the efficacy of LNP decreased with increasing hydrophobicity of cationic lipids []. The presence of the phospholipid DOPE was generally the strongest predictor of in vivo efficacy [,,]. The specific combination of ionizable lipid and phospholipid in the LNP design provides high transfection efficiency in vitro [].
The DOE approach has been used in most of the optimization works; however, the full QbD approach has been described in only a few papers [,,,,], and a few articles refer only to some aspects of QbD—the definition of QTPP and CQA [,,,,].
We have seen different approaches and innovations in DOE and statistical analysis. Traditional statistical tests and modeling based on ANOVA and regression analysis are slowly being replaced by artificial intelligence and machine learning methods, e.g., neural networks []. From a methodological point of view, the most interesting new development in pharmacy is the development of self-validated ensemble models (SVEM), especially with the connection of mixture design [].
5. Conclusions
Over the past 10 years, many research articles have described the development of studies of RNA-loaded lipid nanoparticles for various clinical purposes, e.g., prophylaxis of infectious diseases, treatment of rare diseases, and gene, cancer, and protein replacement therapy. In addition, the coronavirus disease 2019 (COVID-19) pandemic and the emergence of safe and effective RNA vaccines have brought RNA technology to the forefront of medical innovation. Due to the mechanism of action, the therapeutic scope of RNA technology is wide, and the manufacturing processes are versatile. Different products could be produced using the same raw materials, consumables, equipment, unit operations, and analytical methods. In parallel with the development of new drug delivery methods, the QbD approach is becoming increasingly widespread in pharmaceutical manufacturing.
This review could be very useful for researchers and pharmaceutical manufacturers to apply the quality by design approach to the development of lipid nanosystems loaded with different types of RNA, following the latest trends and regulatory requirements and using modern mathematical and statistical design methods.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines11102752/s1, Table S1: Optimization of RNA-LNP system using the QbD and/or DOE approach—summary and results.
Author Contributions
Conceptualization, L.G.-B. and W.M.; methodology, L.G.-B. and D.A.S.; software, L.G.-B.; formal analysis, L.G.-B. and W.M.; investigation L.G.-B. and D.A.S.; resources, D.A.S. and K.D. and M.W.; data curation, W.M.; writing—original draft preparation, L.G.-B.; writing—review and editing, L.G.-B. and W.M.; visualization, L.G.-B.; supervision, W.M. and K.D.; project administration, K.D.; funding acquisition, K.D. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by Celon Pharma S.A. and the Medical Research No. 2021/ABM/05/00005 entitled “Development of Innovative Therapeutic Solutions using RNA technology (TransformRNA—mRNA Therapeutics generation platform)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All contributors to this work at the time of their direct involvement in the project were the full-time employees of Celon Pharma S.A. M. Wieczorek is the CEO of Celon Pharma S.A. Some of the authors are the shareholders of Celon Pharma S.A.
References
- Deidda, R.; Orlandini, S.; Hubert, P.; Hubert, C. Risk-based approach for method development in pharmaceutical quality control context: A critical review. J. Pharm. Biomed. Anal. 2018, 161, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Winkle, H. Quality by design for biopharmaceuticals. Nat. Biotechnol. 2009, 27, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2007, 25, 781–791. [Google Scholar] [CrossRef]
- Csoka, I.; Pallagi, E.; Paal, T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today 2018, 23, 1340–1343. [Google Scholar]
- Food and Drug Administration. Pharmaceutical cGMPs for the 21st Century—A Risk-Based Approach; Final Report; Food and Drug Administration: Silver Spring, MD, USA, 2004.
- Zhang, L.; Mao, S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- ICH. Q8(2R), Pharmaceutical Development; International Conference on Harmonization: London, UK, 2009. [Google Scholar]
- ICH. Q9, Quality Risk Management; International Conference on Harmonization: London, UK, 2005. [Google Scholar]
- ICH. Q10, Pharmaceutical Quality System; International Conference on Harmonization: London, UK, 2008. [Google Scholar]
- ICH. Q11, Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities); International Conference on Harmonization: London, UK, 2011. [Google Scholar]
- Simon, D.; Kis, Z.; Kontoravdi, C.; Shah, N. Quality by Design for enabling RNA platform production processes. Trends Biotechnol. 2022, 40, 1213–1228. [Google Scholar] [CrossRef]
- Ditzel, H.J.; Tuttolomondo, M.; Kauppinen, S. Design and Delivery of SiRNA Therapeutics; Humana Press: Hertfordshire, UK, 2021; Chapter 9; p. 139. [Google Scholar]
- Thanki, K.; Zeng, X.; Justesen, S.; Tejlmann, S.; Falkenberg, E.; Van Driessche, E.; Nielsen, H.M.; Franzyk, H.; Foged, C. Engineering of small interfering RNA-loaded lipidoid-poly(DL-Lactic-Co-Glycolic Acid) hybrid nanoparticles for highly efficient and safe gene silencing: A quality by design-based approach. Eur. J. Pharm. Biopharm. 2017, 120, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Papai, S.; Lokras, A.; Rose, F.; Falkenberg, E.; Franzyk, H.; Foged, C. Application of a Quality-By-Design Approach to Optimise Lipid-Polymer Hybrid Nanoparticles Loaded with a Splice-Correction Antisense Oligonucleotide: Maximising Loading and Intracellular Delivery. Pharm. Res. 2019, 36, 37. [Google Scholar] [CrossRef]
- Lokras, A.; Thakur, A.; Wadhwa, A.; Thanki, K.; Franzyk, H.; Foged, C. Optimizing the Intracellular Delivery of Therapeutic Anti-inflammatory TNF-a siRNA to Activated Macrophages Using Lipidoid-Polymer Hybrid Nanoparticles. Front. Bioeng. Biotechnol. 2021, 8, 601155. [Google Scholar] [CrossRef]
- Zheng, Q.; Qin, F.; Luo, R.; Jin, C.; Huang, H.; Xi, H.; Xiao, W.; Guo, M.; Yang, S.; He, S.; et al. mRNA-Loaded Lipid-Like Nanoparticles for Liver Base Editing Via the Optimization of Central Composite Design. Adv. Funct. Mater. 2021, 31, 2011068. [Google Scholar] [CrossRef]
- van de Berg, D.; Kis, Z.; Behmer, C.F.; Samnuan, K.; Blakney, A.K.; Kontoravdi, C.; Shattock, R.; Shah, N. Quality by design modelling to support rapid RNA vaccine production against emerging infectious diseases. NPJ Vaccines 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yen, C.W.; Lin, H.C.; Hou, W.; Estevez, A.; Sarode, A.; Goyon, A.; Bian, J.; Lin, J.; Koenig, S.G.; et al. Automated high-throughput preparation and characterization of oligonucleotide-loaded lipid nanoparticles. Int. J. Pharm. 2021, 599, 120392. [Google Scholar] [CrossRef] [PubMed]
- Bevers, S.; Kooijmans, S.A.A.; Van de Velde, E.; Evers, M.J.W.; Seghers, S.; Gitz-Francois, J.J.J.M.; van Kronenburg, N.C.H.; Fens, M.H.A.M.; Mastrobattista, E.; Hassler, L.; et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol. Ther. 2022, 30, 3078–3094. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.T.; Essex, A.S.; Wisnowski, J.; Rushing, H. A Workflow for Lipid Nanoparticle (LNP) Formulation Optimization Using Designed Mixture-Process Experiments and Self-Validated Ensemble Models (SVEM). arXiv 2022. [Google Scholar] [CrossRef]
- Schmidt, A.; Helgers, H.; Vetter, F.L.; Zobel-Roos, S.; Hengelbrock, A.; Strube, J. Process Automation and Control Strategy by Quality-by-Design in Total Continuous mRNA Manufacturing Platforms. Processes 2022, 10, 1783. [Google Scholar] [CrossRef]
- Toma, I.; Porfire, A.S.; Tefas, L.R.; Berindan-Neagoe, I.; Tomut, I. A Quality by Design Approach in Pharmaceutical Development of Non-Viral Vectors with a Focus on miRNA. Pharmaceutics 2022, 14, 1482. [Google Scholar] [CrossRef]
- Young, R.E.; Nelson, K.M.; Hofbauer, S.I.; Vijayakumar, T.; Alameh, M.G.; Weissman, D.; Papachristou, C.; Gleghorn, J.P.; Riley, R.S. Lipid Nanoparticle Composition Drives Delivery of mRNA to the Placenta. bioRxiv. 2022. [Google Scholar] [CrossRef]
- Nag, K.; Sarker, E.H.; Kumar, S.; Khan, H.; Chakraborty, S.; Islam, J.; Baray, J.C.; Khan, M.R.; Mahmud, A.; Barman, U.; et al. DoE-derived continuous and robust process for manufacturing of pharmaceutical-grade wide-range LNPs for RNA-vaccine/ drug delivery. Sci. Rep. 2022, 12, 9394. [Google Scholar] [CrossRef]
- Ly, H.H.; Daniel, S.; Soriano, S.K.V.; Kis, Z.; Blakney, A.K. Optimization of Lipid Nanoparticles for saRNA Expression and Cellular Activation Using a Design-of-Experiment Approach. Mol. Pharm. 2022, 19, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef] [PubMed]
- Bastogne, T.; Hassler, L.; Bruniaux, J.; Thomassin, M.; Gidrol, X.; Sulpice, E.; Navarro, F.P. A Bayesian implementation of Quality-by-Design for the development of Cationic Nano-Lipid for siRNA Transfection. IEEE Trans. NanoBiosci. 2023, 22, 455–466. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Kohli, K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov. Today 2019, 24, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Cuhna, S.; Costa, C.P.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. Nanomed. Nanotechnol. Biol. Med. 2020, 27, 102206. [Google Scholar] [CrossRef]
- Short, S.M.; Cogdill, R.P.; Drennen, J.K., III; Anderson, C.A. Performance-Based Quality Specifications: The Relationship Between Process Critical Control Parameters, Critical Quality Attributes and Clinical Performance. Eur. J. Pharm. Sci. 2010, 100, 1566–1575. [Google Scholar] [CrossRef]
- Aksu, B.; Mesut, B. Quality by design (QbD) for pharmaceutical area. J. Fac. Pharm. Istanbul. 2015, 45, 233–251. [Google Scholar]
- Cun, D.; Jensen, D.K.; Maltesen, M.J.; Bunker, M.; Whiteside, P.; Scurr, D.; Foged, C.; Nielsen, H.M. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: Quality by design optimization and characterization. Eur. J. Pharm. Biopharm. 2011, 77, 26–35. [Google Scholar] [CrossRef]
- Chen, D.; Love, K.T.; Chen, Y.; Eltoukhy, A.A.; Kastrup, C.; Sahay, G.; Jeon, A.; Dong, Y.; Whitehead, K.A.; Anderson, D.G. Rapid Discovery of Potent siRNA-Containing Lipid Nanoparticles Enabled by Controlled Microfluidic Formulation. J. Am. Chem. Soc. 2012, 134, 6948–6951. [Google Scholar] [CrossRef]
- Li, B.; Luo, X.; Deng, B.; Wang, J.; McComb, D.W.; Shi, Y.; Gaensler, K.M.L.; Tan, X.; Dunn Amy, L.; Kerlin, B.A.; et al. An Orthogonal Array Optimization of Lipid-like Nanoparticles for mRNA Delivery in Vivo. Nano Lett. 2015, 15, 8099–8107. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Yus, B.I.; Hunter, J.E.; Dex, E.A.; Shattock, R.J. The Skin You Are In: Design-of-Experiments Optimization of Lipid Nanoparticle Self-Amplifying RNA Formulations in Human Skin Explants. ACS Nano 2019, 13, 5920–5930. [Google Scholar] [CrossRef]
- Hashiba, A.; Toyooka, M.; Sato, Y.; Masatoshi, M.; Tokeshi, M.; Harashima, H. The use of design of experiments with multiple responses to determine optimal formulations for in vivo hepatic mRNA delivery. J. Control. Release 2020, 327, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Kulkarni, J.A.; Huynh, A.; Chen, S.; van der Meel, R.; Tam, Y.Y.C.; Cullis, P.R. Characterization of Lipid Nanoparticles Containing Ionizable Cationic Lipids Using Design-of-Experiments Approach. Langmuir 2021, 37, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).