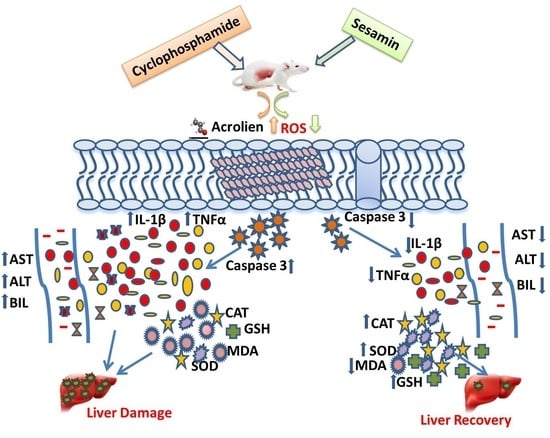
Sesamin’s Therapeutic Actions on Cyclophosphamide-Induced Hepatotoxicity, Molecular Mechanisms, and Histopathological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drug
2.2. Animal Models and Experimental Design
2.3. Biochemical Assay for Liver Functions
2.4. Inflammatory Cytokines and Apoptosis Marker Test
2.5. Oxidative Stress Assay for Liver Tissue
2.6. Histopathological Assessment
2.7. Protein Evaluation
2.8. Statistical Analysis
3. Results
3.1. Effects of Sesamin on Liver Weight and Liver Function Tests
3.2. Effects of Sesamin on MDA and Antioxidant Enzymes
3.3. Effects of Sesamin on Cytokines and Apoptotic Markers
3.4. Effects of Sesamin on Liver Architecture
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Małyszko, J.; Kozłowska, K.; Kozłowski, L.; Małyszko, J. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2017, 32, 924–936. [Google Scholar] [CrossRef]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology—strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Choti, M.A. Chemotherapy-associated hepatotoxicity, do we need to be concerned? Ann. Surg. Oncol. 2009, 16, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002, 65, 166–176. [Google Scholar] [CrossRef]
- King, P.D.; Perry, M.C. Hepatotoxicity of chemotherapy. Oncologist 2001, 6, 162–176. [Google Scholar] [CrossRef]
- Floyd, J.; Mirza, I.; Sachs, B.; Perry, M.C. Hepatotoxicity of chemotherapy. Semin. Oncol. 2006, 33, 50–67. [Google Scholar] [CrossRef]
- Grigorian, A.O.; Brien, C.B. Hepatotoxicity secondary to chemotherapy. J. Clin. Transl. Hepatol. 2014, 2, 95–102. [Google Scholar]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2, protective effect of 18β-Glycyrrhetinic acid. Chem. Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Valsala, G.A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Fan, D.; Yang, Z.; Yuan, Y.; Wu, Q.Q.; Xu, M.; Jin, Y.G.; Tang, Q.Z. Sesamin prevents apoptosis and inflammation after experimental myocardial infarction by JNK and NF-κB pathways. Food Funct. 2017, 8, 2875–2885. [Google Scholar] [CrossRef]
- Takada, S.; Kinugawa, S.; Matsushima, S.; Takemoto, D.; Furihata, T.; Mizushima, W.; Fukushima, A.; Yokota, T.; Ono, Y.; Shibata, H.; et al. Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochondrial function in mice high-fat diet-induced diabetes. Exp. Physiol. 2015, 100, 1319–1330. [Google Scholar] [CrossRef]
- Penalvo, J.L.; Hopia, A.; Adlercreutz, H. Effect of sesamin on serum cholesterol and triglycerides levels in LDL receptor-deficient mice. Eur. J. Nutr. 2006, 45, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kita, S.; Matsumura, Y.; Morimoto, S.; Akimoto, K.; Furuya, M.; Oka, N.; Tanaka, T. Antihypertensive effect of sesamin. II. Protection against two-kidney, one-clip renal hypertension and cardiovascular hypertrophy. Biol. Pharm. Bull. 1995, 18, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Kitagawa, Y.; Akamatsu, T.; Hirose, N.; Sugano, M.; Shimizu, S.; Yamada, H. Protective effects of sesamin against liver damage caused by alcohol or carbon tetrachloride in rodents. Ann. Nutr. Metab. 1993, 37, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Piao, H.M.; Zheng, M.Y.; Lin, Z.H.; Li, G.; Yan, G.H. Sesamin attenuates mast cell-mediated allergic responses by suppressing the activation of p38 and nuclear factor-κB. Mol. Med. Rep. 2016, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gou, X.; Cai, P.; Xu, C.; Cao, L.; Zhao, Z.; Huang, M.; Jin, J. Sesamin enhances Nrf2-mediated protective defense against oxidative stress and inflammation in colitis via AKT and ERK activation. Oxid. Med. Cell. Longev. 2019, 2019, 2432416. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.; Ali Thubab, H.M.; Ali Zaeri, A.M.; Anwer, T.; Ahmed, R.A.; Jali, A.M.; Qadri, M.; Nomier, Y.; Moni, S.S.; Alam, M.F. The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats. Int. J. Mol. Sci. 2022, 23, 11615. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhu, C.Q.; Liu, L. Sesamin ameliorates oxidative liver injury induced by carbon tetrachloride in rat. Int. J. Clin. Exp. Pathol. 2015, 8, 5733–5738. [Google Scholar]
- Utley, H.G.; Bernheim, F.; Hochstein, P. Effect of sulfhydryl reagent on peroxidation in microsome. Arch. Biochem. Biophys. 1967, 260, 521–531. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Stevens, M.J.; Obrosova, I.; Cao, X.; Van Huysen, C.; Greene, D.A. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 2000, 49, 1006–1015. [Google Scholar] [CrossRef]
- Alam, M.F.; Ajeibi, A.O.; Safhi, M.H.; Alabdly, A.J.A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Jali, A.M.; Alqahtani, S.; Nomier, Y.; et al. Therapeutic Potential of Capsaicin against cyclophosphamide -Induced Liver Damage. J. Clin. Med. 2023, 12, 911. [Google Scholar] [CrossRef]
- Hales, B.F. Comparison of the mutagenicity and teratogenicity of and its active metabolites, 4-hydroxy, phosphoramide mustard, and acrolein. Cancer Res. 1982, 42, 3016–3021. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Mohammed, H.A.; Faris, T.M.; Hassan, A.S.; Mohamed, H.B.; El Dosoky, M.I.; Aboubakr, E.M. Nano-Structured Lipid Carrier-Based Oral Glutathione Formulation Mediates Renoprotection against Cyclophosphamide-Induced Nephrotoxicity, and Improves Oral Bioavailability of Glutathione Confirmed through RP-HPLC Micellar Liquid Chromatography. Molecules 2021, 26, 7491. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Slattery, J.T.; Bouvier, M.E.; Ren, S.; Batchelder, A.L.; Kalhorn, T.F.; Schoch, H.G.; Anasetti, C.; Gooley, T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 2003, 101, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Muratori, L.; Ferrari, R.; Muratori, P.; Granito, A.; Bianchi, F.B. Acute icteric hepatitis induced by a short course of low-dose in a patient with lupus nephritis. Dig. Dis. Sci. 2005, 50, 2364–2365. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M. Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology 1987, 45, 79–91. [Google Scholar] [CrossRef]
- Sánchez-Valle, V.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis, A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Kocahan, S.; Dogan, Z.; Erdemli, E.; Taskin, E. Protective Effect of Quercetin Against Oxidative Stress-induced Toxicity Associated With Doxorubicin and Cyclophosphamide in Rat Kidney and Liver Tissue. Iran. J. Kidney Dis. 2017, 11, 124–131. [Google Scholar]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Are free radicals involved in thiol-based redox signaling? Free Radic. Biol. Med. 2015, 80, 164–170. [Google Scholar] [CrossRef]
- Frank, L.; Price, L.T.; Whitney, P.L. Possible mechanism for late gestational development of the antioxidant enzymes in the fetal rat lung. Biol. Neonate 1996, 70, 116–127. [Google Scholar] [CrossRef]
- Chabra, A.; Shokrzadeh, M.; Naghshvar, F.; Salehi, F.; Ahmadi, A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum. Exp. Toxicol. 2014, 33, 185–195. [Google Scholar] [CrossRef]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Ashique, M.; Manoj, P.; Sudhadevi, M.; Al Tobi, M.; Nemmar, A. Ameliorative effect of sesamin in cisplatin-induced nephrotoxicity in rats by suppressing inflammation, oxidative/nitrosative stress, and cellular damage. Physiol. Res. 2020, 69, 61–72. [Google Scholar] [CrossRef]
- Cengiz, M.; Yildiz, S.C.; Demir, C.; Sahin, I.K.; Teksoy, Ö.; Ayhanci, A. Hepato-preventive and anti-apoptotic role of boric acid against liver injury induced by cyclophosphamide. Trace Elements Med. Biol. 2019, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Asiri, Y.A. Probucol attenuates cyclophosphamide-induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxidative Med. Cell. Longev. 2010, 3, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Syed, M.; Ali, J.; Najmi, A.K.; Haque, M.M. Nerolidol protects the liver against cyclophosphamide-induced hepatic inflammation, apoptosis, and fibrosis via modulation of Nrf2, NF-kB p65, and caspase-3 signaling molecules in Swiss albino mice. BioFactors 2020, 46, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.M.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- ALHaithloul, H.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef]
- Qadri, M.M.; Alam, M.F.; Khired, Z.A.; Alaqi, R.O.; Khardali, A.A.; Alasmari, M.M.; Alrashah, A.S.S.; Muzafar, H.M.A.; Qahl, A.M. Thymoquinone Ameliorates Carfilzomib-Induced Renal Impairment by Modulating Oxidative Stress Markers, Inflammatory/Apoptotic Mediators, and Augmenting Nrf2 in Rats. Int. J. Mol. Sci. 2023, 24, 10621. [Google Scholar] [CrossRef]
- Alqahtani, S.; Mahmoud, A.M. Gamma-Glutamylcysteine Ethyl Ester Protects against Cyclophosphamide-Induced Liver Injury and Hematologic Alterations via Upregulation of PPAR and Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Oxidative Med. Cell. Longev. 2016, 2016, 4016209. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res. 2018, 25, 20968–20984. [Google Scholar] [CrossRef]
- Horvath, J.J.; Witmer, C.M.; Witz, G. Nephrotoxicity of the 1,1 acrolein-glutathione adduct in the rat. Toxicol. Appl. Pharmacol. 1992, 117, 200–207. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Devaki, T.; Manohar, B.M.; Babu, M.S. Effect of squalene on cyclophosphamide-induced toxicity. Clin. Chim. Acta 2006, 364, 335–342. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jali, A.M.; Alam, M.F.; Hanbashi, A.; Mawkili, W.; Abdlasaed, B.M.; Alshahrani, S.; Qahl, A.M.; Alrashah, A.S.S.; Shahi, H.A. Sesamin’s Therapeutic Actions on Cyclophosphamide-Induced Hepatotoxicity, Molecular Mechanisms, and Histopathological Characteristics. Biomedicines 2023, 11, 3238. https://doi.org/10.3390/biomedicines11123238
Jali AM, Alam MF, Hanbashi A, Mawkili W, Abdlasaed BM, Alshahrani S, Qahl AM, Alrashah ASS, Shahi HA. Sesamin’s Therapeutic Actions on Cyclophosphamide-Induced Hepatotoxicity, Molecular Mechanisms, and Histopathological Characteristics. Biomedicines. 2023; 11(12):3238. https://doi.org/10.3390/biomedicines11123238
Chicago/Turabian StyleJali, Abdulmajeed M., Mohammad Firoz Alam, Ali Hanbashi, Wedad Mawkili, Basher M. Abdlasaed, Saeed Alshahrani, Abdullah M. Qahl, Ahmad S. S. Alrashah, and Hamad Al Shahi. 2023. "Sesamin’s Therapeutic Actions on Cyclophosphamide-Induced Hepatotoxicity, Molecular Mechanisms, and Histopathological Characteristics" Biomedicines 11, no. 12: 3238. https://doi.org/10.3390/biomedicines11123238
APA StyleJali, A. M., Alam, M. F., Hanbashi, A., Mawkili, W., Abdlasaed, B. M., Alshahrani, S., Qahl, A. M., Alrashah, A. S. S., & Shahi, H. A. (2023). Sesamin’s Therapeutic Actions on Cyclophosphamide-Induced Hepatotoxicity, Molecular Mechanisms, and Histopathological Characteristics. Biomedicines, 11(12), 3238. https://doi.org/10.3390/biomedicines11123238