Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Drug Administration
2.3. Neuropathic Pain Induction
2.4. Behavioral Assessment
2.4.1. Burrowing Test
2.4.2. Von Frey Test
2.4.3. Light/Dark Box Test
2.5. Tissue Collection
2.6. Immunofluorescence
2.7. Multiplex Assay
2.8. Statistical Analyses
3. Results
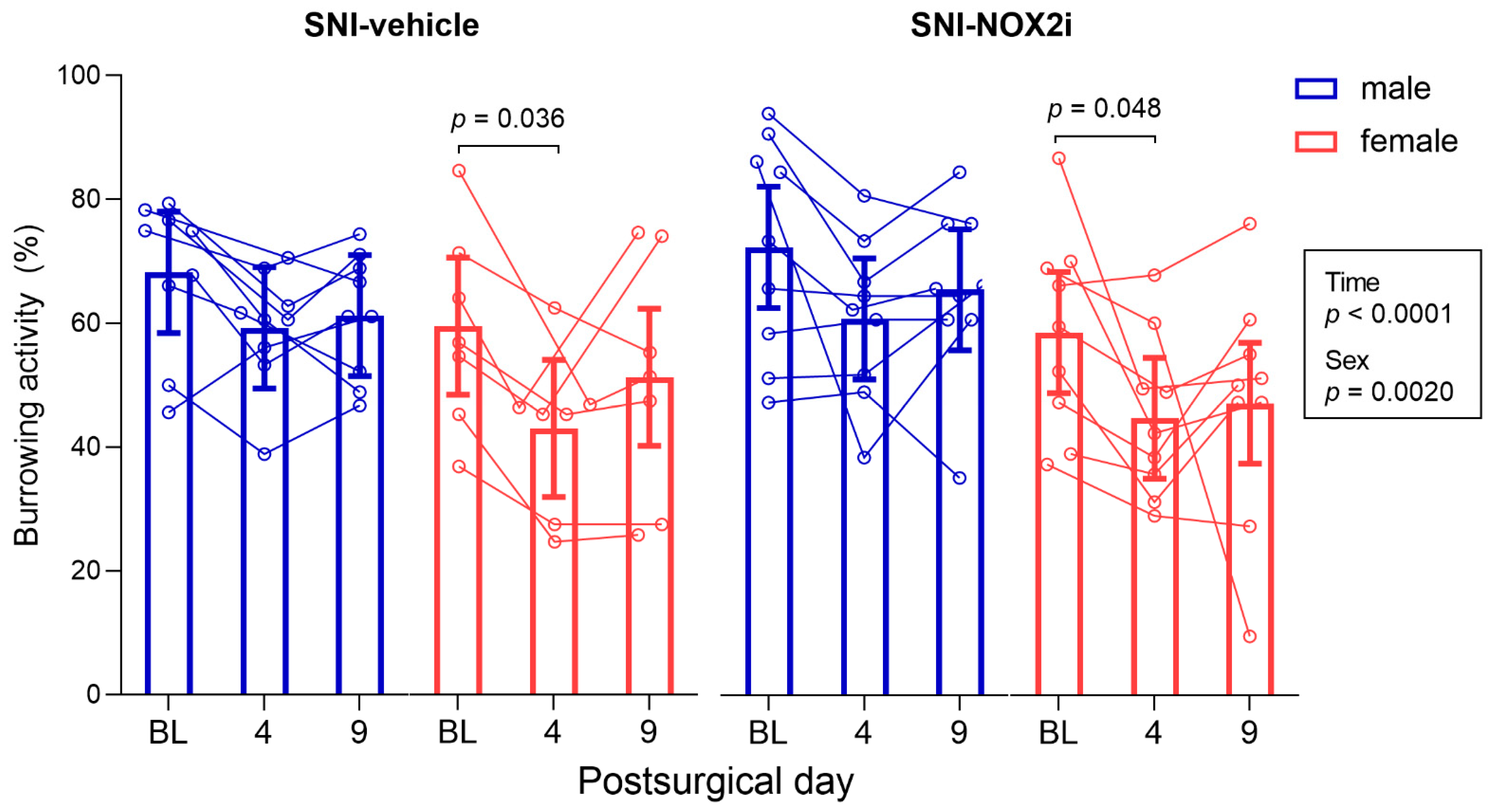
3.1. NOX2i Treatment Did Not Increase the Well-Being of SNI-Mice
3.2. NOX2i Treatment Reduced SNI-Induced Mechanical Hypersensitivity in Both Male and Female Mice
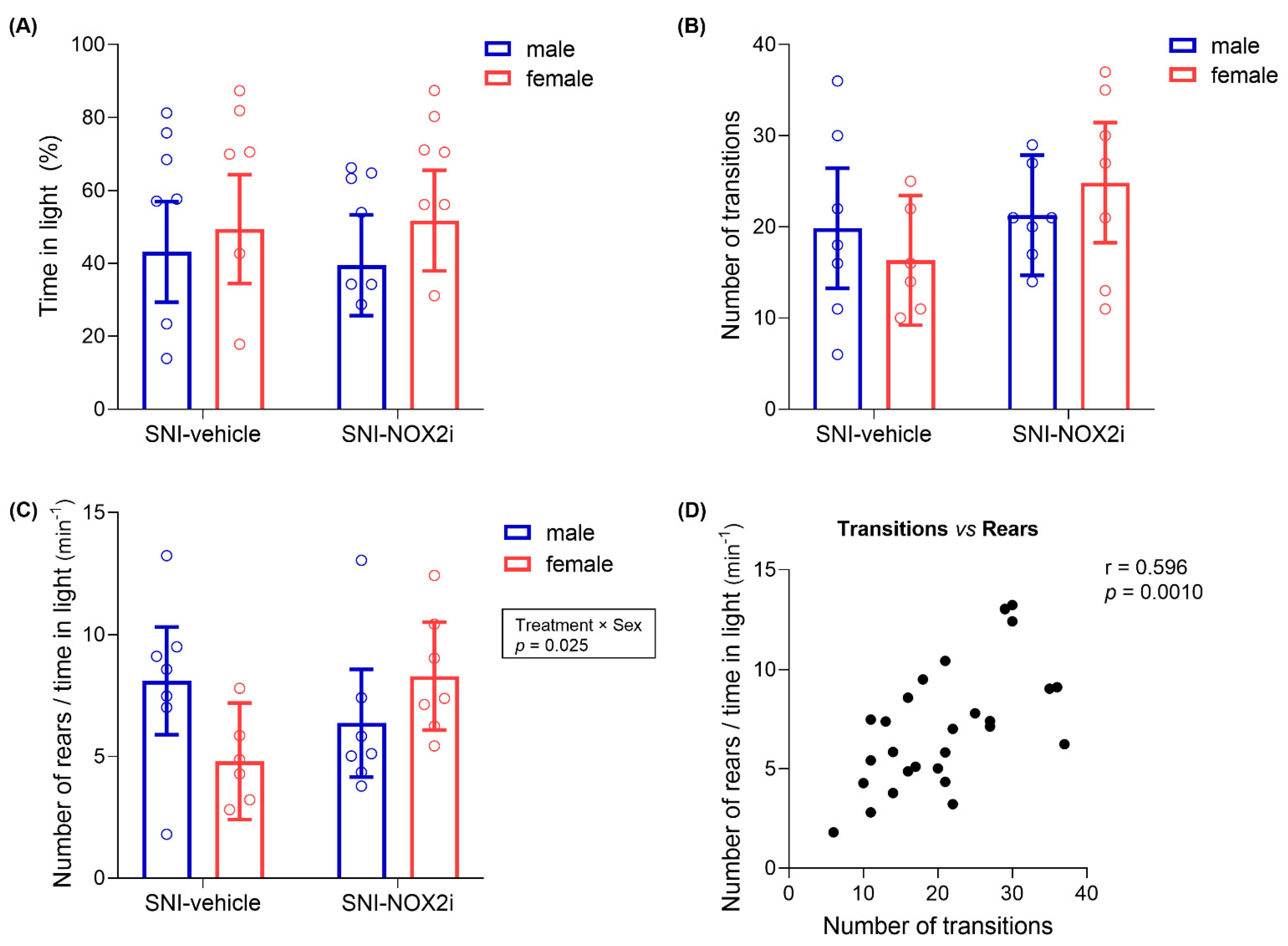
3.3. NOX2i Treatment Appeared to Improve Anxiety-Like Behavior on SNI Female Mice
3.4. NOX2i Treatment Reduced Early SNI-Induced Spinal Microglial Activation in Both Male and Female Mice
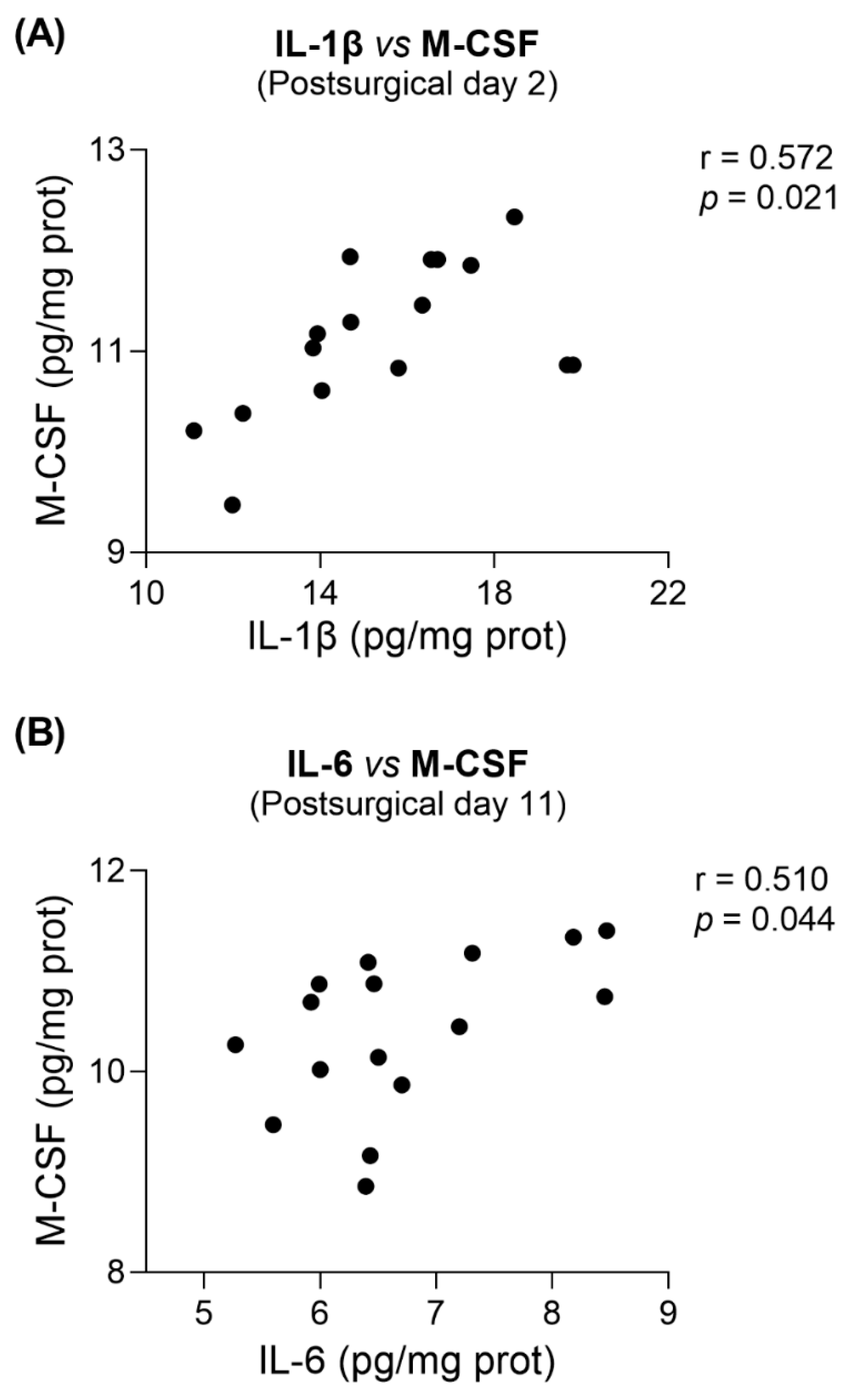
3.5. NOX2i Treatment Marginally Decreased IL-6 in SNI Female and Increased M-CSF in SNI Male Mice on Day 11
4. Discussion
4.1. On the Behavioral Assessment of Analgesic and Anxiolytic Effects of NOX2i Treatment
4.2. On the Neuroinflammatory Mechanisms Underlying NOX2i Actions
4.3. On the Limitations of the Study and Future Research Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Gaudet, A.D.; Staikopoulos, V.; Maier, S.F.; Hutchinson, M.R.; Salvemini, D.; Watkins, L.R. Nitroxidative Signaling Mechanisms in Pathological Pain. Trends Neurosci. 2016, 39, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Basbaum, A.; Guan, Z. Contribution of colony-stimulating factor 1 to neuropathic pain. Pain Rep. 2021, 6, e883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The role of microglia in the pathobiology of neuropathic pain development: What do we know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.-J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2021, 162, 2186–2200. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Schmidt, H.H.; Stocker, R.; Vollbracht, C.; Paulsen, G.; Riley, D.; Daiber, A.; Cuadrado, A. Antioxidants in Translational Medicine. Antioxid. Redox Signal. 2015, 23, 1130–1143. [Google Scholar] [CrossRef]
- Dao, V.T.-V.; Casas, A.I.; Maghzal, G.J.; Seredenina, T.; Kaludercic, N.; Robledinos-Anton, N.; Di Lisa, F.; Stocker, R.; Ghezzi, P.; Jaquet, V. Pharmacology and clinical drug candidates in redox medicine. Antioxid. Redox Signal. 2015, 23, 1113–1129. [Google Scholar] [CrossRef]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801. [Google Scholar] [CrossRef]
- Rastogi, R.; Geng, X.; Li, F.; Ding, Y. NOX Activation by Subunit Interaction and Underlying Mechanisms in Disease. Front. Cell. Neurosci. 2017, 10, 301. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schroder, K.; Geisslinger, G.; Schmidtko, A. NOXious signaling in pain processing. Pharmacol. Ther. 2013, 137, 309–317. [Google Scholar] [CrossRef]
- Kim, D.; You, B.; Jo, E.K.; Han, S.K.; Simon, M.I.; Lee, S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14851–14856. [Google Scholar] [CrossRef]
- Lim, H.; Kim, D.; Lee, S.J. Toll-like receptor 2 mediates peripheral nerve injury-induced NADPH oxidase 2 expression in spinal cord microglia. J. Biol. Chem. 2013, 288, 7572–7579. [Google Scholar] [CrossRef]
- Choi, S.-R.; Roh, D.-H.; Yoon, S.-Y.; Kang, S.-Y.; Moon, J.-Y.; Kwon, S.-G.; Choi, H.-S.; Han, H.-J.; Beitz, A.J.; Oh, S.-B.; et al. Spinal sigma-1 receptors activate NADPH oxidase 2 leading to the induction of pain hypersensitivity in mice and mechanical allodynia in neuropathic rats. Pharmacol. Res. 2013, 74, 56–67. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Hohmann, S.W.; Syhr, K.M.; Schroder, K.; Sisignano, M.; Weigert, A.; Lorenz, J.E.; Lu, R.; Brune, B.; Brandes, R.P.; et al. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 2014, 155, 2161–2170. [Google Scholar] [CrossRef]
- Geis, C.; Geuss, E.; Sommer, C.; Schmidt, H.H.; Kleinschnitz, C. NOX4 is an early initiator of neuropathic pain. Exp. Neurol. 2017, 288, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCε in rat dorsal root ganglion neurons. J. Neuroinflammation 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Sabirzhanov, B.; Li, Y.; Coll-Miro, M.; Matyas, J.J.; He, J.; Kumar, A.; Ward, N.; Yu, J.; Faden, A.I.; Wu, J. Inhibition of NOX2 signaling limits pain-related behavior and improves motor function in male mice after spinal cord injury: Participation of IL-10/miR-155 pathways. Brain. Behav. Immun. 2019, 80, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Choi, G.-E.; Kim, Y.-J.; Lee, G.-H.; Hyun, K.-Y. Neuroprotective properties of ethanolic extract of Citrus unshiu Markovich peel through NADPH oxidase 2 inhibition in chemotherapy-induced neuropathic pain animal model. Phytother. Res. 2021, 35, 6918–6931. [Google Scholar] [CrossRef] [PubMed]
- Aldieri, E.; Riganti, C.; Polimeni, M.; Gazzano, E.; Lussiana, C.; Campia, I.; Ghigo, D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr. Drug Metab. 2008, 9, 686–696. [Google Scholar] [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mucke, H.; Schmidt, H.H.H.W. NOX Inhibitors: From Bench to Naxibs to Bedside. In Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 145–168. [Google Scholar]
- Hirano, K.; Chen, W.S.; Chueng, A.L.; Dunne, A.A.; Seredenina, T.; Filippova, A.; Ramachandran, S.; Bridges, A.; Chaudry, L.; Pettman, G. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid. Redox Signal. 2015, 23, 358–374. [Google Scholar] [CrossRef]
- Nelson, D.a.M.; Fasbender, E.K.; Jakubiak, M.C.; Lindsay, A.; Lowe, D.A.; Ervasti, J.M. Rapid, redox-mediated mechanical susceptibility of the cortical microtubule lattice in skeletal muscle. Redox Biol. 2020, 37, 101730. [Google Scholar] [CrossRef]
- Zeng, Q.; Ye, L.; Ling, M.; Ma, R.; Li, J.; Chen, H.; Pan, L. TLR4/TRAF6/NOX2 signaling pathway is involved in ventilation-induced lung injury via endoplasmic reticulum stress in murine model. Int. Immunopharmacol. 2021, 96, 107774. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Y.; Li, G.G.; Yu, H.H.; Bai, S.; Guo, G.Y.; Guo, W.L.; Ma, Y.; Wang, J.H.; Liu, N.; et al. P2X7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage. Neural Regen. Res. 2021, 16, 1582–1591. [Google Scholar] [CrossRef]
- Hu, X.F.; Xiang, G.; Wang, T.J.; Ma, Y.B.; Zhang, Y.; Yan, Y.B.; Zhao, X.; Wu, Z.X.; Feng, Y.F.; Lei, W. Impairment of type H vessels by NOX2-mediated endothelial oxidative stress: Critical mechanisms and therapeutic targets for bone fragility in streptozotocin-induced type 1 diabetic mice. Theranostics 2021, 11, 3796–3812. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, W.; Huang, C.; Jian, J.; Zhang, Y.; Liu, Q.; Chen, P.; Zhu, X. Ursolic acid alleviates Kupffer cells pyroptosis in liver fibrosis by the NOX2/NLRP3 inflammasome signaling pathway. Int. Immunopharmacol. 2022, 113, 109321. [Google Scholar] [CrossRef]
- Wang, M.; Luo, L. An Effective NADPH Oxidase 2 Inhibitor Provides Neuroprotection and Improves Functional Outcomes in Animal Model of Traumatic Brain Injury. Neurochem. Res. 2020, 45, 1097–1106. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Lin, L.; Ren, J.; He, R.; Sun, K. Inhibition of NADPH oxidase 2 (NOX2) reverses cognitive deficits by modulating excitability and excitatory transmission in the hippocampus after traumatic brain injury. Biochem. Biophys. Res. Commun. 2022, 617, 1–7. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Bourquin, A.-F.; Süveges, M.; Pertin, M.; Gilliard, N.; Sardy, S.; Davison, A.C.; Spahn, D.R.; Decosterd, I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 2006, 122, 14.e1–14.e14. [Google Scholar] [CrossRef]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; King, T.; Morgan, M.M. Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci. Biobehav. Rev. 2019, 100, 335–343. [Google Scholar] [CrossRef]
- Shepherd, A.J.; Cloud, M.E.; Cao, Y.-Q.; Mohapatra, D.P. Deficits in Burrowing Behaviors Are Associated With Mouse Models of Neuropathic but Not Inflammatory Pain or Migraine. Front. Behav. Neurosci. 2018, 12, 124. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Burrowing in rodents: A sensitive method for detecting behavioral dysfunction. Nat. Protoc. 2006, 1, 118–121. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Richner, M.; Bjerrum, O.J.; Nykjaer, A.; Vaegter, C.B. The spared nerve injury (SNI) model of induced mechanical allodynia in mice. J. Vis. Exp. 2011, 54, e3092. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bouwknecht, J.A.; Paylor, R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav. Pharmacol. 2008, 19, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Piasecki, C.C.; Lonstein, J.S. Use of the light–dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol. Biochem. Behav. 2011, 100, 130–137. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Wen, Y.-R.; Suter, M.R.; Kawasaki, Y.; Huang, J.; Pertin, M.; Kohno, T.; Berde, C.B.; Decosterd, I.; Ji, R.-R. Nerve Conduction Blockade in the Sciatic Nerve Prevents but Does Not Reverse the Activation of p38 Mitogen-activated Protein Kinase in Spinal Microglia in the Rat Spared Nerve Injury Model. Anesthesiology 2007, 107, 312–321. [Google Scholar] [CrossRef]
- Amo-Aparicio, J.; Martínez-Muriana, A.; Sánchez-Fernández, A.; López-Vales, R. Neuroinflammation Quantification for Spinal Cord Injury. Curr. Protoc. Immunol. 2018, 123, e57. [Google Scholar] [CrossRef]
- Clark, R.A.; Shoaib, M.; Hewitt, K.N.; Stanford, S.C.; Bate, S.T. A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J. Psychopharm. 2012, 26, 1136–1142. [Google Scholar] [CrossRef]
- Mills, C.; LeBlond, D.; Joshi, S.; Zhu, C.; Hsieh, G.; Jacobson, P.; Meyer, M.; Decker, M. Estimating Efficacy and Drug ED50's Using von Frey Thresholds: Impact of Weber’s Law and Log Transformation. J. Pain 2012, 13, 519–523. [Google Scholar] [CrossRef]
- Mogil, J.S.; Chanda, M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef]
- Green-Fulgham, S.M.; Harland, M.E.; Ball, J.B.; Li, J.; Lacagnina, M.J.; D’Angelo, H.; Dreher, R.A.; Willcox, K.F.; Lorca, S.A.; Kwilasz, A.J.; et al. Preconditioning by voluntary wheel running attenuates later neuropathic pain via Nrf2 antioxidant signaling in rats. Pain 2022, 163, 19939–19951. [Google Scholar] [CrossRef]
- Mogil, J.S.; Graham, A.C.; Ritchie, J.; Hughes, S.F.; Austin, J.-S.; Schorscher-Petcu, A.; Langford, D.J.; Bennett, G.J. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol. Pain 2010, 6, 34. [Google Scholar] [CrossRef]
- Norman, G.J.; Karelina, K.; Zhang, N.; Walton, J.C.; Morris, J.S.; DeVries, A.C. Stress and IL-1β contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry 2010, 15, 404–414. [Google Scholar] [CrossRef]
- Guida, F.; De Gregorio, D.; Palazzo, E.; Ricciardi, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Infantino, R.; Formato, F.; Marabese, I.; et al. Behavioral, Biochemical and Electrophysiological Changes in Spared Nerve Injury Model of Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 3396. [Google Scholar] [CrossRef]
- Sartori, S.B.; Landgraf, R.; Singewald, N. The clinical implications of mouse models of enhanced anxiety. Future Neurol. 2011, 6, 531–571. [Google Scholar] [CrossRef]
- Griebel, G.; Belzung, C.; Perrault, G.; Sanger, D.J. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology 2000, 148, 164–170. [Google Scholar] [CrossRef]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. S1), S10–S28. [Google Scholar] [CrossRef]
- Schwabenland, M.; Brück, W.; Priller, J.; Stadelmann, C.; Lassmann, H.; Prinz, M. Analyzing microglial phenotypes across neuropathologies: A practical guide. Acta Neuropathol. 2021, 142, 923–936. [Google Scholar] [CrossRef]
- Zhang, J.; De Koninck, Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006, 97, 772–783. [Google Scholar] [CrossRef]
- Scholz, J.; Abele, A.; Marian, C.; Häussler, A.; Herbert, T.A.; Woolf, C.J.; Tegeder, I. Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats. Pain 2008, 138, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, S.; Shi, X.Q.; Rivest, S.; Zhang, J. Peripheral nerve injury alters blood–spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J. Neurosci. 2011, 31, 10819–10828. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Savarese, L.; Colangelo, A.M.; Bianco, M.R.; Cirillo, G.; Alberghina, L.; Papa, M. Astrocytes and Microglia-Mediated Immune Response in Maladaptive Plasticity is Differently Modulated by NGF in the Ventral Horn of the Spinal Cord Following Peripheral Nerve Injury. Cell. Mol. Neurobiol. 2016, 36, 37–46. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Backonja, M.-M.; Stacey, B. Neuropathic pain symptoms relative to overall pain rating. J. Pain 2004, 5, 491–497. [Google Scholar] [CrossRef]
- Cunha, A.M.; Pereira-Mendes, J.; Almeida, A.; Guimarães, M.R.; Leite-Almeida, H. Chronic pain impact on rodents’ behavioral repertoire. Neurosci. Biobehav. Rev. 2020, 119, 101–127. [Google Scholar] [CrossRef]
- González-Cano, R.; Montilla-García, Á.; Ruiz-Cantero, M.C.; Bravo-Caparrós, I.; Tejada, M.Á.; Nieto, F.R.; Cobos, E.J. The search for translational pain outcomes to refine analgesic development: Where did we come from and where are we going? Neurosci. Biobehav. Rev. 2020, 113, 238–261. [Google Scholar] [CrossRef]
- Sadler, K.E.; Mogil, J.S.; Stucky, C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2022, 23, 70–85. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxidative Med. Cell. Longev. 2009, 2, 623654. [Google Scholar] [CrossRef]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef]
- Huang, X.; Xiaokaiti, Y.; Yang, J.; Pan, J.; Li, Z.; Luria, V.; Li, Y.; Song, G.; Zhu, X.; Zhang, H.-T.; et al. Inhibition of phosphodiesterase 2 reverses gp91phox oxidase-mediated depression- and anxiety-like behavior. Neuropharmacology 2018, 143, 176–185. [Google Scholar] [CrossRef]
- Yan, J.; Huang, J.; Liu, A.; Wu, J.; Fan, H.; Shen, M.; Lai, X.; Ma, H.; Sun, W.; Yang, J.; et al. Atorvastatin improves motor function, anxiety and depression by NOX2-mediated autophagy and oxidative stress in MPTP-lesioned mice. Aging 2020, 13, 831–845. [Google Scholar] [CrossRef]
- Andrews, N.; Legg, E.; Lisak, D.; Issop, Y.; Richardson, D.; Harper, S.; Pheby, T.; Huang, W.; Burgess, G.; Machin, I.; et al. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur. J. Pain 2012, 16, 485–495. [Google Scholar] [CrossRef]
- Huang, W.; Calvo, M.; Karu, K.; Olausen, H.R.; Bathgate, G.; Okuse, K.; Bennett, D.L.H.; Rice, A.S.C. A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain 2013, 154, 560–575. [Google Scholar] [CrossRef]
- Lau, W.; Dykstra, C.; Thevarkunnel, S.; Silenieks, L.B.; de Lannoy, I.A.M.; Lee, D.K.H.; Higgins, G.A. A back translation of pregabalin and carbamazepine against evoked and non-evoked endpoints in the rat spared nerve injury model of neuropathic pain. Neuropharmacology 2013, 73, 204–215. [Google Scholar] [CrossRef]
- Muralidharan, A.; Kuo, A.; Jacob, M.; Lourdesamy, J.S.; Carvalho, L.M.S.P.D.; Nicholson, J.R.; Corradini, L.; Smith, M.T. Comparison of Burrowing and Stimuli-Evoked Pain Behaviors as End-Points in Rat Models of Inflammatory Pain and Peripheral Neuropathic Pain. Front. Behav. Neurosci. 2016, 10, 88. [Google Scholar] [CrossRef]
- Rutten, K.; Gould, S.A.; Bryden, L.; Doods, H.; Christoph, T.; Pekcec, A. Standard analgesics reverse burrowing deficits in a rat CCI model of neuropathic pain, but not in models of type 1 and type 2 diabetes-induced neuropathic pain. Behav. Brain Res. 2018, 350, 129–138. [Google Scholar] [CrossRef]
- Turner, P.V.; Pang, D.S.J.; Lofgren, J.L.S. A Review of Pain Assessment Methods in Laboratory Rodents. Comp. Med. 2019, 69, 451–467. [Google Scholar] [CrossRef]
- Bravo-Caparrós, I.; Ruiz-Cantero, M.C.; Perazzoli, G.; Cronin, S.J.F.; Vela, J.M.; Hamed, M.F.; Penninger, J.M.; Baeyens, J.M.; Cobos, E.J.; Nieto, F.R. Sigma-1 receptors control neuropathic pain and macrophage infiltration into the dorsal root ganglion after peripheral nerve injury. FASEB J. 2020, 34, 5951–5966. [Google Scholar] [CrossRef]
- Donner, N.C.; Lowry, C.A. Sex differences in anxiety and emotional behavior. Pflugers Arch. 2013, 465, 601–626. [Google Scholar] [CrossRef]
- An, X.-L.; Zou, J.-X.; Wu, R.-Y.; Yang, Y.; Tai, F.-D.; Zeng, S.-Y.; Jia, R.; Zhang, X.; Liu, E.-Q.; Broders, H. Strain and Sex Differences in Anxiety-Like and Social Behaviors in C57BL/6J and BALB/cJ Mice. Exp. Anim. 2011, 60, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; González, M.I.; Wilson, C.A.; File, S.E. Factor Analysis Shows That Female Rat Behaviour Is Characterized Primarily by Activity, Male Rats Are Driven by Sex and Anxiety. Pharmacol. Biochem. Behav. 1999, 64, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.L.; Afzal, A.; Fox, L.C.; Watt, M.J.; Forster, G.L. Sex differences in anxiety-like behaviors in rats. Physiol. Behav. 2019, 211, 112670. [Google Scholar] [CrossRef] [PubMed]
- Mutso, A.A.; Radzicki, D.; Baliki, M.N.; Huang, L.; Banisadr, G.; Centeno, M.V.; Radulovic, J.; Martina, M.; Miller, R.J.; Apkarian, A.V. Abnormalities in Hippocampal Functioning with Persistent Pain. J. Neurosci. 2012, 32, 5747. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yue, N.; Liu, S.-B.; Wang, Z.-F.; Mi, W.-L.; Jiang, J.-W.; Wu, G.-C.; Yu, J.; Wang, Y.-Q. Effects of Chronic Electroacupuncture on Depression- and Anxiety-Like Behaviors in Rats with Chronic Neuropathic Pain. Evid. Based Complement. Alternat. Med. 2014, 2014, 158987. [Google Scholar] [CrossRef]
- González-Sepúlveda, M.; Pozo, O.J.; Marcos, J.; Valverde, O. Chronic pain causes a persistent anxiety state leading to increased ethanol intake in CD1 mice. J. Psychopharm. 2015, 30, 188–203. [Google Scholar] [CrossRef]
- Pitzer, C.; La Porta, C.; Treede, R.-D.; Tappe-Theodor, A. Inflammatory and neuropathic pain conditions do not primarily evoke anxiety-like behaviours in C57BL/6 mice. Eur. J. Pain 2019, 23, 285–306. [Google Scholar] [CrossRef]
- Urban, R.; Scherrer, G.; Goulding, E.H.; Tecott, L.H.; Basbaum, A.I. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011, 152, 990–1000. [Google Scholar] [CrossRef]
- Kremer, M.; Becker, L.J.; Barrot, M.; Yalcin, I. How to study anxiety and depression in rodent models of chronic pain? Eur. J. Neurosci. 2021, 53, 236–270. [Google Scholar] [CrossRef]
- Sieberg, C.B.; Taras, C.; Gomaa, A.; Nickerson, C.; Wong, C.; Ward, C.; Baskozos, G.; Bennett, D.L.H.; Ramirez, J.D.; Themistocleous, A.C.; et al. Neuropathic pain drives anxiety behavior in mice, results consistent with anxiety levels in diabetic neuropathy patients. Pain Rep. 2018, 3, e651. [Google Scholar] [CrossRef]
- Gu, N.; Peng, J.; Murugan, M.; Wang, X.; Eyo Ukpong, B.; Sun, D.; Ren, Y.; DiCicco-Bloom, E.; Young, W.; Dong, H.; et al. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep. 2016, 16, 605–614. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.-R. Sex-dependent glial signaling in pathological pain: Distinct roles of spinal microglia and astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef]
- Inyang, K.E.; Szabo-Pardi, T.; Wentworth, E.; McDougal, T.A.; Dussor, G.; Burton, M.D.; Price, T.J. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol. Res. 2019, 139, 1–16. [Google Scholar] [CrossRef]
- Neher, J.J.; Cunningham, C. Priming Microglia for Innate Immune Memory in the Brain. Trends Immunol. 2019, 40, 358–374. [Google Scholar] [CrossRef]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef]
- Haley, M.J.; Brough, D.; Quintin, J.; Allan, S.M. Microglial Priming as Trained Immunity in the Brain. Neuroscience 2019, 405, 47–54. [Google Scholar] [CrossRef]
- Walker, D.J.; Spencer, K.A. Glucocorticoid programming of neuroimmune function. Gen. Comp. Endocrinol. 2018, 256, 80–88. [Google Scholar] [CrossRef]
- Lajqi, T.; Lang, G.P.; Haas, F.; Williams, D.L.; Hudalla, H.; Bauer, M.; Groth, M.; Wetzker, R.; Bauer, R. Memory-Like Inflammatory Responses of Microglia to Rising Doses of LPS: Key Role of PI3Kγ. Front. Immunol. 2019, 10, 2492. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Parvathenani, L.K.; Tertyshnikova, S.; Greco, C.R.; Roberts, S.B.; Robertson, B.; Posmantur, R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 2003, 278, 13309–13317. [Google Scholar] [CrossRef]
- Schmidt, C.; Schneble-Löhnert, N.; Lajqi, T.; Wetzker, R.; Müller, J.P.; Bauer, R. PI3Kγ Mediates Microglial Proliferation and Cell Viability via ROS. Cells 2021, 10, 2534. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Bohren, Y.; Timbolschi, D.I.; Muller, A.; Barrot, M.; Yalcin, I.; Salvat, E. Platelet-rich plasma and cytokines in neuropathic pain: A narrative review and a clinical perspective. Eur. J. Pain 2022, 26, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, Z.; Liu, Z.H.; Chen, S.P.; Li, M.; Shahveranov, A.; Ye, D.W.; Tian, Y.K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflammation 2016, 13, 141. [Google Scholar] [CrossRef]
- Xu, X.-J.; Hao, J.-X.; Andell-Jonsson, S.; Poli, V.; Bartfai, T.; Wiesenfeld-Hallin, Z. Nociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve section. Cytokine 1997, 9, 1028–1033. [Google Scholar] [CrossRef]
- Chen, F.-F.; Huo, F.-Q.; Xiong, H.; Wan, Q.; Zheng, Y.-N.; Du, W.-J.; Mei, Z.-N. Analgesic effect of total flavonoids from Sanguis draxonis on spared nerve injury rat model of neuropathic pain. Phytomedicine 2015, 22, 1125–1132. [Google Scholar] [CrossRef]
- Wang, X.-y.; Ma, H.-j.; Xue, M.; Sun, Y.-l.; Ren, A.; Li, M.-q.; Huang, Z.-h.; Huang, C. Anti-nociceptive effects of Sedum Lineare Thunb. on spared nerve injury-induced neuropathic pain by inhibiting TLR4/NF-κB signaling in the spinal cord in rats. Biomed. Pharmacother. 2021, 135, 111215. [Google Scholar] [CrossRef]
- Fang, X.; Zhan, G.; Zhang, J.; Xu, H.; Zhu, B.; Hu, Y.; Yang, C.; Luo, A. Abnormalities in inflammatory cytokines confer susceptible to chronic neuropathic pain-related anhedonia in a rat model of spared nerve injury. Clin. Psychopharmacol. Neurosci. 2019, 17, 189. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Jia, P.; Liu, F.-F.; Chen, K.; Meng, F.-Y.; Hong, J.-H.; Zhang, T.; Jin, X.-H.; Shi, J. The analgesic action of larixyl acetate, a potent TRPC6 inhibitor, in rat neuropathic pain model induced by spared nerve injury. J. Neuroinflammation 2020, 17, 1–20. [Google Scholar] [CrossRef]
- Fontinele, L.L.; Heimfarth, L.; Pereira, E.W.M.; Rezende, M.M.; Lima, N.T.; de Carvalho, Y.M.B.G.; de Moura Pires, E.A.; Guimarães, A.G.; Carvalho, M.T.B.; Barreto, R.d.S.S. Anti-hyperalgesic effect of (-)-α-bisabolol and (-)-α-bisabolol/β-Cyclodextrin complex in a chronic inflammatory pain model is associated with reduced reactive gliosis and cytokine modulation. Neurochem. Int. 2019, 131, 104530. [Google Scholar] [CrossRef]
- Fonseca, M.M.; Davoli-Ferreira, M.; Santa-Cecília, F.; Guimarães, R.M.; Oliveira, F.F.B.; Kusuda, R.; Ferreira, D.W.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M. IL-27 Counteracts Neuropathic Pain Development Through Induction of IL-10. Front. Immunol. 2020, 10, 3059. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Y.-W.; Liu, L.; Wang, W.; Zhong, X.-X.; Wei, X.-H.; Liu, X.-G. Liver X Receptor α Is Involved in Counteracting Mechanical Allodynia by Inhibiting Neuroinflammation in the Spinal Dorsal Horn. Anesthesiology 2017, 127, 534–547. [Google Scholar] [CrossRef]
- Okubo, M.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Kanda, H.; Yagi, H.; Noguchi, K. Macrophage-Colony Stimulating Factor Derived from Injured Primary Afferent Induces Proliferation of Spinal Microglia and Neuropathic Pain in Rats. PLoS ONE 2016, 11, e0153375. [Google Scholar] [CrossRef]
- Wang, Y.; Zeigler, M.M.; Lam, G.K.; Hunter, M.G.; Eubank, T.D.; Khramtsov, V.V.; Tridandapani, S.; Sen, C.K.; Marsh, C.B. The Role of the NADPH Oxidase Complex, p38 MAPK, and Akt in Regulating Human Monocyte/Macrophage Survival. Am. J. Respir. Cell Mol. Biol. 2007, 36, 68–77. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, T.H.; Jhon, G.-J.; Lee, S.Y. Reactive oxygen species regulate M-CSF-induced monocyte/macrophage proliferation through SHP1 oxidation. Cell. Signal. 2011, 23, 1633–1639. [Google Scholar] [CrossRef]
- Kuhn, J.A.; Vainchtein, I.D.; Braz, J.; Hamel, K.; Bernstein, M.; Craik, V.; Dahlgren, M.W.; Ortiz-Carpena, J.; Molofsky, A.B.; Molofsky, A.V.; et al. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. eLife 2021, 10, e69056. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, H.; Lee, S.J. Distinct roles of GT1b and CSF-1 in microglia activation in nerve injury-induced neuropathic pain. Mol. Pain 2021, 17, 17448069211020918. [Google Scholar] [CrossRef]
- Latrémolière, A.; Mauborgne, A.; Masson, J.; Bourgoin, S.; Kayser, V.; Hamon, M.; Pohl, M. Differential Implication of Proinflammatory Cytokine Interleukin-6 in the Development of Cephalic versus Extracephalic Neuropathic Pain in Rats. J. Neurosci. 2008, 28, 8489. [Google Scholar] [CrossRef]
- Brifault, C.; Kwon, H.; Campana, W.M.; Gonias, S.L. LRP1 deficiency in microglia blocks neuro-inflammation in the spinal dorsal horn and neuropathic pain processing. Glia 2019, 67, 1210–1224. [Google Scholar] [CrossRef]
- Sassetti, E.; Clausen, M.H.; Laraia, L. Small-Molecule Inhibitors of Reactive Oxygen Species Production. J. Med. Chem. 2021, 64, 5252–5275. [Google Scholar] [CrossRef]
- Howard, R.F.; Walker, S.M.; Mota, P.M.; Fitzgerald, M. The ontogeny of neuropathic pain: Postnatal onset of mechanical allodynia in rat spared nerve injury (SNI) and chronic constriction injury (CCI) models. Pain 2005, 115, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.; Beggs, S.; Vega-Avelaira, D.; Costigan, M.; Hathway, G.J.; Salter, M.W.; Fitzgerald, M. Spinal microglia and neuropathic pain in young rats. Pain 2007, 128, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Vega-Avelaira, D.; Moss, A.; Fitzgerald, M. Age-related changes in the spinal cord microglial and astrocytic response profile to nerve injury. Brain Behav Immun 2007, 21, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Vega-Avelaira, D.; McKelvey, R.; Hathway, G.; Fitzgerald, M. The Emergence of Adolescent Onset Pain Hypersensitivity following Neonatal Nerve Injury. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, R.; Berta, T.; Old, E.; Ji, R.-R.; Fitzgerald, M. Neuropathic Pain Is Constitutively Suppressed in Early Life by Anti-Inflammatory Neuroimmune Regulation. J. Neurosci. 2015, 35, 457. [Google Scholar] [CrossRef]
- Lajqi, T.; Stojiljkovic, M.; Williams, D.L.; Hudalla, H.; Bauer, M.; Witte, O.W.; Wetzker, R.; Bauer, R.; Schmeer, C. Memory-Like Responses of Brain Microglia Are Controlled by Developmental State and Pathogen Dose. Front. Immunol. 2020, 11, 546415. [Google Scholar] [CrossRef]
- Zhang, B.; Bailey, W.M.; McVicar, A.L.; Gensel, J.C. Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiology of Aging 2016, 47, 157–167. [Google Scholar] [CrossRef]
- von Leden, R.E.; Khayrullina, G.; Moritz, K.E.; Byrnes, K.R. Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury. J. Neuroinflammation 2017, 14, 161. [Google Scholar] [CrossRef]
- Stewart, A.N.; Lowe, J.L.; Glaser, E.P.; Mott, C.A.; Shahidehpour, R.K.; McFarlane, K.E.; Bailey, W.M.; Zhang, B.; Gensel, J.C. Acute inflammatory profiles differ with sex and age after spinal cord injury. J. Neuroinflammation 2021, 18, 113. [Google Scholar] [CrossRef]
- Geng, L.; Fan, L.M.; Liu, F.; Smith, C.; Li, J.M. Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Sci. Rep. 2020, 10, 1582. [Google Scholar] [CrossRef]
- Stewart, A.N.; Gensel, J.C.; Zhang, B. Therapeutic implications of advanced age at time of spinal cord injury. Neural Regen Res 2019, 14, 1895–1896. [Google Scholar] [CrossRef]
- Zhang, B.; Bailey, W.M.; McVicar, A.L.; Stewart, A.N.; Veldhorst, A.K.; Gensel, J.C. Reducing age-dependent monocyte-derived macrophage activation contributes to the therapeutic efficacy of NADPH oxidase inhibition in spinal cord injury. Brain. Behav. Immun. 2019, 76, 139–150. [Google Scholar] [CrossRef]
- Padilha, E.C.; Shah, P.; Rai, G.; Xu, X. NOX2 inhibitor GSK2795039 metabolite identification towards drug optimization. J. Pharm. Biomed. Anal. 2021, 201, 114102. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira-Santos, L.; Veríssimo, E.; Martins, S.; Sousa, T.; Albino-Teixeira, A.; Pinho, D. Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain. Biomedicines 2023, 11, 416. https://doi.org/10.3390/biomedicines11020416
Teixeira-Santos L, Veríssimo E, Martins S, Sousa T, Albino-Teixeira A, Pinho D. Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain. Biomedicines. 2023; 11(2):416. https://doi.org/10.3390/biomedicines11020416
Chicago/Turabian StyleTeixeira-Santos, Luísa, Eduardo Veríssimo, Sandra Martins, Teresa Sousa, António Albino-Teixeira, and Dora Pinho. 2023. "Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain" Biomedicines 11, no. 2: 416. https://doi.org/10.3390/biomedicines11020416
APA StyleTeixeira-Santos, L., Veríssimo, E., Martins, S., Sousa, T., Albino-Teixeira, A., & Pinho, D. (2023). Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain. Biomedicines, 11(2), 416. https://doi.org/10.3390/biomedicines11020416





