The Potassium Channel Blocker β-Bungarotoxin from the Krait Bungarus multicinctus Venom Manifests Antiprotozoal Activity
Abstract
:1. Introduction
- -
- To isolate this compound in pure form;
- -
- To determine its antiprotozoal activity;
- -
- To establish its amino acid sequence;
- -
- To reveal functional characteristics essential for its antiprotozoal activity.
2. Materials and Methods
2.1. Materials
2.2. Cultivation of Tetrahymena pyriformis
2.3. Measurement of Toxicity for Tetrahymena pyriformis
2.4. Venom Fractionation and Isolation of Active Compound
2.5. Mass Spectrometry
2.5.1. MALDI-TOF Mass Spectrometry
2.5.2. High Resolution Mass Spectrometry
2.6. Modification of β-Bgt with p-Bromophenacyl Bromide
2.7. Influence of Tetraethylammonium on T. pyriformis Cells
2.8. Statistical Analysis
3. Results
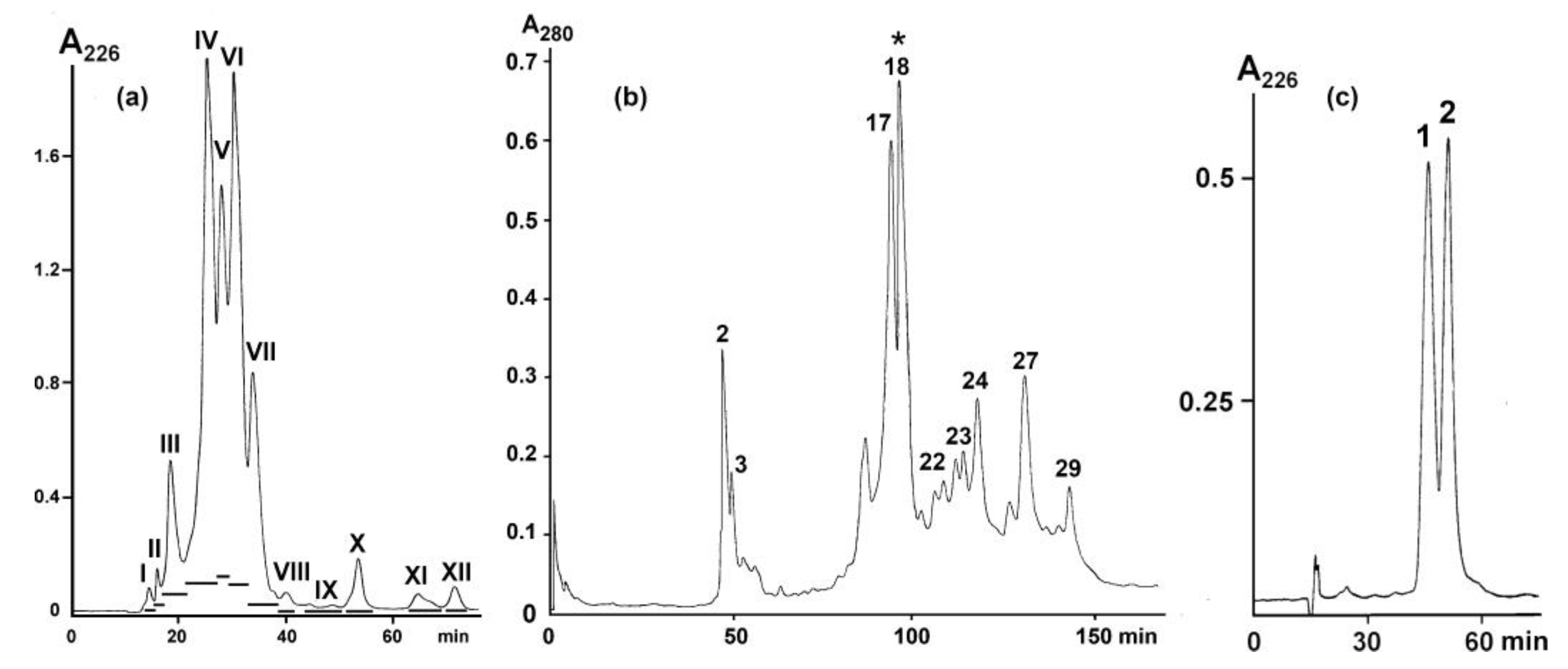
3.1. Isolation of Active Compound
3.2. Determination of the Structure of Active Protein
3.3. Influence of β-Bgt Modification with p-Bromophenacyl Bbromide on Its Antiprotozoal Activity
3.4. Influence of Tetraethylammonium (TEA) on Tetrahymena Survival and Motility
4. Discussion
4.1. Isolation and Structural Characterization of the Active Compound
4.2. Antiprotozoal Activity of β-Bgt
4.3. The Elucidation of the Mechanism of Antiprotozoal β-Bgt Activity
4.4. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saadeh, K.; Nantha Kumar, N.; Fazmin, I.T.; Edling, C.E.; Jeevaratnam, K. Anti-malarial drugs: Mechanisms underlying their proarrhythmic effects. Br. J. Pharmacol. 2022, 179, 5237–5258. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Holt, H.R.; Selby, R.; Guitian, J. Past and Ongoing Tsetse and Animal Trypanosomiasis Control Operations in Five African Countries: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0005247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adade, C.M.; Souto-Padrón, T. Venoms as Sources of Novel Anti-Parasitic Agents. In Toxins and Drug Discovery; Cruz, L., Luo, S., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 1–31. [Google Scholar] [CrossRef]
- Hassan, E.A.; Abdel-Rahman, M.A.; Ibrahim, M.M.; Soliman, M.F. In vitro antischistosomal activity of venom from the Egyptian snake Cerastes cerastes. Rev. Soc. Bras. Med. Trop. 2016, 49, 752–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkov, V.G.; Osipov, A.V.; Utkin, Y.N. Toxicity of venoms from vipers of Pelias group to crickets Gryllus assimilis and its relation to snake entomophagy. Toxicon 2007, 49, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.; Siniavin, A.; Kasheverov, I.; Tsetlin, V. Antiviral Effects of Animal Toxins: Is There a Way to Drugs? Int. J. Mol. Sci. 2022, 23, 3634. [Google Scholar] [CrossRef]
- Teodoro, A.; Gonçalves, F.J.M.; Oliveira, H.; Marques, S. Venom of Viperidae: A Perspective of its Antibacterial and Antitumor Potential. Curr. Drug Targets 2022, 23, 126–144. [Google Scholar] [CrossRef]
- Abdullahi, Z.U.; Musa, S.S.; He, D.; Bello, U.M. Antiprotozoal Effect of Snake Venoms and Their Fractions: A Systematic Review. Pathogens 2021, 10, 1632. [Google Scholar] [CrossRef]
- de Barros, E.; Gonçalves, R.M.; Cardoso, M.H.; Santos, N.C.; Franco, O.L.; Cândido, E.S. Snake Venom Cathelicidins as Natural Antimicrobial Peptides. Front. Pharmacol. 2019, 10, 1415. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.R.; Palacios, A.L.V.; Patiño, R.S.P.; Mendes, B.; Teixeira, C.A.S.; Gomes, P.; da Silva, S.L. Harnessing snake venom phospholipases A2 to novel approaches for overcoming antibiotic resistance. Drug Dev. Res. 2019, 80, 68–85. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, S.C.; Borges, B.C.; Oliveira, V.Q.; Carregosa, L.S.; Bastos, L.A.; Santos, I.A.; Jardim, A.C.G.; Melo, F.F.; Freitas, L.M.; Rodrigues, V.M.; et al. Insights into the antiviral activity of phospholipases A2 (PLA2s) from snake venoms. Int. J. Biol. Macromol. 2020, 164, 616–625. [Google Scholar] [CrossRef]
- Fallahi, N.; Shahbazzadeh, D.; Maleki, F.; Aghdasi, M.; Tabatabaie, F.; Khanaliha, K. The In Vitro Study of Anti-leishmanial Effect of Naja naja oxiana Snake Venom on Leishmania major. Infect. Disord. Drug Targets. 2020, 20, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, I.; Tabatabaie, F.; Nikpour, S.; Mostafavi, M.; Tavakoli Oliaee, R.; Sharifi, F.; Babaei, Z.; Jafari, E.; Salarkia, E.; Shahbazzadeh, D. The Effect of Naja naja oxiana Snake Venom Against Leishmania tropica Confirmed by Advanced Assays. Acta Parasitol. 2021, 66, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kuleshina, O.N.; Kruykova, E.V.; Cheremnykh, E.G.; Kozlov, L.V.; Andreeva, T.V.; Starkov, V.G.; Osipov, A.V.; Ziganshin, R.H.; Tsetlin, V.I.; Utkin, Y.N. Screening Snake Venoms for Toxicity to Tetrahymena Pyriformis Revealed Anti-Protozoan Activity of Cobra Cytotoxins. Toxins 2020, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Cheremnykh, E.G.; Osipov, A.V.; Starkov, V.G.; Trang, N.T.T.; Khoa, N.C.; Anh, H.N.; Dung, L.T.; Tsetlin, V.I.; Utkin, Y.N. Comparative Study of the Effect of Snake Venoms on the Growth of Ciliates Tetrahymena pyriformis: Identification of Venoms with High Antiprotozoal Activity. Dokl. Biochem. Biophys. 2022, 503, 98–103. [Google Scholar] [CrossRef]
- Barbosa, L.G.; Costa, T.R.; Borges, I.P.; Costa, M.S.; Carneiro, A.C.; Borges, B.C.; Silva, M.J.B.; Amorim, F.G.; Quinton, L.; Yoneyama, K.A.G.; et al. A comparative study on the leishmanicidal activity of the L-amino acid oxidases BjussuLAAO-II and BmooLAAO-II isolated from Brazilian Bothrops snake venoms. Int. J. Biol. Macromol. 2021, 167, 267–278. [Google Scholar] [CrossRef]
- Bregge-Silva, C.; Nonato, M.C.; de Albuquerque, S.; Ho, P.L.; Junqueira de Azevedo, I.L.; Vasconcelos Diniz, M.R.; Lomonte, B.; Rucavado, A.; Díaz, C.; Gutiérrez, J.M.; et al. Isolation and biochemical, functional and structural characterization of a novel L-amino acid oxidase from Lachesis muta snake venom. Toxicon 2012, 60, 1263–1276. [Google Scholar] [CrossRef]
- Costa, T.R.; Menaldo, D.L.; Prinholato da Silva, C.; Sorrechia, R.; de Albuquerque, S.; Pietro, R.C.L.R.; Ghisla, S.; Antunes, L.M.G.; Sampaio, S.V. Evaluating the microbicidal, antiparasitic and antitumor effects of CR-LAAO from Calloselasma rhodostoma venom. Int. J. Biol. Macromol. 2015, 80, 489–497. [Google Scholar] [CrossRef]
- Martos-Esteban, A.; Macleod, O.J.S.; Maudlin, I.; Kalogeropoulos, K.; Jürgensen, J.A.; Carrington, M.; Laustsen, A.H. Black-necked spitting cobra (Naja nigricollis) phospholipases A2 may cause Trypanosoma brucei death by blocking endocytosis through the flagellar pocket. Sci. Rep. 2022, 12, 6394. [Google Scholar] [CrossRef]
- Bastos, L.M.; Júnior, R.J.O.; Silva, D.A.O.; Mineo, J.R.; Vieira, C.U.; Teixeira, D.N.S.; Homsi-Brandeburgo, M.I.; Rodrigues, V.D.M.; Hamaguchi, A. Toxoplasma gondii: Effects of neuwiedase, a metalloproteinase from Bothrops neuwiedi snake venom, on the invasion and replication of human fibroblasts in vitro. Exp. Parasitol. 2008, 120, 391–396. [Google Scholar] [CrossRef]
- Martins, G.G.; de Jesus Holanda, R.; Alfonso, J.; Gómez Garay, A.F.; Dos Santos, A.P.A.; de Lima, A.M.; Francisco, A.F.; Teles, C.B.G.; Zanchi, F.B.; Soares, A. Identification of a peptide derived from a Bothrops moojeni metalloprotease with in vitro inhibitory action on the Plasmodium falciparum purine nucleoside phosphorylase enzyme (PfPNP). Biochimie 2019, 62, 97–106. [Google Scholar] [CrossRef]
- Aranda-Souza, M.Â.; de Lorena, V.M.B.; Dos Santos Correia, M.T.; de Figueiredo, R.C.B.Q. In vitro effect of Bothrops leucurus lectin (BLL) against Leishmania amazonensis and Leishmania braziliensis infection. Int. J. Biol. Macromol. 2018, 120, 431–439. [Google Scholar] [CrossRef]
- Allane, D.; Oussedik-Oumehdi, H.; Harrat, Z.; Seve, M.; Laraba-Djebari, F. Isolation and characterization of an anti-leishmanial disintegrin from Cerastes cerastes venom. J. Biochem. Mol. Toxicol. 2018, 32, e22018. [Google Scholar] [CrossRef] [PubMed]
- El Chamy Maluf, S.; Dal Mas, C.; Oliveira, E.B.; Melo, P.M.; Carmona, A.K.; Gazarini, M.L.; Hayashi, M.A. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides 2016, 78, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.C.J.; Bandeira-Lima, D.; Mello, C.P.; Pereira, T.P.; De Menezes, R.R.P.P.B.; Sampaio, T.L.; Falcão, C.B.; Rádis-Baptista, G.; Martins, A.M.C. Antichagasic effect of crotalicidin, a cathelicidin-like vipericidin, found in Crotalus durissus terrificus rattlesnake’s venom gland. Parasitology 2018, 145, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Dematei, A.; Nunes, J.B.; Moreira, D.C.; Jesus, J.A.; Laurenti, M.D.; Mengarda, A.C.A.; Vieira, M.S.; do Amaral, C.P.; Domingues, M.M.; de Moraes, J.; et al. Mechanistic Insights into the Leishmanicidal and Bactericidal Activities of Batroxicidin, a Cathelicidin-Related Peptide from a South American Viper (Bothrops atrox). J. Nat. Prod. 2021, 84, 1787–1798. [Google Scholar] [CrossRef]
- Adade, C.M.; Carvalho, A.L.; Tomaz, M.A.; Costa, T.F.; Godinho, J.L.; Melo, P.A.; Lima, A.P.C.A.; Rodrigues, J.C.F.; Zingali, R.B.; Souto-Padrón, T. Crovirin, a snake venom cysteine-rich secretory protein (CRISP) with promising activity against Trypanosomes and Leishmania. PLoS Negl. Trop. Dis. 2014, 8, e3252. [Google Scholar] [CrossRef]
- Krüger, T.; Engstler, M. Flagellar motility in eukaryotic human parasites. Semin. Cell Dev. Biol. 2015, 46, 113–127. [Google Scholar] [CrossRef]
- Engstler, M.; Pfohl, T.; Herminghaus, S.; Boshart, M.; Wiegertjes, G.; Heddergott, N.; Overath, P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 2007, 131, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Beneke, T.; Demay, F.; Hookway, E.; Ashman, N.; Jeffery, H.; Smith, J.; Valli, J.; Becvar, T.; Myskova, J.; Lestinova, T.; et al. Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog. 2019, 15, e1007828. [Google Scholar] [CrossRef] [Green Version]
- Nematollahi, A.; Jaberi, S.; Helan, J.A.; Sheikhzadeh, N. Histopathological study on parasites in freshwater ornamental fishes in Iran. J. Parasit. Dis. 2016, 40, 756–759. [Google Scholar] [CrossRef] [Green Version]
- Valentine, M.S.; Van Houten, J. Ion channels of cilia: Paramecium as a model. J. Eukaryot. Microbiol. 2022, 69, e12884. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Mesones, S. Down the membrane hole: Ion channels in protozoan parasites. PLoS Pathog. 2022, 18, e1011004. [Google Scholar] [CrossRef] [PubMed]
- Ginger, M.L.; Portman, N.; McKean, P.G. Swimming with protists: Perception, motility and flagellum assembly. Nat. Rev. Microbiol. 2008, 6, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.B.; Kraft, L.G.; van Dooren, G.G.; Goodman, C.D.; Ford, K.L.; Cassin, A.M.; Bacic, A.; McFadden, G.I.; Waller, R.F. Ciliate pellicular proteome identifies novel protein families with characteristic repeat motifs that are common to alveolates. Mol. Biol. Evol. 2011, 28, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mühlpfordt, H. Vergleichende elektronenmikroskopische Untersuchung über die Markierung von Leishmania donovani, Leishmania tropica und Leishmania braziliensis mit Ferritin. Tropenmed. Parasitol. 1975, 26, 385–389. [Google Scholar]
- Balber, A.E. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: Functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit. Rev. Immunol. 1990, 10, 177–201. [Google Scholar]
- Salger, S.A.; Cassady, K.R.; Reading, B.J.; Noga, E.J. A Diverse Family of Host-Defense Peptides (Piscidins) Exhibit Specialized Anti-Bacterial and Anti-Protozoal Activities in Fishes. PLoS ONE 2016, 11, e0159423. [Google Scholar] [CrossRef] [Green Version]
- Preparation of the Live Ciliate Tetrahymena Pyriformis Stained with a Mitochondria-Specific Probe (Mitotracker green) and Viewed with the CSU-10 Confocal Microscope. Available online: http://www.cellimagelibrary.org/images/11972#cite (accessed on 8 March 2023).
- Sauvant, M.; Pepin, D.; Piccinni, E. Tetrahymena pyriformis: A tool for toxicological studies: A review. Chemosphere 1999, 38, 1631–1669. [Google Scholar] [CrossRef]
- Kuleshina, O.N.; Kozlov, L.V.; Cheremnykh, E.G. A universal method for measuring functional activity of complement in humans, laboratory, domestic, and agricultural animals, amphibians, and birds. Bull. Exp. Biol. Med. 2014, 157, 285–287. [Google Scholar] [CrossRef]
- Ziganshin, R.H.; Kovalchuk, S.I.; Arapidi, G.P.; Starkov, V.G.; Hoang, A.N.; Thi Nguyen, T.T.; Nguyen, K.C.; Shoibonov, B.B.; Tsetlin, V.I.; Utkin, Y.N. Quantitative proteomic analysis of Vietnamese krait venoms: Neurotoxins are the major components in Bungarus multicinctus and phospholipases A2 in Bungarus fasciatus. Toxicon 2015, 107, 197–209. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Spirova, E.N.; Serebryakova, M.V.; Shelukhina, I.V.; Kudryavtsev, D.S.; Kryukova, E.V.; Starkov, V.G.; Kopylova, N.V.; Zhmak, M.N.; Ivanov, I.A.; et al. Peptides from puff adder Bitis arietans venom, novel inhibitors of nicotinic acetylcholine receptors. Toxicon 2016, 121, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Radvanyi, F.; Jordan, L.; Russo-Marie, F.; Bon, C. A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal. Biochem. 1989, 177, 103–109. [Google Scholar] [CrossRef]
- Schnaible, V.; Wefing, S.; Resemann, A.; Suckau, D.; Bücker, A.; Wolf-Kümmeth, S.; Hoffmann, D. Screening for disulfide bonds in proteins by MALDI in-source decay and LIFT-TOF/TOF-MS. Anal. Chem. 2002, 74, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ghosh, P.; De, T.; Gomes, A.; Gomes, A.; Dungdung, S.R. In vivo and in vitro antileishmanial activity of Bungarus caeruleus snake venom through alteration of immunomodulatory activity. Exp. Parasitol. 2013, 135, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Muth, T.; Renard, B.Y. Evaluating de novo sequencing in proteomics: Already an accurate alternative to database-driven peptide identification? Brief. Bioinform. 2018, 19, 954–970. [Google Scholar] [CrossRef]
- Chu, C.C.; Li, S.H.; Chen, Y.H. Resolution of isotoxins in the beta-bungarotoxin family. J. Chromatogr. A 1995, 694, 492–497. [Google Scholar] [CrossRef]
- Kondo, K.; Hiroko, I.; Narita, K.; Lee, C.Y. Amino acid sequence of beta 2-bungarotoxin from Bungarus multicinctus venom. The amino acid substitutions in the B chains. J. Biochem. 1982, 91, 1519–1530. [Google Scholar] [CrossRef]
- Chu, C.C.; Chu, S.T.; Chen, S.W.; Chen, Y.H. The non-phospholipase A2 subunit of beta-bungarotoxin plays an important role in the phospholipase A2-independent neurotoxic effect: Characterization of three isotoxins with a common phospholipase A2 subunit. Biochem. J. 1994, 303, 171–176. [Google Scholar] [CrossRef]
- Teixeira, S.C.; da Silva, M.S.; Gomes, A.A.S.; Moretti, N.S.; Lopes, D.S.; Ferro, E.A.V.; Rodrigues, V.M. Panacea within a Pandora’s box: The antiparasitic effects of phospholipases A2 (PLA2s) from snake venoms. Trends Parasitol. 2022, 38, 80–94. [Google Scholar] [CrossRef]
- Chakraborti, T.; Das, P.; Choudhury, R.; De, T. Effect of different serine protease inhibitors in validating the 115 kDa Leishmania donovani secretory serine protease as chemotherapeutic target. Indian J. Biochem. Biophys. 2015, 52, 14–22. [Google Scholar]
- de Almeida Nogueira, N.P.; Morgado-Díaz, J.A.; Menna-Barreto, R.F.; Paes, M.C.; da Silva-López, R.E. Effects of a marine serine protease inhibitor on viability and morphology of Trypanosoma cruzi, the agent of Chagas disease. Acta Trop. 2013, 128, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Babu, C.R. Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: Insight into structural and antimalarial features. Phytochemistry 2009, 70, 703–712. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, R.; Peigneur, S.; Pons, T.; Alvarez, C.; González, L.; Chávez, M.A.; Tytgat, J. The Kunitz-Type Protein ShPI-1 Inhibits Serine Proteases and Voltage-Gated Potassium Channels. Toxins 2016, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Vandermosten, L.; Peigneur, S.; Moreels, L.; Rozenski, J.; Tytgat, J.; Herdewijn, P.; Van den Steen, P.E.; De Jonghe, S. Astemizole analogues with reduced hERG inhibition as potent antimalarial compounds. Bioorg. Med. Chem. 2017, 25, 6332–6344. [Google Scholar] [CrossRef]
- Palomo-Ligas, L.; Gutiérrez-Gutiérrez, F.; Ochoa-Maganda, V.Y.; Cortés-Zárate, R.; Charles-Niño, C.L.; Castillo-Romero, A. Identification of a novel potassium channel (GiK) as a potential drug target in Giardia lamblia: Computational descriptions of binding sites. PeerJ 2019, 7, e6430. [Google Scholar] [CrossRef] [Green Version]
- Rowan, E.G.; Harvey, A.L. Potassium channel blocking actions of beta-bungarotoxin and related toxins on mouse and frog motor nerve terminals. Br. J. Pharmacol. 1988, 94, 839–847. [Google Scholar] [CrossRef]
- Benishin, C.G. Potassium channel blockade by the B subunit of beta-bungarotoxin. Mol. Pharmacol. 1990, 38, 164–169. [Google Scholar]
- Oosawa, Y.; Sokabe, M.; Kasai, M. A cation channel for K+ and Ca2+ from Tetrahymena cilia in planar lipid bilayers. Cell Struct. Funct. 1988, 13, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Schwab, A.; Hanley, P.; Fabian, A.; Stock, C. Potassium channels keep mobile cells on the go. Physiology 2008, 23, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Stanfield, P.R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev. Physiol. Biochem. Pharmacol. 1983, 97, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Schechter, L.E. The potassium channel blockers 4-aminopyridine and tetraethylammonium increase the spontaneous basal release of [3H]5-hydroxytryptamine in rat hippocampal slices. J. Pharmacol. Exp. Ther. 1997, 282, 262–270. [Google Scholar] [PubMed]
- Quintana, J.; Chacón, A.M.; Vargas, L.; Segura, C.; Gutiérrez, J.M.; Alarcón, J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012, 124, 126–132. [Google Scholar] [CrossRef] [PubMed]
- de Moura, A.A.; Kayano, A.M.; Oliveira, G.A.; Setúbal, S.S.; Ribeiro, J.G.; Barros, N.B.; Nicolete, R.; Moura, L.A.; Fuly, A.L.; Nomizo, A.; et al. Purification and biochemical characterization of three myotoxins from Bothrops mattogrossensis snake venom with toxicity against Leishmania and tumor cells. Biomed. Res. Int. 2014, 2014, 195356. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.C.; Vargas, L.J.; Segura, C.; Gutiérrez, J.M.; Pérez, J.C. In vitro antiplasmodial activity of phospholipases A2 and a phospholipase homologue isolated from the venom of the snake Bothrops asper. Toxins 2012, 4, 1500–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, K.; Toda, H.; Narita, K. Characterization of phospholipase A activity of beta1-bungarotoxin from Bungarus multicinctus venom. II. Identification of the histidine residue of beta1-bungarotoxin modified by p-bromophenacyl bromide. J. Biochem. 1978, 84, 1301–1308. [Google Scholar] [CrossRef]
- Valentim-Silva, J.R.; de Barros, N.B.; Macedo, S.R.A.; Ferreira, A.D.S.; Silva, R.S.; Dill, L.S.M.; Zanchi, F.B.; do Nascimento, J.R.; do Nascimento, F.R.F.; Lourenzoni, M.R.; et al. Antileishmanial activity, cytotoxicity and cellular response of amphotericin B in combination with crotamine derived from Crotalus durissus terrificus venom using in vitro and in silico approaches. Toxicon 2022, 217, 96–106. [Google Scholar] [CrossRef]
- de Barros, N.B.; Aragão Macedo, S.R.; Ferreira, A.S.; Tagliari, M.P.; Kayano, A.M.; Nicolete, L.D.F.; Soares, A.M.; Nicolete, R. ASP49-phospholipase A2-loaded liposomes as experimental therapy in cutaneous leishmaniasis model. Int. Immunopharmacol. 2018, 55, 128–132. [Google Scholar] [CrossRef]
- Podešvová, L.; Leštinová, T.; Horáková, E.; Lukeš, J.; Volf, P.; Yurchenko, V. Suicidal Leishmania. Pathogens 2020, 9, 79. [Google Scholar] [CrossRef] [Green Version]








| Chain | Molecular Masses | |
|---|---|---|
| HR 1 | MALDI 2 | |
| B-chain, this work | 7264.52 3 | 7264 |
| B-chain, known | 7292.48 3 gi|82207097 4 Mono 5 | 7291.35 gi|82207097 Av 6 |
| A-chain, this work | 13,408 | |
| A-chain, calculated from HR data 7 | 13,416.96 | |
| A-chain, known | 13,417.02 gi|82206358 Mono | 13,426.16 gi|82206358 Av |
| Native compound | 20,681.48 | 20,672 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, A.V.; Cheremnykh, E.G.; Ziganshin, R.H.; Starkov, V.G.; Nguyen, T.T.T.; Nguyen, K.C.; Le, D.T.; Hoang, A.N.; Tsetlin, V.I.; Utkin, Y.N. The Potassium Channel Blocker β-Bungarotoxin from the Krait Bungarus multicinctus Venom Manifests Antiprotozoal Activity. Biomedicines 2023, 11, 1115. https://doi.org/10.3390/biomedicines11041115
Osipov AV, Cheremnykh EG, Ziganshin RH, Starkov VG, Nguyen TTT, Nguyen KC, Le DT, Hoang AN, Tsetlin VI, Utkin YN. The Potassium Channel Blocker β-Bungarotoxin from the Krait Bungarus multicinctus Venom Manifests Antiprotozoal Activity. Biomedicines. 2023; 11(4):1115. https://doi.org/10.3390/biomedicines11041115
Chicago/Turabian StyleOsipov, Alexey V., Elena G. Cheremnykh, Rustam H. Ziganshin, Vladislav G. Starkov, Trang Thuy Thi Nguyen, Khoa Cuu Nguyen, Dung Tien Le, Anh Ngoc Hoang, Victor I. Tsetlin, and Yuri N. Utkin. 2023. "The Potassium Channel Blocker β-Bungarotoxin from the Krait Bungarus multicinctus Venom Manifests Antiprotozoal Activity" Biomedicines 11, no. 4: 1115. https://doi.org/10.3390/biomedicines11041115
APA StyleOsipov, A. V., Cheremnykh, E. G., Ziganshin, R. H., Starkov, V. G., Nguyen, T. T. T., Nguyen, K. C., Le, D. T., Hoang, A. N., Tsetlin, V. I., & Utkin, Y. N. (2023). The Potassium Channel Blocker β-Bungarotoxin from the Krait Bungarus multicinctus Venom Manifests Antiprotozoal Activity. Biomedicines, 11(4), 1115. https://doi.org/10.3390/biomedicines11041115







