A Combination of an Angiotensin II Receptor and a Neprilysin Inhibitor Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation
Abstract
1. Introduction
2. Materials and Methods
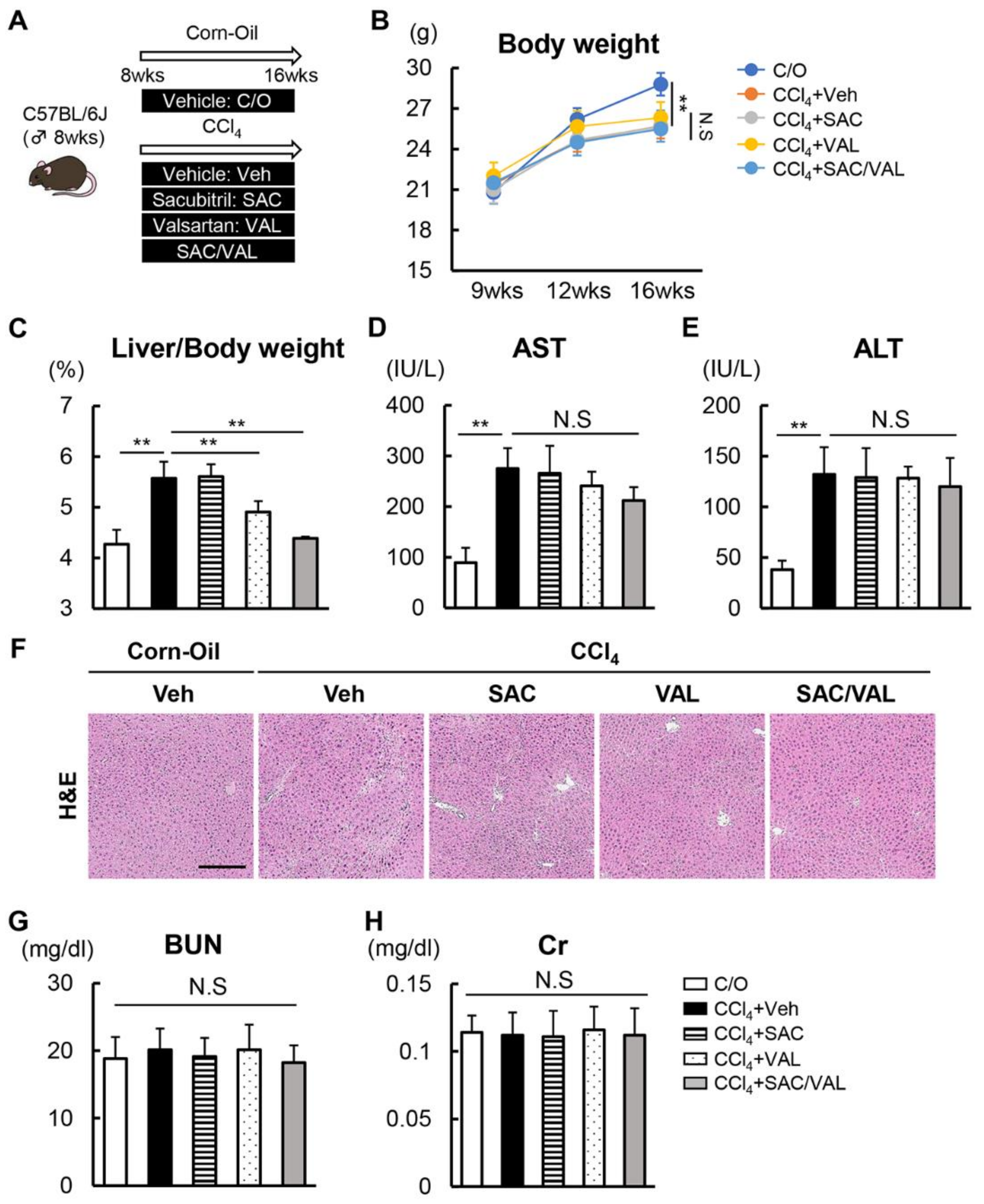
2.1. In Vivo Experimental Design
2.2. Ethics of the Animal Study
2.3. Mouse Plasma AT-II, ANP, and CNP Measurement
2.4. Histological and Immunohistochemical Analyses
2.5. Cell Culture
2.6. Cell Proliferation Assay
2.7. Intracellular cGMP Assay
2.8. PKG Activity Assay
2.9. Protein Kinase C (PKC) Activity Assay
2.10. RNA Isolation and Real-Time Quantitative PCR
2.11. Western Blotting Assay
2.12. Statistical Analyses
3. Results
3.1. Combination of NEP-i with ARB Suppresses CCl4-Induced Liver Fibrosis
3.2. NEP-i and ARB Increase the Serum Levels of ANP and CNP in the CCl4-Treated Mice
3.3. Increased ANP Levels Inhibit Proliferative and Fibrogenic Activity through cGMP/PKG Signaling
3.4. ARB Suppresses AT-II-Induced HSC Proliferation and Pro-Fibrogenic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE-I | angiotensin-converting enzyme inhibitor |
| NP/ANP/CNP | natriuretic peptide/atrial NP/C-type NP |
| ANRI | angiotensin II receptor and neprilysin inhibitor |
| AT-II/AT1R/ARB | angiotensin II/AT-II receptor type 1/AT-II receptor blocker |
| CCl4 | carbon tetrachloride |
| cGMP | cyclic guanosine monophosphate |
| CTGF | connective tissue growth factor |
| ECM | extracellular matrix |
| ELISA | enzyme-linked immunosorbent assay |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GC | guanylyl cyclases |
| H&E | hematoxylin and eosin |
| HRP | horseradish peroxidase |
| HSC | hepatic stellate cell |
| MMP | matrix metalloproteinase |
| NEP-i | neprilysin inhibitor |
| NPR | natriuretic peptide receptor |
| NPY | neuropeptide Y |
| PKC/G | protein kinase C/G |
| RAAS | renin–angiotensin–aldosterone system |
| α-SMA | α-smooth muscle actin |
| SAC | sacubitril |
| TGF | transforming growth factor |
| TIMP | tissue inhibitors of metalloproteinase |
| VAL | valsartan |
References
- Friedman, S.L. Liver fibrosis–from bench to bedside. J. Hepatol. 2003, 38 (Suppl. 1), S38–S53. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef]
- Turco, L.; Garcia-Tsao, G. Portal Hypertension: Pathogenesis and Diagnosis. Clin. Liver Dis. 2019, 23, 573–587. [Google Scholar] [CrossRef]
- Jalan, R.; D’amico, G.; Trebicka, J.; Moreau, R.; Angeli, P.; Arroyo, V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J. Hepatol. 2021, 75 (Suppl. 1), S14–S26. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Bataller, R.; Sancho-Bru, P.; Ginês, P.; Brenner, D.A. Liver Fibrogenesis: A New Role for the Renin–Angiotensin System. Antioxid. Redox Signal. 2005, 7, 1346–1355. [Google Scholar] [CrossRef]
- Munshi, M.K.; Uddin, M.N.; Glaser, S.S. The role of the renin–angiotensin system in liver fibrosis. Exp. Biol. Med. 2011, 236, 557–566. [Google Scholar] [CrossRef]
- Lee, K.-C.; Wu, P.-S.; Lin, H.-C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77–98. [Google Scholar] [CrossRef]
- Bataller, R.; Ginès, P.; Nicolás, J.M.; Görbig, M.N.; Garcia–Ramallo, E.; Gasull, X.; Bosch, J.; Arroyo, V.; Rodés, J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology 2000, 118, 1149–1156. [Google Scholar] [CrossRef]
- Bataller, R.; Gäbele, E.; Schoonhoven, R.; Morris, T.; Lehnert, M.; Yang, L.; Brenner, D.A.; Rippe, R.A. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G642–G651. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Fukui, H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 2001, 34, 745–750. [Google Scholar] [CrossRef]
- Gu, Z.; Fang, L.; Ma, P. The angiotensin-converting enzyme inhibitor, captopril, suppressed hepatic stellate cell activation via NF-kappaB or wnt3α/β-catenin pathway. Bioengineered 2021, 12, 8370–8377. [Google Scholar] [CrossRef]
- Huang, Z.; Khalifa, M.O.; Li, P.; Huang, Y.; Gu, W.; Li, T.-S. Angiotensin receptor blocker alleviates liver fibrosis by altering the mechanotransduction properties of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G446–G456. [Google Scholar] [CrossRef]
- Epstein, F.H.; Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic Peptides. N. Engl. J. Med. 1998, 30, 321–328. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Sangaralingham, S.J.; Kuhn, M.; Cannone, V.; Chen, H.H.; Burnett, J.C. Natriuretic peptide pathways in heart failure: Further therapeutic possibilities. Cardiovasc. Res. 2023, 118, 3416–3433. [Google Scholar] [CrossRef]
- Ishigaki, N.; Yamamoto, N.; Jin, H.; Uchida, K.; Terai, S.; Sakaida, I. Continuos intravenous infusion of atrial natriuretic peptide (ANP) prevented liver fibrosis in rat. Biochem. Biophys. Res. Commun. 2009, 378, 354–359. [Google Scholar] [CrossRef]
- Tao, J.; Mallat, A.; Gallois, C.; Belmadani, S.; Méry, P.-F.; Nhieu, J.T.-V.; Pavoine, C.; Lotersztajn, S. Biological Effects of C-type Natriuretic Peptide in Human Myofibroblastic Hepatic Stellate Cells. J. Biol. Chem. 1999, 274, 23761–23769. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Spratt, J.C.; Haynes, W.G.; Webb, D.J. Inhibition of Neutral Endopeptidase Causes Vasoconstriction of Human Resistance Vessels In Vivo. Circulation 1998, 97, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Mcmurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. PARADIGM-HF Investigators and Committees. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, Y.; Tashiro, K.; Morita, H.; Ideishi, A.; Kuwano, T.; Miura, S.-I. Angiotensin Receptor Blocker and Neprilysin Inhibitor Suppresses Cardiac Dysfunction by Accelerating Myocardial Angiogenesis in Apolipoprotein E-Knockout Mice Fed a High-Fat Diet. J. Renin-Angiotensin-Aldosterone Syst. 2021, 2021, 9916789. [Google Scholar] [CrossRef] [PubMed]
- Myakala, K.; Jones, B.A.; Wang, X.X.; Levi, M. Sacubitril/valsartan treatment has differential effects in modulating diabetic kidney disease in db/db mice and KKAy mice compared with valsartan treatment. Am. J. Physiol. Renal. Physiol. 2021, 320, F1133–F1151. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Zhang, C.; Zhang, C.; Bu, P. Lcz696 Alleviates Myocardial Fibrosis After Myocardial Infarction Through the sFRP-1/Wnt/β-Catenin Signaling Pathway. Front. Pharmacol. 2021, 12, 724147. [Google Scholar] [CrossRef]
- Xu, X.; Yan, Q.; Liu, X.; Li, P.; Li, X.; Chen, Y.; Simoncini, T.; Liu, J.; Zhu, D.; Fu, X. 17β-Estradiol nongenomically induces vascular endothelial H2S release by promoting phosphorylation of cystathionine γ-lyase. J. Biol. Chem. 2019, 294, 15577–15592. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef]
- Huang, J.-S.; Chuang, L.-Y.; Guh, J.-Y.; Chen, C.-J.; Yang, Y.-L.; Chiang, T.-A.; Hung, M.-Y.; Liao, T.-N. Effect of Nitric Oxide-cGMP-Dependent Protein Kinase Activation on Advanced Glycation End-Product–Induced Proliferation in Renal Fibroblasts. J. Am. Soc. Nephrol. 2005, 16, 2318–2329. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Yanase, K.; Namisaki, T.; Yamazaki, M.; Tsujinoue, H.; Imazu, H.; et al. Angiotensin-II induces the tissue inhibitor of metalloproteinases-1 through the protein kinase-C signaling pathway in rat liver fibrosis development. Hepatol. Res. 2003, 27, 51–56. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhang, X.; Wang, J.; Wang, S.; Xiao, X.; Wang, R.; Li, P.; Wang, Y. Angiotensin II induces connective tissue growth factor expression in human hepatic stellate cells by a transforming growth factor β-independent mechanism. Sci. Rep. 2017, 7, 7841. [Google Scholar] [CrossRef]
- Klaiber, M.; Dankworth, B.; Kruse, M.; Hartmann, M.; Nikolaev, V.O.; Yang, R.-B.; Völker, K.; Gaßner, B.; Oberwinkler, H.; Feil, R.; et al. A cardiac pathway of cyclic GMP-independent signaling of guanylyl cyclase A, the receptor for atrial natriuretic peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 18500–18505. [Google Scholar] [CrossRef]
- Li, P.; Wang, D.; Lucas, J.; Oparil, S.; Xing, D.; Cao, X.; Novak, L.; Renfrow, M.B.; Chen, Y.-F. Atrial Natriuretic Peptide Inhibits Transforming Growth Factor β–Induced Smad Signaling and Myofibroblast Transformation in Mouse Cardiac Fibroblasts. Circ. Res. 2008, 102, 185–192. [Google Scholar] [CrossRef]
- Nishikimi, T.; Inaba-Iemura, C.; Ishimura, K.; Tadokoro, K.; Koshikawa, S.; Ishikawa, K.; Akimoto, K.; Hattori, Y.; Kasai, K.; Minamino, N.; et al. Natriuretic peptide/natriuretic peptide receptor-A (NPR-A) system has inhibitory effects in renal fibrosis in mice. Regul. Pept. 2009, 154, 44–53. [Google Scholar] [CrossRef]
- Görbig, M.N.; Ginès, P.; Bataller, R.; Nicolás, J.M.; Garcia-Ramallo, E.; Tobías, E.; Titos, E.; Rey, M.J.; Clària, J.; Arroyo, V.; et al. Atrial natriuretic peptide antagonizes endothelin-induced calcium increase and cell contraction in cultured human hepatic stellate cells. Hepatology 1999, 30, 501–509. [Google Scholar] [CrossRef]
- Franko, A.; Kovarova, M.; Feil, S.; Feil, R.; Wagner, R.; Heni, M.; Königsrainer, A.; Ruoß, M.; Nüssler, A.K.; Weigert, C.; et al. cGMP-dependent protein kinase I (cGKI) modulates human hepatic stellate cell activation. Metabolism 2018, 88, 22–30. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Liu, L.; Lara, L.S.; Seth, D.M.; Navar, L.G.; Prieto, M.C. Angiotensin II Stimulates Renin in Inner Medullary Collecting Duct Cells via Protein Kinase C and Independent of Epithelial Sodium Channel and Mineralocorticoid Receptor Activity. Hypertension 2011, 57, 594–599. [Google Scholar] [CrossRef]
- Bataller, R.; Schwabe, R.F.; Choi, Y.H.; Yang, L.; Paik, Y.H.; Lindquist, J.; Qian, T.; Schoonhoven, R.; Hagedorn, C.H.; Lemasters, J.J.; et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J. Clin. Investig. 2003, 112, 1383–1394. [Google Scholar] [CrossRef]
- Ortiz, C.; Klein, S.; Reul, W.H.; Magdaleno, F.; Gröschl, S.; Dietrich, P.; Schierwagen, R.; Uschner, F.E.; Torres, S.; Hieber, C.; et al. Neprilysin-dependent neuropeptide Y cleavage in the liver promotes fibrosis by blocking NPY-receptor 1. Cell Rep. 2023, 42, 112059. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, J.; Kaji, K.; Nishimura, N.; Kubo, T.; Tomooka, F.; Shibamoto, A.; Iwai, S.; Tsuji, Y.; Fujinaga, Y.; Kitagawa, K.; et al. A Combination of an Angiotensin II Receptor and a Neprilysin Inhibitor Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation. Biomedicines 2023, 11, 1295. https://doi.org/10.3390/biomedicines11051295
Suzuki J, Kaji K, Nishimura N, Kubo T, Tomooka F, Shibamoto A, Iwai S, Tsuji Y, Fujinaga Y, Kitagawa K, et al. A Combination of an Angiotensin II Receptor and a Neprilysin Inhibitor Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation. Biomedicines. 2023; 11(5):1295. https://doi.org/10.3390/biomedicines11051295
Chicago/Turabian StyleSuzuki, Junya, Kosuke Kaji, Norihisa Nishimura, Takahiro Kubo, Fumimasa Tomooka, Akihiko Shibamoto, Satoshi Iwai, Yuki Tsuji, Yukihisa Fujinaga, Koh Kitagawa, and et al. 2023. "A Combination of an Angiotensin II Receptor and a Neprilysin Inhibitor Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation" Biomedicines 11, no. 5: 1295. https://doi.org/10.3390/biomedicines11051295
APA StyleSuzuki, J., Kaji, K., Nishimura, N., Kubo, T., Tomooka, F., Shibamoto, A., Iwai, S., Tsuji, Y., Fujinaga, Y., Kitagawa, K., Namisaki, T., Akahane, T., & Yoshiji, H. (2023). A Combination of an Angiotensin II Receptor and a Neprilysin Inhibitor Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation. Biomedicines, 11(5), 1295. https://doi.org/10.3390/biomedicines11051295





