RETRACTED: Sinapic Acid Attenuate Liver Injury by Modulating Antioxidant Activity and Inflammatory Cytokines in Thioacetamide-Induced Liver Cirrhosis in Rats
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemical and Standard Drug
2.2. Sinapic Acid (SA)
2.3. Acute Toxicity Study and Experimental Animals
2.4. Experimental Animals for Hepatoprotective Activity
2.5. Biochemical Parameters (Liver Function Test)
2.6. Macroscopic Views of Liver
2.7. Histopathology of Liver Tissue
2.8. Immunohistochemistry Investigation
2.9. Liver Tissue Homogenate for Endogenous (CAT, SOD) Enzymes and Oxidative Stress (MDA)
2.10. Estimation of Inflammatory Cytokines
2.11. Statistical Analysis of Data
3. Results
3.1. Acute Toxicity Study
3.2. Effect of SA in TAA-Induced Liver Cirrhosis
3.2.1. Body Heaviness, Liver Mass, and Liver Index
3.2.2. Gross Appearance of Liver
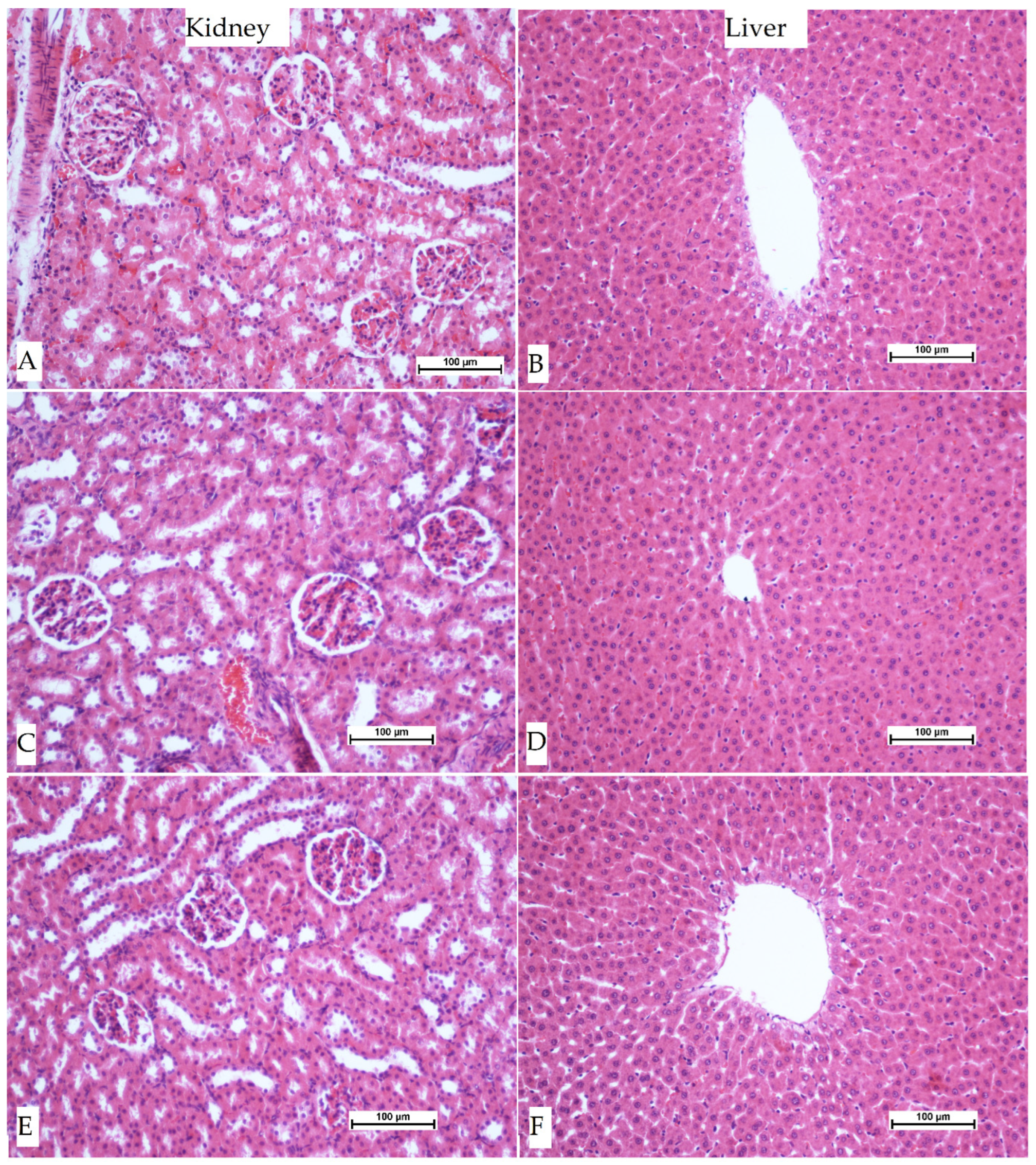
3.3. Histopathological Investigation of Liver Sections
3.4. Immunohistochemical Stain of Hepatocyte
3.5. Effects of SA on Endogenous Antioxidant Enzymes in TAA-Induced Liver Cirrhosis in Rats
3.6. Effect of SA on Cytokines (TNF-α, IL-6, and IL-10) in TAA-Induced Liver Cirrhosis in Rats
3.7. Effect of SA on Liver Biochemistry in TAA-Induced Liver Cirrhosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Kamalidehghan, B.; Ahmadipour, F.; Yehye, W.A. PASS-predicted hepatoprotective activity of Caesalpinia sappan in thioacetamide-induced liver fibrosis in rats. Sci. World J. 2014, 2014, 301879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Medhtiy, M.H.; Jabbar, A.A.; Shareef, S.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Abdulla, M.A. Histopathological Evaluation of Annona muricata in TAA-Induced Liver Injury in Rats. Processes 2022, 10, 1613. [Google Scholar] [CrossRef]
- da Silva, B.S.; Paulino, A.M.B.; Taffarel, M.; Borba, I.G.; Telles, L.O.; Lima, V.V.; Aguiar, D.H.; Dias, M.C.; Nascimento, A.F.; Sinhorin, V.D.G.; et al. High sucrose diet attenuates oxidative stress, inflammation and liver injury in thioacetamide-induced liver cirrhosis. Life Sci. 2021, 267, 118944. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.M.; Ibrahim, I.A.A.; Shahzad, N.; Al-Ghamdi, S.; Ayoub, N.; AlRashdi, A.S.; Abdulla, M.A.; Salehen, N.; Bilgen, M. Hepatoprotectivity of Panduratin A against liver damage: In vivo demonstration with a rat model of cirrhosis induced by thioacetamide. APMIS 2018, 126, 710–721. [Google Scholar] [CrossRef]
- Bradosty, S.W.; Hamad, S.W.; Agha, N.F.S.; Shaikh, F.K.; Qadir Nanakali, N.M.; Aziz, P.Y.; Salehen, N.; Suzergoz, F.; Abdulla, M.A.; Salehen, N.A.; et al. In vivo hepatoprotective effect of Morinda elliptica stem extract against liver fibrosis induced by thioacetamide. Environ. Toxicol. 2021, 36, 2404–2413. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Gad, I.B. Sinapic acid restores blood parameters, serum antioxidants, and liver and kidney functions in obesity. J. Diabetes Metab. Disord. 2022, 21, 293–303. [Google Scholar] [CrossRef]
- Altındağ, F.; Ergen, H. Sinapic acid alleviates cisplatin-induced acute kidney injury by mitigating oxidative stress and apoptosis. Environ. Sci. Pollut. Res. 2023, 30, 12402–12411. [Google Scholar] [CrossRef]
- Mirza, A.C.; Panchal, S.S.; Allam, A.A.; Othman, S.I.; Satia, M.; Mandhane, S.N. Mandhane, Syringic Acid Ameliorates Cardiac, Hepatic, Renal and Neuronal Damage Induced by Chronic Hyperglycaemia in Wistar Rats: A Behavioural, Biochemical and Histological Analysis. Molecules 2022, 27, 6722. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Ansari, M.A.; Raish, M.; Alkharfy, K.M.; Ahad, A.; Al-Jenoobi, F.I.; Haq, N.; Khan, M.R.; Ahmad, A. Sinapic Acid Ameliorates Oxidative Stress, Inflammation, and Apoptosis in Acute Doxorubicin-Induced Cardiotoxicity via the NF-κB-Mediated Pathway. BioMed Res. Int. 2020, 2020, 3921796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, M.; Kim, H.J.; Heo, H.; Kim, M.; Jeon, Y.; Lee, H.; Lee, J. Comparison of the Antihypertensive Activity of Phenolic Acids. Molecules 2022, 27, 6185. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Mainzen Prince, P.S. Protective effects of sinapic acid on cardiac hypertrophy, dyslipidaemia and altered electrocardiogram in isoproterenol-induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 699, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Aldubayan, M.A.; Ahmed, A.S.; Emara, A.M.; Ahmed, A.A.; Elgharabawy, R.M. Sinapic Acid Attenuates Cardiovascular Disorders in Rats by Modulating Reactive Oxygen Species and Angiotensin Receptor Expression. Oxid. Med. Cell. Longev. 2020, 2020, 1436858. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Vergara, P.; Moreno, M.G.; Muriel, P. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim. Biophys. Acta 2007, 1770, 989–996. [Google Scholar] [CrossRef]
- Niederreiter, L.; Tilg, H. Cytokines and fatty liver diseases. Liver Res. 2018, 2, 14–20. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Wang, H.; Lin, Z.; Shao, K.; Xu, J.; Zhao, Y. Ameliorative effect of white tea from 50-year-old tree of Camellia sinensis L. (Theaceae) on kidney damage in diabetic mice via SIRT1/AMPK pathway. J. Ethnopharmacol. 2021, 272, 113919. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, V.; Pandit, K.; Tuli, H.S.; Sak, K.; Jain, S.K.; Kaur, S. Antioxidant Phytoconstituents From Onosma bracteata Wall. (Boraginaceae) Ameliorate the CCl4 Induced Hepatic Damage: In Vivo Study in Male Wistar Rats. Front. Pharmacol. 2020, 11, 1301. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Rohani, M.; Pourshafiea, M.R. The effects of the probiotic cocktail on modulation of the NF-kB and JAK/STAT signaling pathways involved in the inflammatory response in bowel disease model. BMC Immunol. 2022, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.A.; Abdullah, F.O.; Abdoulrahman, K.; Galali, Y.; Ibrahim, I.A.; Alzahrani, A.R.; Hassan, R.R. Gastroprotective, Biochemical, and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats. Processes 2022, 10, 1985. [Google Scholar] [CrossRef]
- Latief, U.; Tung, G.K.; Singh, H.; Per, T.S.; Jain, S.K. Bergenia ciliata as a future candidate for liver diseases: A concise review. J. Basic Appl. Zool. 2022, 83, 7028314 . [Google Scholar] [CrossRef]
- Salama, S.; Kue, C.S.; Mohamad, H.; Omer, F.; Ibrahim, M.Y.; Abdulla, M.; Ali, H.; Mariod, A.; Jayash, S.N. Hepatoprotective potential of a novel quinazoline derivative in thioacetamide-induced liver toxicity. Front. Pharmacol. 2022, 13, 943340. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.M.; Abdulla, M.A.; AlRashdi, A.S.; Ismail, S.; Alkiyumi, S.S.; Golbabapour, S. Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complement. Altern. Med. 2013, 13, 56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phyther. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Wong, W.L.; Abdulla, M.A.; Chua, K.H.; Kuppusamy, U.R.; Tan, Y.S.; Sabaratnam, V. Hepatoprotective effects of Panus giganteus (Berk.) corner against thioacetamide-(TAA-) induced liver injury in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 170303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Yehye, W.A. Corrigendum to: Hepatoprotective role of ethanolic extract of vitex negundo in thioacetamide-induced liver fibrosis in male rats. Evid. Based Complement. Altern. Med. 2018, 2018, 8464628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salama, S.M.; Abdulla, M.A.; Alrashdi, A.S.; Hadi, A.H.A. Mechanism of hepatoprotective effect of Boesenbergia rotunda in thioacetamide-induced liver damage in rats. Evid. Based Complement. Altern. Med. 2013, 2013, 157456. [Google Scholar] [CrossRef][Green Version]
- Alshawsh, M.A.; Amin, Z.A.; Ismail, S.; and Abdulla, M.A. Gene Expression Profiling Reveals Underlying Molecular Mechanism of Hepatoprotective Effect of Orthosiphon stamineus and Morinda citrifolia on Thioacetamide-Induced Hepatotoxicity in Rats. Public Health Genom. 2015, 18, 20–25. [Google Scholar] [CrossRef]
- Abood, W.N.; Bradosty, S.W.; Shaikh, F.K.; Salehen, N.A.; Farghadani, R.; Agha, N.F.S.; Al-Medhtiy, M.H.; Kamil, T.D.A.; Agha, A.S.; Abdulla, M.A. Garcinia mangostana peel extracts exhibit hepatoprotective activity against thioacetamide-induced liver cirrhosis in rats. J. Funct. Foods 2020, 74, 104200. [Google Scholar] [CrossRef]
- Salama, S.M.; Bilgen, M.; Al Rashdi, A.S.; Abdulla, M.A. Efficacy of Boesenbergia rotunda treatment against thioacetamide-induced liver cirrhosis in a rat model. Evid. Based Complement. Altern. Med. 2012, 2012, 137083. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jabbar, A.A.; Abdulrahman, K.K.; Abdulsamad, P.; Mojarrad, S.; Mehmetçik, G. Phytochemical profile, Antioxidant, Enzyme inhibitory and acute toxicity activity of Astragalus bruguieri. Baghdad Sci. J. 2022, 20, 157–165. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdullah, F.O.; Abdulrahman, K.K.; Galali, Y.; Sardar, A.S. GC-MS Analysis of Bioactive Compounds in Methanolic Extracts of Papaver decaisnei and Determination of Its Antioxidants and Anticancer Activities. J. Food Qual. 2022, 2022, 1405157. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.; Salama, R.A.A. Therapeutic effect of dunaliella salina microalgae on thioacetamide-(taa-) induced hepatic liver fibrosis in rats: Role of tgf-β and mmp9. BioMed Res. Int. 2019, 2019, 17. [Google Scholar] [CrossRef][Green Version]
- Hamad Shareef, S.; Abdel Aziz Ibrahim, I.; Alzahrani, A.R.; Al-Medhtiy, M.H.; Ameen Abdulla, M. Hepatoprotective effects of methanolic extract of green tea against Thioacetamide-Induced liver injury in Sprague Dawley rats. Saudi. J. Biol. Sci. 2022, 29, 564–573. [Google Scholar] [CrossRef]
- Rouhollahi, E.; Moghadamtousi, S.Z.; Baig, S.; Looi, C.Y.; Abdulla, M.A.; Mohamed, Z. Dichloromethane Curcuma purpurascens BI Rhizome Extract Prevents Thioacetamide-Induced Liver Cirrhosis in Rats. Public Health Genom. 2015, 18, 33–39. [Google Scholar]
- Osman Mahmud, S.; Hamad Shareef, S.; Jabbar, A.A.J.; Hassan, R.R.; Jalal, H.K.; Abdulla, M.A. Green Synthesis of Silver Nanoparticles from Aqueous Extract of Tinospora crispa Stems Accelerate Wound Healing in Rats. Int. J. Low Extrem. Wounds 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Wang, P.-F.; Xie, K.; Cao, Y.-X.; Zhang, A. Hepatoprotective Effect of Mitochondria-Targeted Antioxidant Mito-TEMPO against Lipopolysaccharide-Induced Liver Injury in Mouse. Mediat. Inflamm. 2022, 2022, 6394199. [Google Scholar] [CrossRef]
- Bagheri, E.; Saremi, K.; Hajiaghaalipour, F.; Faraj, F.L.; Ali, H.M.; Abdulla, M.A.; Khaing, S.L.; Salehen, N. Synthesis of Novel Derivatives of Quinazoline Schiff base Compound Promotes Epithelial Wound Healing. Curr. Pharm. Des. 2018, 24, 1395–1404. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdullah, F.O.; Hassan, A.O.; Galali, Y.; Hassan, R.R.; Rashid, E.Q.; Salih, M.I.; Aziz, K.F. Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules 2022, 27, 8687. [Google Scholar] [CrossRef] [PubMed]
- Abdulaziz Bardi, D.; Halabi, M.F.; Abdullah, N.A.; Rouhollahi, E.; Hajrezaie, M.; Abdulla, M.A. In vivo evaluation of ethanolic extract of Zingiber officinale rhizomes for its protective effect against liver cirrhosis. BioMed Res. Int. 2013, 2013, 918460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azab, A.E.; Albasha, M.O. Hepatoprotective Effect of Some Medicinal Plants and Herbs against Hepatic Disorders Induced by Hepatotoxic Agents. J. Biotechnol. Bioeng. 2018, 2, 8–23. [Google Scholar]
- Unsal, V.; Kolukcu, E.; Gevrek, F.; Firat, F. Sinapic acid reduces ischemia/reperfusion injury due to testicular torsion/detorsion in rats. Andrologia 2021, 53, e14117. [Google Scholar] [CrossRef]
- Yang, M.; Xiong, J.; Zou, Q.; Wang, X.; Hu, K.; Zhao, Q. Sinapic Acid Attenuated Cardiac Remodeling After Myocardial Infarction by Promoting Macrophage M2 Polarization Through PPARγ Pathway. Front. Cardiovasc. Med. 2022, 9, 915903. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef][Green Version]
- Shin, D.S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.O. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch. Pharm. Res. 2013, 36, 626–633. [Google Scholar] [CrossRef]
- Lee, S.-B.; Yang, H.O. Sinapic Acid Ameliorates REV-ERB α Modulated Mitochondrial Fission against MPTP-Induced Parkinson’s Disease Model. Biomol. Ther. 2022, 30, 409–417. [Google Scholar] [CrossRef]
- Amin, Z.A.; Bilgen, M.; Alshawsh, M.A.; Ali, H.M.; Hadi, A.H.A.; Abdulla, M.A. Protective Role of Phyllanthus niruri Extract against Thioacetamide-Induced Liver Cirrhosis in Rat Model. Evid. Based Complement. Alternat. Med. 2012, 2012, 241583. [Google Scholar] [CrossRef][Green Version]
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective Effect of Sinapic Acid on Ethanol-Induced Gastric Ulcers in Rats: Involvement of Nrf2/HO-1 and NF-κB Signaling and Antiapoptotic Role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Ibrahim, I.A.A.; Abdullah, F.O.; Aziz, K.F.; Alzahrani, A.R.; Abdulla, M.A. Chemopreventive Effects of Onosma mutabilis against Azoxymethane-Induced Colon Cancer in Rats via Amendment of Bax/Bcl-2 and NF-κB Signaling Pathways. Curr. Issues Mol. Biol. 2023, 45, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M. Hematological and biochemical investigations on the effect of curcumin and Thymoquinone in male mice exposed to Thioacetamide. Saudi. J. Biol. Sci. 2022, 29, 660–665. [Google Scholar] [CrossRef] [PubMed]
- ElBaset, M.A.; Salem, R.S.; Ayman, F.; Ayman, N.; Shaban, N.; Afifi, S.M.; Esatbeyoglu, T.; Abdelaziz, M.; Elalfy, Z.S. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Kim, D.I.; Nam, H.C.; Chang, U.I.; Yang, J.M.; Song, D.S. Association between serum tumor necrosis factor-α and sarcopenia in liver cirrhosis. Clin. Mol. Hepatol. 2022, 28, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Ciciliato, M.P.; de Souza, M.C.; Tarran, C.M.; de Castilho, A.L.; Vieira, A.J.; Rozza, A.L. Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation. Pharmaceutics 2022, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Yan, R.; Wang, S.; Li, G.; Kang, X.; Hu, Y.; Lin, M.; Shan, W.; Zhao, Y.; Wang, Z.; et al. Sinapic Acid Reduces Oxidative Stress and Pyroptosis via Inhibition of BRD4 in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 668708. [Google Scholar] [CrossRef] [PubMed]






| (A) | ||||||
| Animals Groups | ALP (IU/L) | ALT (IU/L) | AST (IU/L) | T. Bilirubin (µmol/L) | T. Protein (g/L) | Albumin (g/L) |
| Control 10% Tween 20 | 79 ± 2.34 | 39.1 ± 3.62 | 59.2 ± 1.22 | 1.23 ± 0.23 | 71.2 ± 2.19 | 26.2 ± 1.3 |
| SA 2 g/kg | 71.16 ± 2.10 | 48.16 ± 2.59 | 62.23 ± 2.12 | 1.35 ± 0.03 | 70.30 ± 2.72 | 24.54 ± 3.2 |
| SA 4 g/kg | 75 ± 2.30 | 38.2 ± 1.76 | 58 ± 2.15 | 1.30 ± 0.04 | 74.25 ± 3.51 | 25.36 ± 3.30 |
| (B) | ||||||
| Animals Groups | Sodium mmol/L | Potassium mmol/L | Chloride mmol/L | Urea mmol/L | Creatinine µmol/L | |
| Control 10% Tween 20 | 148 ± 2.37 | 5.1 ± 0.32 | 107 ± 3.039 | 4.41 ± 0.21 | 42.22 ± 3.60 | |
| SA 2 g/kg | 145.21 ± 2.35 | 5.32 ± 3.65 | 136.5 ± 3.82 | 5.28 ± 0.32 | 37.23 ± 2.72 | |
| SA 4 g/kg | 141.22 ± 3.32 | 5.1 ± 0.30 | 100.23 ± 2.41 | 4.72 ± 0.45 | 40.32 ± 3.20 | |
| Groups | Body Weight (gm) | Liver Weight (gm) | Liver Index LW/BW % |
|---|---|---|---|
| Negative control | 343.14 ± 5.75 a | 8.25 ± 0.03 a | 2.40 |
| TAA control (200 mg/kg) | 210.34 ± 3.30 d | 11.28 ± 0.04 b | 5.36 |
| Silymarin (50 mg/kg) + TAA(200 mg/kg) | 323.20 ± 6.40 b | 9.45 ± 0.07 a | 2.92 |
| SA (20 mg)/kg + TAA(200 mg/kg) | 264.42 ± 4.54 c | 10.27 ± 0.04 a | 3.88 |
| SA (40 mg/kg) + TAA(200 mg/kg) | 334.5 ± 5.33 c | 8.9 ± 0.05 a | 2.66 |
| Groups | ALP IU/L | ALT IU/L | AST IU/L | T. Bilirubin (µmol/L) | Protein g/L | Albumin g/L |
|---|---|---|---|---|---|---|
| Control (10% Tween 20) | 73.54 ± 3.30 a | 40.3 ± 3.18 a | 63.15 ± 2.5 a | 11.20 ± 0.19 a | 70.14 ± 1.20 a | 24.27 ± 1.24 a |
| TAA control (200 mg/kg) | 257.3 ± 3.41 d | 177 ± 2.01 d | 255.0 ± 3.4 d | 6.21 ± 0.03 d | 43.30 ± 3.41 c | 14.81 ± 2.07 e |
| Silymarin (50 mg/kg) + TAA(200 mg/kg) | 79.45 ± 1.0 a | 46.4 ± 1.21 a | 69.87 ± 1.6 a | 1.42 ± 0.07 b | 63.45 ± 1.32 b | 21.53 ± 2.04 b |
| SA (20 mg/kg) + TAA(200 mg/kg) | 94.75 ± 1.54 c | 84.22 ± 1.3 c | 86.42 ± 1.5 c | 2.50 ± 0.07 c | 59.12 ± 1.2 b | 16.56 ± 1.17 d |
| SA (40 mg/kg) + TAA(200 mg/kg) | 81.57 ± 3.23 b | 55.55 ± 3.5 b | 74.45 ± 2.0 b | 1.22 ± 0.7 b | 62.22 ± 1.1 b | 19.47 ± 0.99 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbar, A.A.J.; Alamri, Z.Z.; Abdulla, M.A.; AlRashdi, A.S.; Najmaldin, S.K.; Zainel, M.A. RETRACTED: Sinapic Acid Attenuate Liver Injury by Modulating Antioxidant Activity and Inflammatory Cytokines in Thioacetamide-Induced Liver Cirrhosis in Rats. Biomedicines 2023, 11, 1447. https://doi.org/10.3390/biomedicines11051447
Jabbar AAJ, Alamri ZZ, Abdulla MA, AlRashdi AS, Najmaldin SK, Zainel MA. RETRACTED: Sinapic Acid Attenuate Liver Injury by Modulating Antioxidant Activity and Inflammatory Cytokines in Thioacetamide-Induced Liver Cirrhosis in Rats. Biomedicines. 2023; 11(5):1447. https://doi.org/10.3390/biomedicines11051447
Chicago/Turabian StyleJabbar, Ahmed A. J., Zaenah Zuhair Alamri, Mahmood Ameen Abdulla, Ahmed S. AlRashdi, Soran Kayfi Najmaldin, and Mustafa AbdulMonam Zainel. 2023. "RETRACTED: Sinapic Acid Attenuate Liver Injury by Modulating Antioxidant Activity and Inflammatory Cytokines in Thioacetamide-Induced Liver Cirrhosis in Rats" Biomedicines 11, no. 5: 1447. https://doi.org/10.3390/biomedicines11051447
APA StyleJabbar, A. A. J., Alamri, Z. Z., Abdulla, M. A., AlRashdi, A. S., Najmaldin, S. K., & Zainel, M. A. (2023). RETRACTED: Sinapic Acid Attenuate Liver Injury by Modulating Antioxidant Activity and Inflammatory Cytokines in Thioacetamide-Induced Liver Cirrhosis in Rats. Biomedicines, 11(5), 1447. https://doi.org/10.3390/biomedicines11051447









