Performance of 11 Host Biomarkers Alone or in Combination in the Diagnosis of Late-Onset Sepsis in Hospitalized Neonates: The Prospective EMERAUDE Study
Abstract
:1. Introduction
2. Materials and Methods
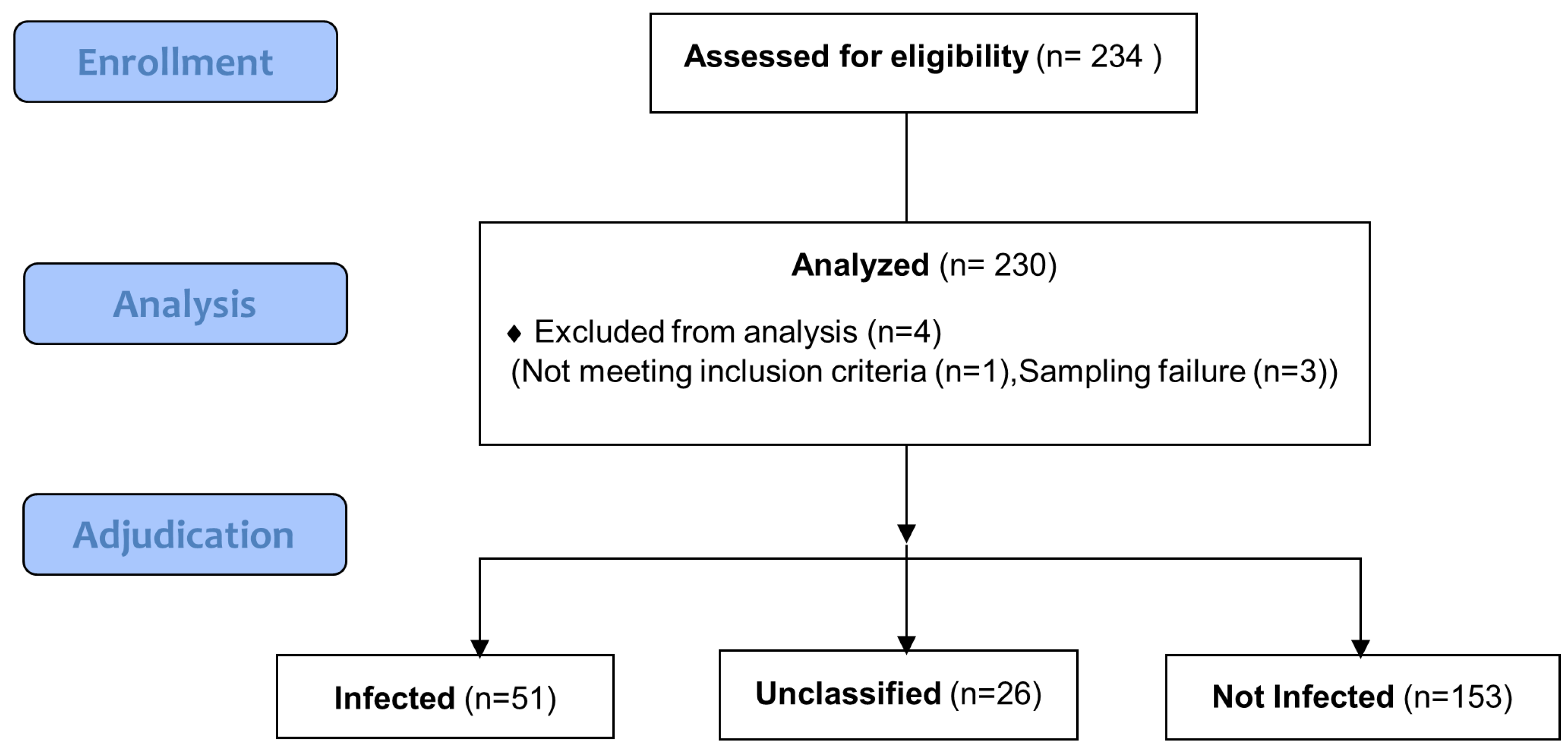
2.1. Study Design and Participants
2.2. Sample Collection and Biomarker Measurement
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Demographics and Microbiological Characteristics According to Infection Status
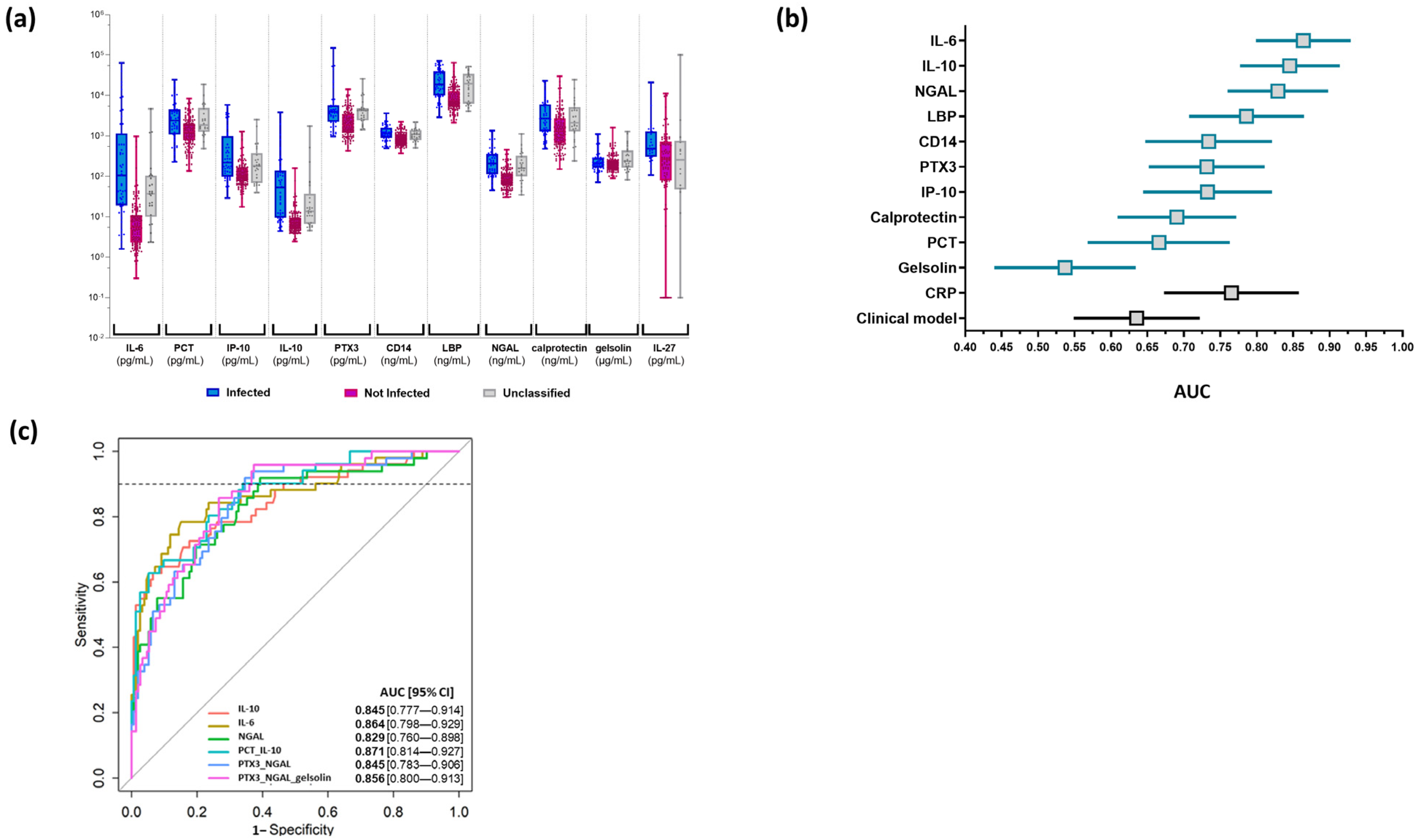
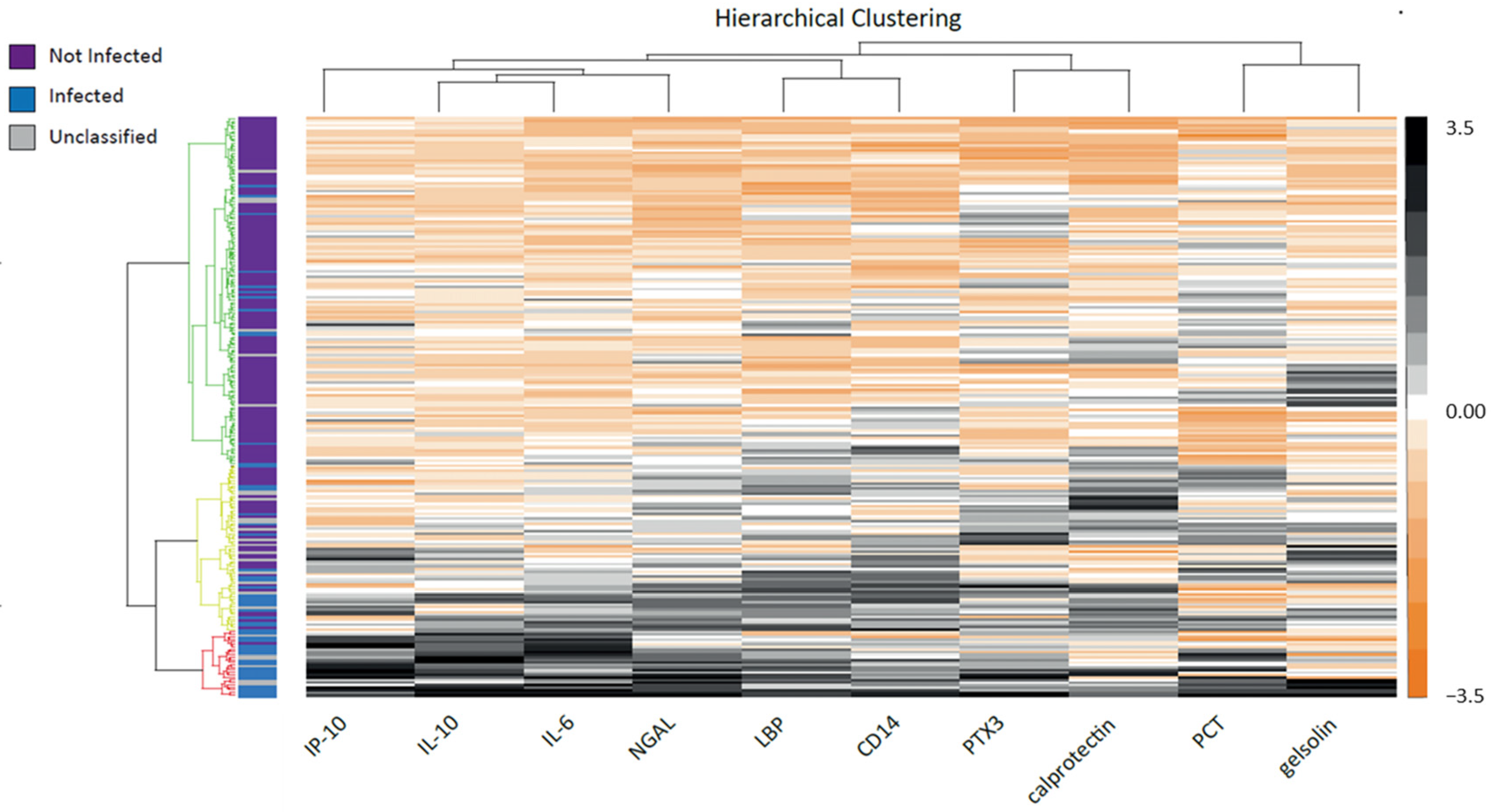
3.3. Biomarkers’ Characteristics
3.4. Application of the Best Models to the Cohort
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Assessment of Outcomes by the Adjudication Committee
| Case Id | Expert 1 | Expert 2 | Expert 3 | Conclusion |
|---|---|---|---|---|
| 1 | Infected | Infected | Infected | Infected |
| 2 | Infected | Infected | Unclassified | Infected |
| 3 | Infected | Unclassified | Unclassified | Unclassified |
| 4 | Not Infected | Not Infected | Not Infected | Not Infected |
| 5 | Not Infected | Not Infected | Unclassified | Not Infected |
| 6 | Not Infected | Unclassified | Unclassified | Unclassified |
| 7 | Infected | Not Infected | Unclassified | Unclassified |
| 9 | Unclassified | Unclassified | Unclassified | Unclassified |
| 10 | Infected | Infected | Not Infected | To review |
| 11 | Not Infected | Not Infected | Infected | To review |
References
- Boghossian, N.S.; Page, G.P.; Bell, E.F.; Stoll, B.J.; Murray, J.C.; Cotten, C.M.; Shankaran, S.; Walsh, M.C.; Laptook, A.R.; Newman, N.S.; et al. Late-Onset Sepsis in Very Low Birth Weight Infants from Singleton and Multiple-Gestation Births. J. Pediatr. 2013, 162, 1120–1124.e1. [Google Scholar] [CrossRef] [Green Version]
- Bekhof, J.; Reitsma, J.B.; Kok, J.H.; Van Straaten, I.H.L.M. Clinical signs to identify late-onset sepsis in preterm infants. Eur. J. Pediatr. 2013, 172, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Connell, T.G.; Rele, M.; Cowley, D.; Buttery, J.P.; Curtis, N. How Reliable Is a Negative Blood Culture Result? Volume of Blood Submitted for Culture in Routine Practice in a Children’s Hospital. Pediatrics 2007, 119, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef]
- Ting, J.Y.; Synnes, A.; Roberts, A.; Deshpandey, A.; Dow, K.; Yoon, E.W.; Lee, K.-S.; Dobson, S.; Lee, S.K.; Shah, P.S.; et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016, 170, 1181–1187. [Google Scholar] [CrossRef]
- Penders, J.; Kummeling, I.; Thijs, C. Infant antibiotic use and wheeze and asthma risk: A systematic review and meta-analysis. Eur. Respir. J. 2011, 38, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, J.Y.; Roberts, A. Association of early life antibiotics and health outcomes: Evidence from clinical studies. Semin. Perinatol. 2020, 44, 151322. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N.; Shastri, S.; Sharma, P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J. Matern.-Fetal Neonatal Med. 2017, 31, 1646–1659. [Google Scholar] [CrossRef]
- Perrone, S.; Lotti, F.; Longini, M.; Rossetti, A.; Bindi, I.; Bazzini, F.; Belvisi, E.; Sarnacchiaro, P.; Scapellato, C.; Buonocore, G. C reactive protein in healthy term newborns during the first 48 hours of life. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 103, F163–F166. [Google Scholar] [CrossRef]
- Brown, J.V.E.; Meader, N.; Wright, K.; Cleminson, J.; McGuire, W. Assessment of C-Reactive Protein Diagnostic Test Accuracy for Late-Onset Infection in Newborn Infants: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, 260–268. [Google Scholar] [CrossRef]
- Ng, P.C.; Li, K.; Chui, K.M.; Leung, T.F.; Wong, R.P.O.; Chu, W.C.W.; Wong, E.; Fok, T.F. IP-10 Is an Early Diagnostic Marker for Identification of Late-Onset Bacterial Infection in Preterm Infants. Pediatr. Res. 2007, 61, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, C.; Frezza, S.; Cingolani, A.; De Luca, A.; Puopolo, M.; De Carolis, M.P.; Vento, G.; Antinori, A.; Tortorolo, G. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur. J. Pediatr. 2001, 160, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, I.R.; Yacoub, A.; Smolkin, T.; Sujov, P.; Kassis, I.; Sprecher, H. Values of C-reactive protein, procalcitonin, and Staphylococcus -specific PCR in neonatal late-onset sepsis. Acta Paediatr. 2006, 95, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Du, W.X.; Jiang, H.Y.; Ai, Q.; Feng, J.; Liu, Z.; Yu, J.L. Multiplex Cytokine Profiling Identifies Interleukin-27 as a Novel Biomarker for Neonatal Early Onset Sepsis. Shock 2017, 47, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Khaertynov, K.S.; Boichuk, S.V.; Khaiboullina, S.F.; Anokhin, V.A.; Andreeva, A.A.; Lombardi, V.C.; Satrutdinov, M.A.; Agafonova, E.A.; Rizvanov, A.A. Comparative Assessment of Cytokine Pattern in Early and Late Onset of Neonatal Sepsis. J. Immunol. Res. 2017, 2017, 8601063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smertka, M.; Wroblewska, J.; Suchojad, A.; Majcherczyk, M.; Jadamus-Niebroj, D.; Owsianka-Podlesny, T.; Brzozowska, A.; Maruniak-Chudek, I. Serum and Urinary NGAL in Septic Newborns. BioMed Res. Int. 2014, 2014, 717318. [Google Scholar] [CrossRef] [Green Version]
- Terrin, G.; Passariello, A.; Manguso, F.; Salvia, G.; Rapacciuolo, L.; Messina, F.; Raimondi, F.; Canani, R.B. Serum Calprotectin: An Antimicrobial Peptide as a New Marker for the Diagnosis of Sepsis in Very Low Birth Weight Newborns. J. Immunol. Res. 2011, 2011, 291085. [Google Scholar] [CrossRef]
- Caironi, P.; Masson, S.; Mauri, T.; Bottazzi, B.; Leone, R.; Magnoli, M.; Barlera, S.; Mamprin, F.; Fedele, A.; Mantovani, A.; et al. Pentraxin 3 in patients with severe sepsis or shock: The ALBIOS trial. Eur. J. Clin. Investig. 2017, 47, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Halis, H.; Gunes, T.; Korkut, S.; Saraymen, B.; Şen, A.; Bastug, O.; Öztürk, A.; Kurtoğlu, S. In the diagnosis of neonatal sepsis importance of gelsolin and relationship with mortality and morbidity. Med. Hypotheses 2016, 94, 77–80. [Google Scholar] [CrossRef]
- Leante-Castellanos, J.L.; de Guadiana-Romualdo, L.G.; Fuentes-Gutiérrez, C.; Hernando-Holgado, A.; García-González, A.; Jiménez-Santos, E. The value of lipopolysaccharide binding protein for diagnosis of late-onset neonatal sepsis in very low birth weight infants. J. Périnat. Med. 2015, 43, 253–257. [Google Scholar] [CrossRef]
- Topcuoğlu, S.; Arslanbuga, C.; Gursoy, T.; Aktas, A.; Karatekin, G.; Uluhan, R.; Ovali, F. Role of presepsin in the diagnosis of late-onset neonatal sepsis in preterm infants. J. Matern.-Fetal Neonatal Med. 2016, 29, 1834–1839. [Google Scholar] [CrossRef]
- Ma, H.; Bandos, A.I.; Rockette, H.E.; Gur, D. On use of partial area under the ROC curve for evaluation of diagnostic performance. Stat. Med. 2013, 32, 3449–3458. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain, Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.; Sobh, H.; Abdalla, M.O.; El-Sharkawy, S.; Rezk, A.R.; Khashana, A. Salivary and Serum Interleukin-10, C-Reactive Protein, Mean Platelet Volume, and CRP/MPV Ratio in the Diagnosis of Late-Onset Neonatal Sepsis in Full-Term Neonates. J. Immunol. Res. 2021, 2021, 4884537. [Google Scholar] [CrossRef]
- Sharma, A.A.; Jen, R.; Butler, A.; Lavoie, P.M. The developing human preterm neonatal immune system: A case for more research in this area. Clin. Immunol. 2012, 145, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkqvist, M.; Källman, J.; Fjaertoft, G.; Xu, S.; Venge, P.; Schollin, J. Human neutrophil lipocalin: Normal levels and use as a marker for invasive infection in the newborn. Acta Paediatr. 2004, 93, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Argyri, I.; Xanthos, T.; Varsami, M.; Aroni, F.; Papalois, A.; Dontas, I.; Fanos, V.; Iacovidou, N. The Role of Novel Biomarkers in Early Diagnosis and Prognosis of Acute Kidney Injury in Newborns. Am. J. Perinatol. 2013, 30, 347–352. [Google Scholar] [CrossRef]
- Tosson, A.M.S.; Koptan, D.M.T.; Kamal, M.; Elhady, M.A. Assessment of Serum Interleukin-27 and Mean Platelet Volume in Late-Onset Neonatal Sepsis. Am. J. Perinatol. 2022. [Google Scholar] [CrossRef]
- Zeitoun, A.A.; Gad, S.S.; Attia, F.M.; Abu Maziad, A.S.; Bell, E.F. Evaluation of neutrophilic CD64, interleukin 10 and procalcitonin as diagnostic markers of early- and late-onset neonatal sepsis. Scand. J. Infect. Dis. 2010, 42, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Aldo, P.; Marusov, G.; Svancara, D.; David, J.; Mor, G. Simple PlexTM: A Novel Multi-Analyte, Automated Microfluidic Immunoassay Platform for the Detection of Human and Mouse Cytokines and Chemokines. Am. J. Reprod. Immunol. 2016, 75, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Taneja, R.; Batra, P. Biomarkers as point of care tests (POCT) in neonatal sepsis: A state of science review. J. Neonatal-Perinatal Med. 2021, 14, 331–338. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 230) | Infected (n = 51) | Not Infected (n = 153) | Unclassified (n = 26) | p Value | Adjusted p Value 1 | |
|---|---|---|---|---|---|---|
| Demographic characteristics, No. (%) | ||||||
| Sex, male | 137 (59.6) | 39 (76.5) | 86 (56.2) | 12 (46.2) | 0.011 | 0.231 |
| Gestational age (in weeks), Median (range) | 27 (23–41) | 27 (24–41) | 28 (23–41) | 26.5 (24–38) | 0.610 | 1.000 |
| Birth weight (g), Median (range) | 940 (450–4660) | 960 (530–3400) | 930 (450–4660) | 902.5 (490–3430) | 0.692 | 1.000 |
| Birth weight < 1500 g | 184 (80.0) | 39 (76.5) | 126 (82.4) | 19 (73.1) | 0.393 | 1.000 |
| Apgar Score at 5 min, Median (range) | 8 (1–10) | 9 (1–10) | 8 (1–10) | 8 (4–10) | 0.850 | 1.000 |
| Small for gestational Age | 67 (29.1) | 10 (19.6) | 52 (34.0) | 5 (19.2) | 0.074 | 1.000 |
| C-section birth | 152 (66.1) | 27 (52.9) | 109 (71.2) | 16 (61.5) | 0.051 | 0.816 |
| Histological chorioamnionitis | 36 (16.5) | 7 (14.6) | 26 (17.8) | 3 (12.5) | 0.816 | 1.000 |
| Congenital malformations | 41 (17.8) | 13 (25.5) | 25 (16.3) | 3 (11.5) | 0.260 | 1.000 |
| Surgery prior to inclusion | 35 (15.2) | 17 (33.3) | 15 (9.8) | 3 (11.5) | 0.001 | 0.028 |
| Time from surgery to inclusion(in days), Median (range) | 15.0 (4.0–63.0) | 16 (6–63) | 15 (6–43) | 6 (4–52) | 0.566 | 1.000 |
| Clinical features at inclusion, No. (%) | ||||||
| Calculated age (in days), Median (range) | 14 (7–178) | 11 (7–159) | 15 (7–178) | 14 (7–69) | 0.637 | 1.000 |
| Fever > 38 °C | 84/229 (36.7) | 25/51(50) | 103/153 (67.3) | 17/26 (65.4) | 0.087 | 1.000 |
| Tachycardia > 160 bpm | 124/230 (53.9) | 33/51 (64.7) | 74/153(48.4) | 17/26 (65.4) | 0.065 | 0.975 |
| Capillary refill time > 3 s | 18/226 (8.0) | 10/51 (19.6) | 5/150 (3.3) | 3/25 (12.0) | 0.001 | 0.028 |
| Grey and/or pale skin complexion | 56/227 (24.7) | 18/51 (35.3) | 29/152 (19.1) | 9/24(37.5) | 0.020 | 0.360 |
| Apnea or bradycardia events | 111/229 (48.5) | 23/51 (45.1) | 78/153 (51.0) | 10/25 (40.0) | 0.534 | 1.000 |
| Digestive disorders 2 | 120/230 (52.2) | 26/51 (51.0) | 81/153 (52.9) | 13/26 (50.0) | 0.938 | 1.000 |
| Hypotonia or lethargy | 38/229 (16.6) | 14/51 (27.5) | 17/153 (11.1) | 7/25 (28.0) | 0.006 | 0.132 |
| Increased ventilatory support and/or increased FiO2 | 107/230 (46.5) | 26/51 (51.0) | 63/153 (41.2) | 18/26 (69.2) | 0.022 | 0.374 |
| Cutaneous rash | 5/230 (2.2) | 1/51 (2.0) | 3/153(2.0) | 1/26 (3.8) | 0.782 | 1.000 |
| Presence of a central venous catheter | 146/229 (63.8) | 44/50 (88.0) | 86/153 (56.2) | 16/26 (61.5) | 0.001 | 0.028 |
| Antibiotics at 48 h, No. (%) | ||||||
| No | 117 (50.9) | 0 (0) | 111 (72.5) | 6 (23.1) | 0.001 | 0.028 |
| Yes | 113 (49.1) | 51 (100) | 42 (27.5) | 20 (76.9) | NA | NA |
| Vancomycin | 98 (42.6) | 48 (94.1) | 36 (23.5) | 14 (53.8) | NA | NA |
| Amikacin | 80 (34.8) | 35 (68.6) | 32 (20.9) | 13 (50.0) | NA | NA |
| Cefotaxime | 41 (17.8) | 20 (39.2) | 13 (8.5) | 8 (30.8) | NA | NA |
| Other betalactams | 18 (7.8) | 8 (15.7) | 5 (3.3) | 5 (19.2) | NA | NA |
| Metronidazole | 2 (0.9) | 1 (2.0) | 1 (0.7) | 0 (0) | NA | NA |
| Other | 20 (8.7) | 9 (17.6) | 6 (3.9) | 5 (19.2) | NA | NA |
| Duration of exposure (days), Median (range) | 3 (1–26) | 10 (2–21) | 2 (2–26) | 3 (2–21) | NA | NA |
| Antibiotic exposure > 2 days | 64 (66) | 48 (94) | 7 (17) | 9 (45) | NA | NA |
| Laboratory values | ||||||
| C-reactive protein, mg/L, (n = 187) Median (range) | 1.0 (0.0–207.0) | 13.5 (0–207) | 1 (0–30.0) | 5.6 (0–165.9) | 0.001 | 0.028 |
| White blood cell count, G/L, (n = 133) Median (range) | 13.3 (2.30–40.12) | 14.48 (2.30–40.12) | 12.65 (2.94–38.05) | 16.67 (5.67–33.3) | 0.205 | 1.000 |
| Neutrophils, G/L, (n = 106) Median (range) | 5.13 (0.93–22.45) | 6.50 (0.95–22.07) | 4.61 (0.93–22.45) | 4.70 (1.01–21.98) | 0.015 | 0.285 |
| Lymphocytes, G/L, (n = 106) Median (range) | 5.06 (0.77–14.90) | 3.81 (0.77–6.73) | 5.39 (1.14–14.90) | 5.01 (1.17–7.93) | 0.012 | 0.240 |
| Blood cultures No./n. (%) | ||||||
| Not done | 2/230 (0.9) | 0/51 (0) | 2/153 (1.3) | 0/26 (0) | 0.001 | 0.028 |
| Sterile | 180/230 (78.0) | 8/51 (15.7) | 148/153 (96.7) | 24/26 (92.3) | NA | NA |
| Positive | 48/230 (20.9) | 43/51(84.3) | 3/153 (2) | 2/26 (7.7) | NA | NA |
| Staphylococcus aureus (n = 228) | 8/228(3.5) | 8/51 (15.7) | 0/151 (0) | 0/26 (0) | NA | NA |
| Coagulase-negative staphylococci (n = 228) | 35/228(15.4) | 30/51 (58.8) | 3/151 (2.0) | 2/26 (7.7) | NA | NA |
| Gram-negative bacilli (n = 228) | 3/228(1.3) | 3/51 (5.9) | 0/151 (0) | 0/26 (0) | NA | NA |
| Other Gram-positive organisms (n = 228) | 2/228(0.9) | 2/51 (3.9) | 0/151 (0) | 0/26 (0) | NA | NA |
| Candida albicans (n = 228) | 1/228(0.4) | 1/51 (2.0) | 0/151 (0) | 0/26 (0) | NA | NA |
| Selected Models | Patients Who Received Antibiotics | |
|---|---|---|
| Patients from the Infected Group, Reclassified as Not Infected Using Biomarker Models (n/N, %) | Patients from the Not Infected Group, Also Classified as Not Infected Using Biomarker Models (n/N, %) | |
| IL-6 | 5/51 (9.8%) | 10/42 (23.8%) |
| IL-10 | 5/51 (9.8%) | 26/42 (61.9%) |
| NGAL | 5/49 (10.2%) | 25/42 (59.5%) |
| PCT/IL-10 | 5/51 (9.8%) | 26/42 (61.9%) |
| PTX3/NGAL | 5/49 (10.2%) | 27/42 (64.3%) |
| PTX3/NGAL/gelsolin | 5/49 (10.2%) | 23/41 (56.1%) |
| IL-6 | 5/51 (9.8%) | 10/42 (23.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons, S.; Trouillet-Assant, S.; Subtil, F.; Abbas-Chorfa, F.; Cornaton, E.; Berthiot, A.; Galletti, S.; Plat, A.; Rapin, S.; Trapes, L.; et al. Performance of 11 Host Biomarkers Alone or in Combination in the Diagnosis of Late-Onset Sepsis in Hospitalized Neonates: The Prospective EMERAUDE Study. Biomedicines 2023, 11, 1703. https://doi.org/10.3390/biomedicines11061703
Pons S, Trouillet-Assant S, Subtil F, Abbas-Chorfa F, Cornaton E, Berthiot A, Galletti S, Plat A, Rapin S, Trapes L, et al. Performance of 11 Host Biomarkers Alone or in Combination in the Diagnosis of Late-Onset Sepsis in Hospitalized Neonates: The Prospective EMERAUDE Study. Biomedicines. 2023; 11(6):1703. https://doi.org/10.3390/biomedicines11061703
Chicago/Turabian StylePons, Sylvie, Sophie Trouillet-Assant, Fabien Subtil, Fatima Abbas-Chorfa, Elise Cornaton, Amélie Berthiot, Sonia Galletti, Aurélie Plat, Stephanie Rapin, Laurene Trapes, and et al. 2023. "Performance of 11 Host Biomarkers Alone or in Combination in the Diagnosis of Late-Onset Sepsis in Hospitalized Neonates: The Prospective EMERAUDE Study" Biomedicines 11, no. 6: 1703. https://doi.org/10.3390/biomedicines11061703
APA StylePons, S., Trouillet-Assant, S., Subtil, F., Abbas-Chorfa, F., Cornaton, E., Berthiot, A., Galletti, S., Plat, A., Rapin, S., Trapes, L., Generenaz, L., Brengel-Pesce, K., Callies, A., Plaisant, F., Claris, O., Portefaix, A., Flamant, C., & Butin, M. (2023). Performance of 11 Host Biomarkers Alone or in Combination in the Diagnosis of Late-Onset Sepsis in Hospitalized Neonates: The Prospective EMERAUDE Study. Biomedicines, 11(6), 1703. https://doi.org/10.3390/biomedicines11061703





