Adjuvant Effect of Whole-Cell Pertussis Component on Tetanus Toxoid Potency in Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Challenge Test in Mice
2.1.1. Vaccines Used for Immunization
2.1.2. Immunization of Animals
2.1.3. Intoxication of Animals
2.1.4. Reading and Interpretation of Results
2.1.5. Requirements for a Valid Assay
2.2. Assessment of Anti-TT (Tetanus Toxoid) IgG Levels in Mouse Sera
2.2.1. Immunization of Animals
2.2.2. Blood Collection
2.2.3. Determination of Anti-TT IgG in Mouse Sera by ELISA Assay
2.2.4. Calculation of ELISA Assay Results
2.2.5. Acceptance Criteria
2.3. Statistical Analysis
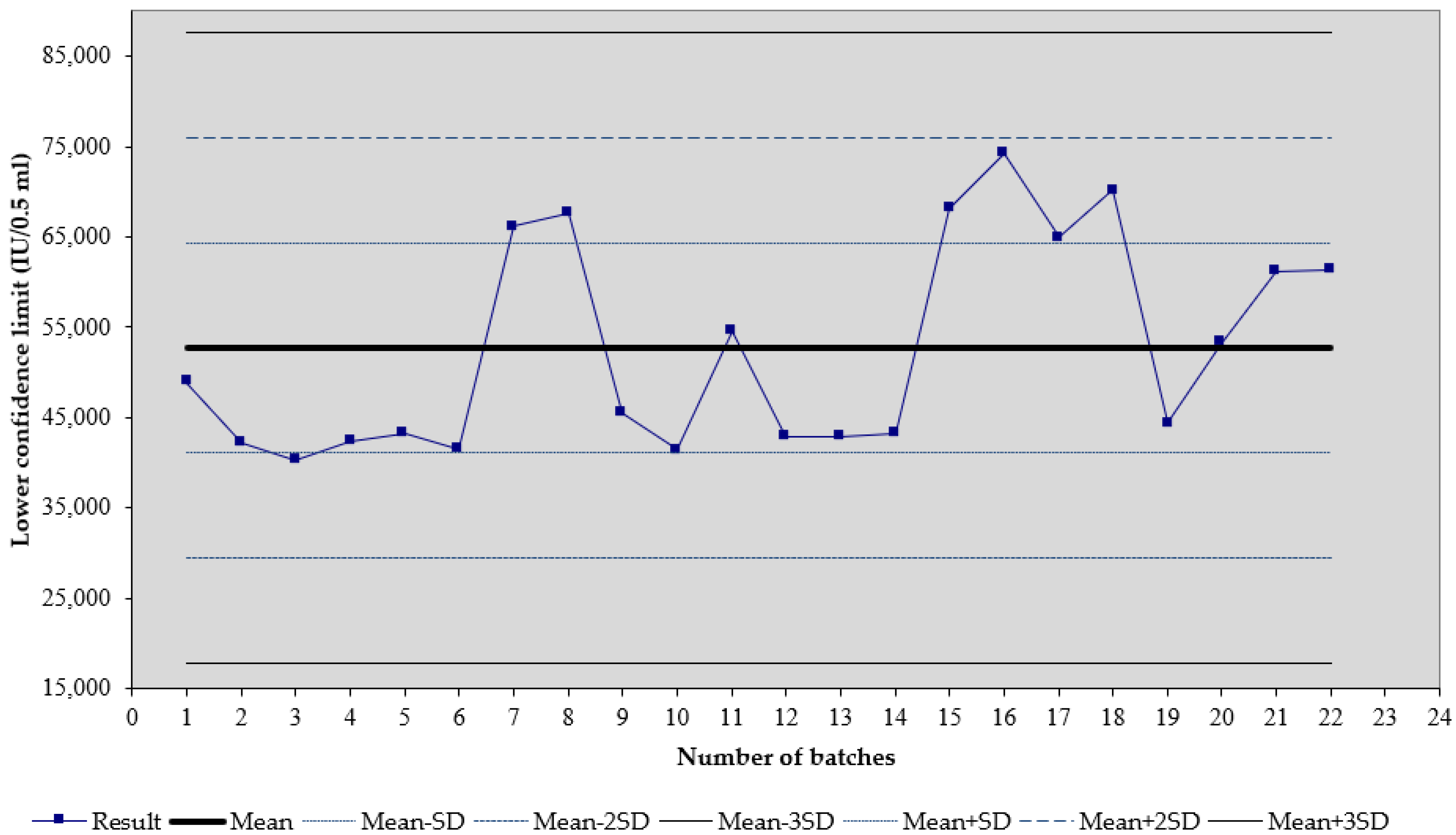
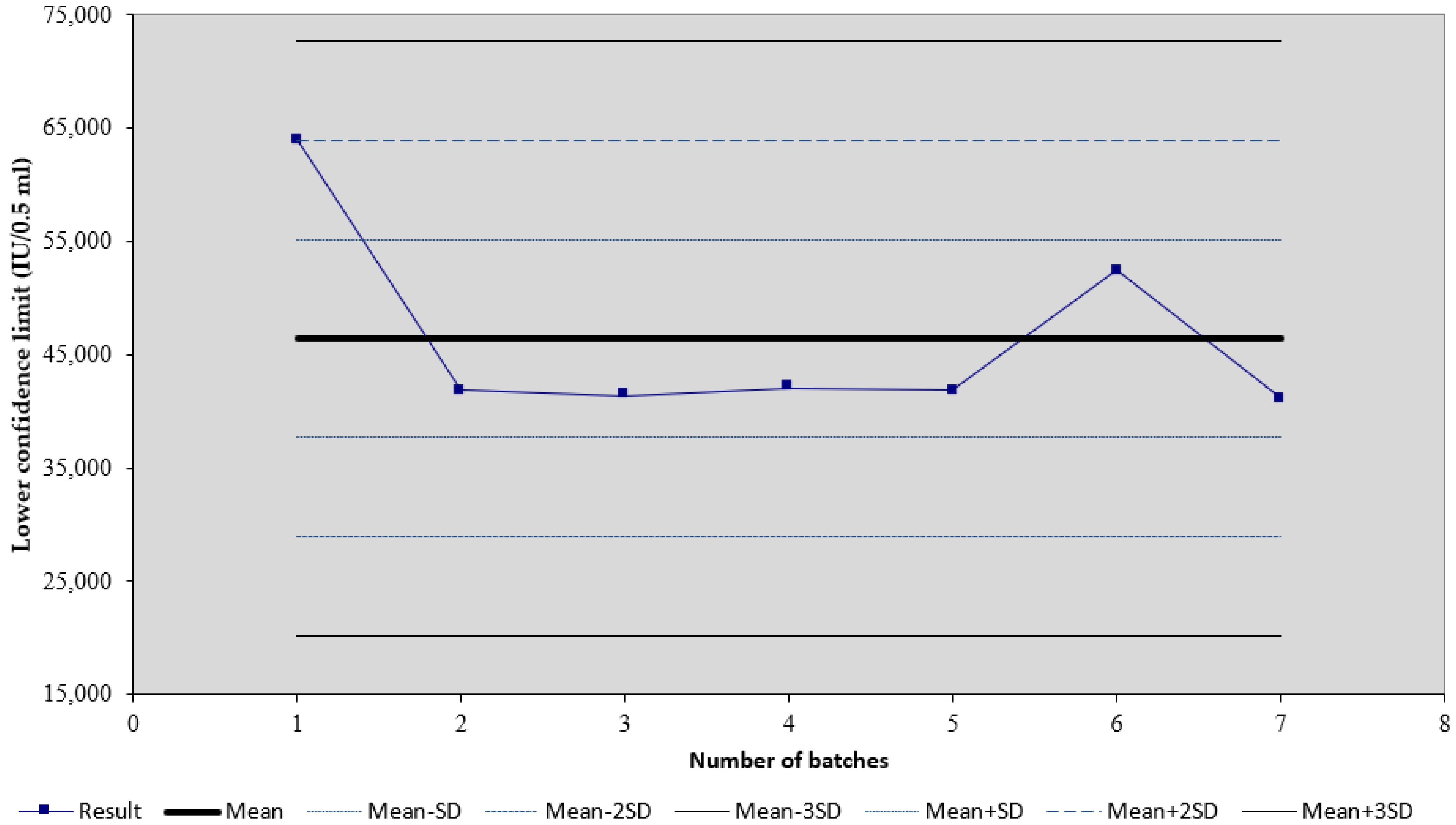
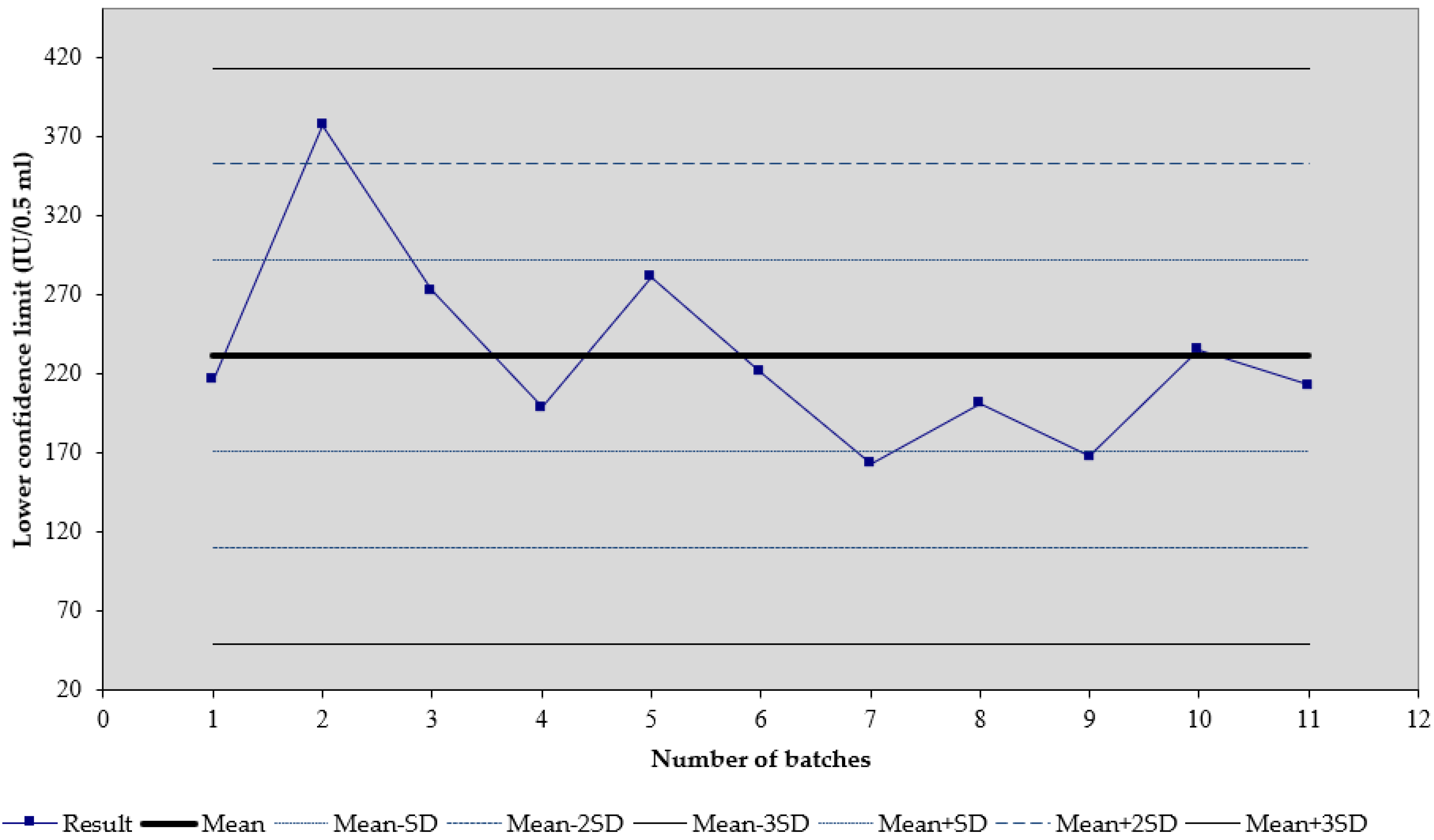
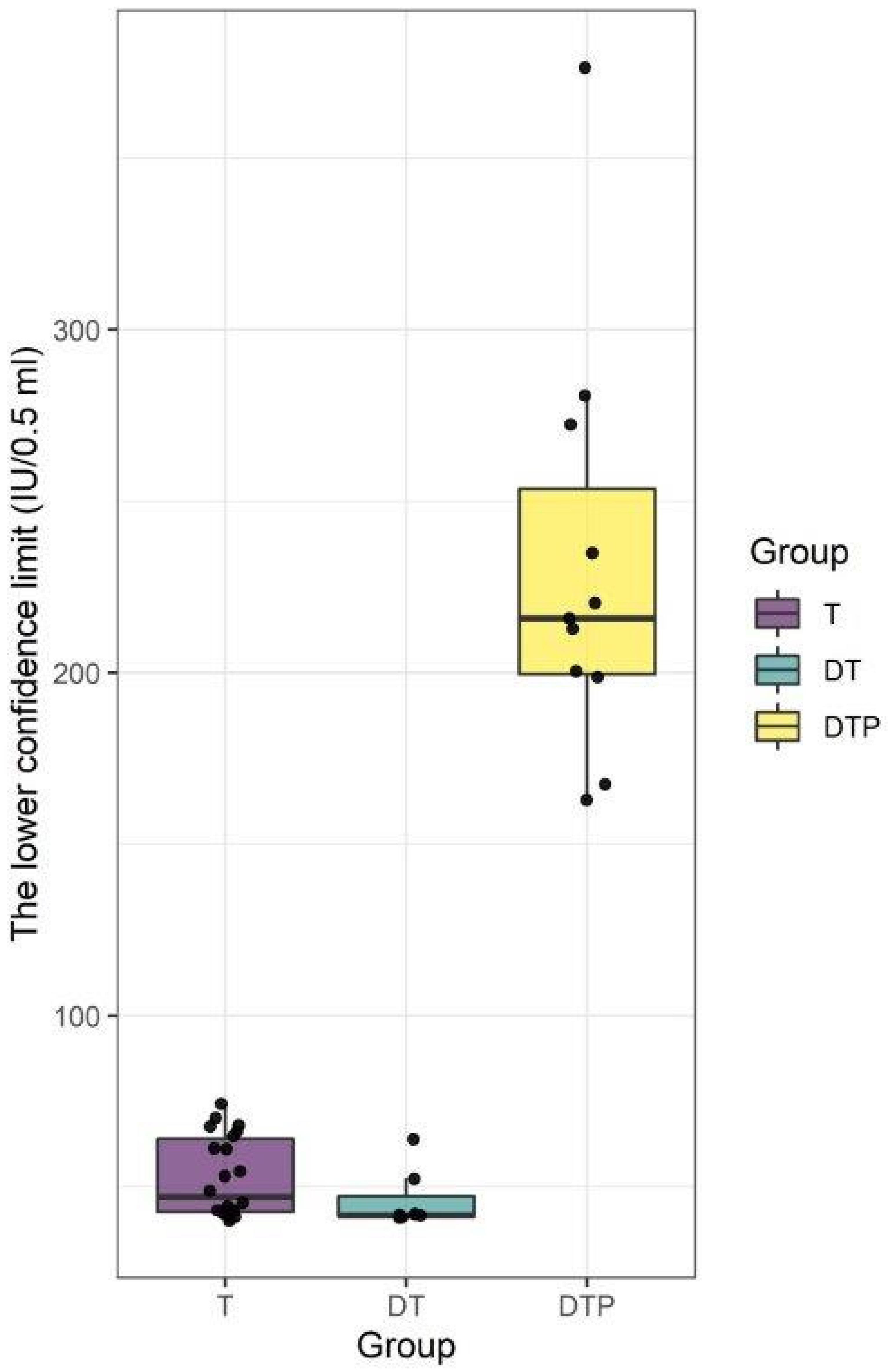
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Prygiel, M.; Mosiej, E.; Górska, P.; Zasada, A.A. Diphtheria-tetanus-pertussis vaccine: Past, current & future. Future Microbiol. 2022, 17, 185–197. [Google Scholar]
- Milstien, J.B.; Gellin, B.G.; Kane, M.; di Fabio, J.L.; Homma, A. Global DTP manufacturing capacity and capability. Status report: January 1995. Vaccine 1996, 14, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A. Plotkin’s Vaccines, 7th ed.; Elsevier: Philadelphia, PA, USA, 2017; pp. 711–761. [Google Scholar]
- Abbasi, A.; Rahbar Saadat, T.; Rahbar Saadat, Y. Microbial exopolysaccharides-β-glucans-as promising postbiotic candidates in vaccine adjuvants. Int. J. Biol. Macromol. 2022, 31, 346–361. [Google Scholar] [CrossRef]
- Greenberg, L.; Fleming, D.S. The immunizing efficiency of diphtheria toxoid when combined with various antigens. Can. J. Public Health 1948, 39, 131–135. [Google Scholar] [PubMed]
- Spiller, V.; Barnes, J.M.; Holt, L.B.; Cullington, D.E. Immunization against diphtheria and whooping-cough; combined v. separate inoculations. Br. J. Med. 1955, 2, 639–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak, M.; Zasada, A.A.; Mosiej, E.; Krysztopa-Grzybowska, K.; Witkowski, L.; Rzeczkowska, M.; Piekarska, K.; Lutyńska, A. Pertactin-deficient Bordetella pertussis isolates in Poland-a country with whole-cell pertussis primary vaccination. Microbes Infect. 2019, 21, 170–175. [Google Scholar] [CrossRef] [PubMed]
- monographs 01/2008:20708; The 9th Edition European Pharmacopoeia, corrected 6.0. European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2017; pp. 253–256.
- Yano, A.; Komatsu, T.; Ishibashi, M.; Udaka, K. Potent CTL induction by a whole cell pertussis vaccine in anti-tumor peptide immunotherapy. Microbiol. Immunol. 2007, 51, 685–699. [Google Scholar] [CrossRef] [Green Version]
- Berstad, A.K.; Andersen, S.R.; Dalseg, R.; Dromtorp, S.; Holst, J.; Namork, E.; Wedege, E.; Haneberg, B. Inactivated meningococci and pertussis bacteria are immunogenic and act as mucosal adjuvants for a nasal inactivated influenza virus vaccine. Vaccine 2000, 18, 1910–1919. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Miyaji, E.N.; Ferreira, D.M.; Moreno, A.T.; Ferreira, P.C.; Lima, F.A.; Santos, F.L.; Sakauchi, M.A.; Takata, C.S.; Higashi, H.G.; et al. Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice. PLoS ONE 2010, 27, 10863. [Google Scholar] [CrossRef] [Green Version]
- Farthing, J.R.; Holt, L.B. Experiments designed to determine the mechanism of the adjuvant activity of Gram-negative organisms upon antibody production. J. Hyg. 1962, 60, 411–426. [Google Scholar] [CrossRef] [Green Version]
- Tamizifar, H.; Jennings, R.; Ali, S.A.; Potter, C.W. Induction of IL-2 and IFN- gamma in BALB/c mice immunised with subunit influenza A vaccine in combination with whole cell or acellular DTP vaccine. J. Med. Microbiol. 1997, 46, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tamizifar, H.; Robinson, A.; Jennings, R.; Potter, C.W. Immune response and protection against influenza A infection in mice immunised with subunit influenza A vaccine in combination with whole cell or acellular DTP vaccine. Vaccine 1995, 13, 1539–1546. [Google Scholar]
- Prikazsky, V.; Bock, H.L. Higher anti-hepatitis B response with combined DTPw-HBV vaccine compared with separate administration in healthy infants at 3, 4 and 5 months of age in Slovakia. Int. J. Clin. Pract. 2001, 55, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Huebner, R.; Mothupi, R.; Kayhty, H.; Mbelle, N.; Khomo, E. Haemophilus influenzae type b conjugate vaccine diluted tenfold in diphtheria- tetanus-whole cell pertussis vaccine: A randomized trial. Pediatr. Infect. Dis. J. 2002, 21, 138–141. [Google Scholar] [CrossRef]
- Reinert, P.; Guy, M.; Girier, B.; Szelechowski, B.; Baudoin, B.; Deberdt, P.; Wollner, A.; Kemeny, G.; Amzallag, M.; Moat, C. The safety and immunogenicity of an heptavalent pneumococcal polysaccharide conjugate vaccine (Prevenar) administered in association with a whole-cell pertussis-based pediatric combination vaccine (DTP-IPV/PRP-T) to French infants with a two-, three-, and four-month schedule]. Arch. Pediatr. 2003, 10, 1048–1055. [Google Scholar] [PubMed]
- Dagan, R.; Goldblatt, D.; Maleckar, J.R.; Yaich, M.; Eskola, J. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect. Immun. 2004, 72, 5383–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, S.C.; Jarnicki, A.G.; Lavelle, E.C.; Mills, K.H. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: Role of IL-17-producing T cells. J. Immunol. 2006, 177, 7980–7989. [Google Scholar] [CrossRef] [Green Version]
- Berstad, A.K.; Oftung, F.; Korsvold, G.E.; Haugen, I.L.; Froholm, L.O.; Holst, J.; Haneberg, B. Induction of antigen-specific T cell responses in human volunteers after intranasal immunization with a whole-cell pertussis vaccine. Vaccine 2000, 18, 2323–2330. [Google Scholar] [CrossRef]
- McGuirk, P.; Mills, K.H. A regulatory role for interleukin 4 in differential inflammatory responses in the lung following infection of mice primed with Th1- or Th2-inducing pertussis vaccines. Infect. Immun. 2000, 68, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Mahon, B.P.; Ryan, M.S.; Griffin, F.; Mills, K.H. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect. Immun. 1996, 64, 5295–5301. [Google Scholar] [CrossRef] [Green Version]
- Quintilio, W.; Kubrusly, F.S.; Iourtov, D.; Miyaki, C.; Sakauchi, M.A.; Lúcio, F.; Dias, S.d.C.; Takata, C.S.; Miyaji, E.N.; Higashi, H.G.; et al. Bordetella pertussis monophosphoryl lipid A as adjuvant for inactivated split virion influenza vaccine in mice. Vaccine 2009, 27, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H. Biochemistry of endotoxins. Annu. Rev. Biochem. 1990, 59, 129–170. [Google Scholar] [CrossRef]
- Hunter, R.L. Over review of vaccine adjuvants: Present and future. Vaccine 2002, 20, 7–12. [Google Scholar] [CrossRef]
- Ohta, M.; Nakashima, I.; Kato, N. Adjuvant action of bacterial lipopolysaccharide in induction of delayed-type hypersensitivity to protein antigens. I1. Relationship of intensity of the action to that of other immunological activities. Immunobiology 1952, 163, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Banus, S.; Stenger, R.M.; Gremmer, E.R.; Dormans, J.A.; Mooi, F.R.; Kimman, T.G.; Vandebriel, R.J. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 2008, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Fedele, G.; Celestino, I.; Spensieri, F.; Frasca, L.; Nasso, M.; Watanabe, M.; Remoli, M.E.; Coccia, E.M.; Altieri, F.; Ausiello, C.M. Lipooligosaccharide from Bordetella pertussis induces mature human mono-cyte-derived dendritic cells and drives a Th2 biased response. Microbes Infect. 2007, 9, 855–863. [Google Scholar] [CrossRef]
- Errea, A.; Moreno, G.; Sisti, F.; Fernandez, J.; Rumbo, M.; Hozbor, D.F. Mucosal innate response stimulation induced by lipopolysaccharide protects against Bordetella pertussis colonization. Med. Microbiol. Immunol. 2010, 199, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Ross, A.C. Antibody response against tetanus toxoid is enhanced by lipopolysaccharide or tumor necrosis factor-alpha in vitamin A-sufficient and deficient rats. Am. J. Clin. Nutr. 1994, 59, 922–928. [Google Scholar] [CrossRef]
- Gery, I.; Kruger, J.; Spiesel, S.Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J. Immunol. 1972, 108, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect. Immun. 1990, 58, 3465–3468. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Kianmehr, Z.; Kaboudanian Ardestani, S.; Gharegozlou, B. Improved immunogenicity of tetanus toxoid by Brucella abortus S19 LPS adjuvant. Iran. J. Immunol. 2014, 11, 189–199. [Google Scholar]
- Kariminia, A.; Kavoossy, G.; Khatami, S.; Zowghi, E.; Ardestani, S.K. Study of interleukin-10 and interleukin-12 productions in response to lipopolysaccharides extracted from two different Brucella strains. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tamora, S.; Yamanaka, A.; Shimomara, M.; Tomita, T.; Komase, K.; Tsuda, Y.; Suzuki, Y.; Nagamine, T.; Kawahara, K.; Danbara, H. Synergistic action of cholera toxin B subunit (and Escherichia co/i heat soluble B subunit) and a trace of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine 1994, 12, 419426. [Google Scholar]
- Redhead, K.; Sesardic, D.; Yost, S.E.; Attwell, A.-M.; Watkins, J.; Hoy, C.S.; Plumb, J.E.; Corbel, M.J. Interaction of Haemophilus influenzae type b conjugate vaccine with diphtheria-tetanus-pertussis vaccine in controlled tests. Vaccine 1994, 12, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Honda, T.; Morokuma, K.; Ginnaga, A.; Ohkuma, K.; Sakoh, M. Enhancing effects of pertussis toxin B oligomer on the immunogenicity of influenza vaccine administered intranasally. Vaccine 1994, 12, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Prygiel, M.; Mosiej, E.; Wdowiak, K.; Górska, P.; Polak, M.; Lis, K.; Krysztopa-Grzybowska, K.; Zasada, A.A. Effectiveness of experimental and commercial pertussis vaccines in the elimination of Bordetella pertussis isolates with different genetic profiles in murine model. Med. Microbiol. Immunol. 2021, 210, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Yu, C.R.; Shi, G.; Vistica, B.P.; Wawrousek, E.F.; Klinman, D.M.; Chan, C.-C.; Egwuagu, C.E.; Gery, I. Pertussis toxin is superior to TLR ligands in enhancing pathogenic autoimmunity, targeted at a neo-self antigen, by triggering robust expansion of Th1 cells and their cytokine production. J. Immunol. 2006, 177, 6896–6903. [Google Scholar] [CrossRef] [Green Version]
- Orr, B.; Douce, G.; Baillie, S.; Parton, R.; Coote, J. Adjuvant effects of adenylate cyclase toxin of Bordetella pertussis after intranasal immunisation of mice. Vaccine 2007, 25, 64–71. [Google Scholar] [CrossRef]
- Tonon, S.; Badran, B.; Benghiat, F.S.; Goriely, S.; Flamand, V.; Willard-Gallo, K.; Willems, F.; Goldman, M.; De Wit, D. Pertussis toxin activates adult and neonatal naive human CD4+ T lymphocytes. Eur. J. Immunol. 2006, 36, 1794–1804. [Google Scholar] [CrossRef]
- Locht, C. A common vaccination strategy to solve unsolved problems of tuberculosis and pertussis? Microbes Infect. 2008, 10, 1051–1056. [Google Scholar] [CrossRef]
- Polak, M.; Zawadka, M.; Mosiej, E.; Rabczenko, D.; Augustynowicz, E.; Lutyńska, A. Effectiveness of experimental whole-cell pertussis vaccines in murine model. Med. Dosw. Mikrobiol. 2014, 66, 79–87. [Google Scholar] [PubMed]
- Altindiş, E.; Tefon, B.E.; Yildirim, V.; Ozcengiz, E.; Becher, D.; Hecker, M.; Ozcengiz, G. Immunoproteomic analysis of Bordetella pertussis and identification of new immunogenic proteins. Vaccine 2009, 27, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wirsing von Koenig, C.H.; Heymer, B.; Hof, H.; Finger, H.; Emmerling, P. Biological activity of Bordetella pertussis in lipopolysaccharide-resistant mice. Infect. Immun. 1981, 33, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef]
- Siskind, G.W.; Benacerraf, B. Cell selection by antigen in the immune response. Adv. Immunol. 1969, 10, 1–50. [Google Scholar]
- Osebold, J.W. Mechanisms of action by immunologic adjuvants. J. Am. Vet. Med. Assoc. 1982, 181, 983–987. [Google Scholar]
- Bungener, L.; Geeraedts, F.; Ter Veer, W.; Medema, J.; Wilschut, J.; Huckriede, A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 2008, 26, 2350–2359. [Google Scholar] [CrossRef]
- Miki, H.; Nakahashi-Oda, C.; Sumida, T.; Shibuya, A. Involvement of CD300a phosphatidylserine immunoreceptor in aluminum salt adjuvant-induced Th2 responses. J. Immunol. 2015, 194, 5069–5076. [Google Scholar] [CrossRef] [Green Version]
- Bruchard, M.; Rebe, C.; Derangere, V.; Togbe, D.; Ryffel, B.; Boidot, R.; Humblin, E.; Hamman, A.; Chalmin, F.; Berger, H.; et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat. Immunol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, F.; Yang, L.; Ge, Y.; Wei, Q.; Ou, Y. EPSAH, an exopolysaccharide from aphanothece halophytica GR02, improves both cellular and humoral immunity as a novel polysaccharide adjuvant. Chin. J. Nat. Med. 2016, 14, 541–548. [Google Scholar]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Woodruff, M.C.; Grigoryan, L.; Maier, B.; Lee, S.H.; Mandal, P.; Cortese, M.; Natrajan, M.S.; Ravindran, R.; Ma, H.; et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 2020, 9, e52687. [Google Scholar] [CrossRef] [PubMed]

| Type of Vaccine | Composition of a Single Human Dose (0.5 mL) |
|---|---|
| T vaccine | • Tetanus toxoid 10 Lf adsorbed on 0.5 mg hydrated aluminum hydroxide |
| DT vaccine | • Tetanus toxoid 10 Lf adsorbed on 0.45 mg hydrated aluminum hydroxide • Diphtheria toxoid 20 Lf adsorbed on 0.45 mg hydrated aluminum hydroxide |
| DTP vaccine | • Tetanus toxoid 10 Lf adsorbed on 0.45 mg hydrated aluminum hydroxide • Diphtheria toxoid 20 Lf adsorbed on 0.45 mg hydrated aluminum hydroxide • Suspension of inactivated Bordetella pertussis strains—15 IOU |
| Tested Vaccines | p-Value 1 | |||
|---|---|---|---|---|
| T, N = 22 | DT, N = 7 | DTP, N = 11 | ||
| Result | <0.001 | |||
| N | 22 | 7 | 11 | |
| Mean (SD) | 52.7 (11.6) | 46.4 (8.7) | 231.2 (60.6) | |
| Median | 47.2 | 41.9 | 215.8 | |
| Range | 40.2–74.3 | 41.2–64.0 | 162.9–376.4 | |
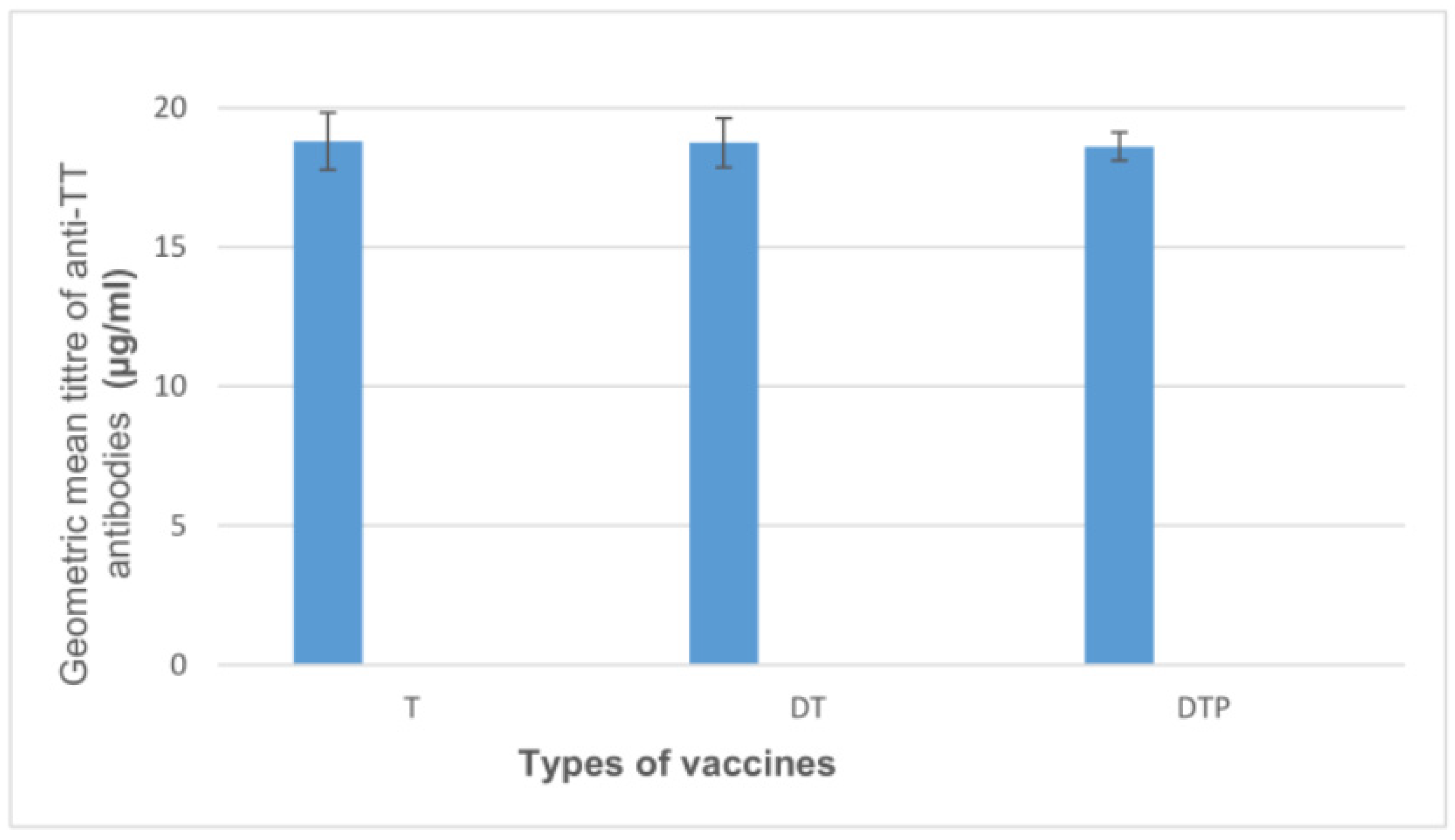
| Tested Vaccines | p-Value 1 | |||
|---|---|---|---|---|
| T, N = 10 | DT, N = 10 | DTP, N = 10 | ||
| Result | >0.9 | |||
| N | 10 | 10 | 10 | |
| Mean (SD) | 18.8 (1.0) | 18.8 (0.9) | 18.6 (0.5) | |
| Median | 18.6 | 18.6 | 18.5 | |
| Range | 17.9–21.3 | 17.3–20.0 | 18.0–19.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prygiel, M.; Mosiej, E.; Wdowiak, K.; Rabczenko, D.; Zasada, A.A. Adjuvant Effect of Whole-Cell Pertussis Component on Tetanus Toxoid Potency in Murine Model. Biomedicines 2023, 11, 1795. https://doi.org/10.3390/biomedicines11071795
Prygiel M, Mosiej E, Wdowiak K, Rabczenko D, Zasada AA. Adjuvant Effect of Whole-Cell Pertussis Component on Tetanus Toxoid Potency in Murine Model. Biomedicines. 2023; 11(7):1795. https://doi.org/10.3390/biomedicines11071795
Chicago/Turabian StylePrygiel, Marta, Ewa Mosiej, Karol Wdowiak, Daniel Rabczenko, and Aleksandra Anna Zasada. 2023. "Adjuvant Effect of Whole-Cell Pertussis Component on Tetanus Toxoid Potency in Murine Model" Biomedicines 11, no. 7: 1795. https://doi.org/10.3390/biomedicines11071795





