Radiomics-Clinical AI Model with Probability Weighted Strategy for Prognosis Prediction in Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Case Selection
2.3. Feature Extraction
2.4. Study Endpoints
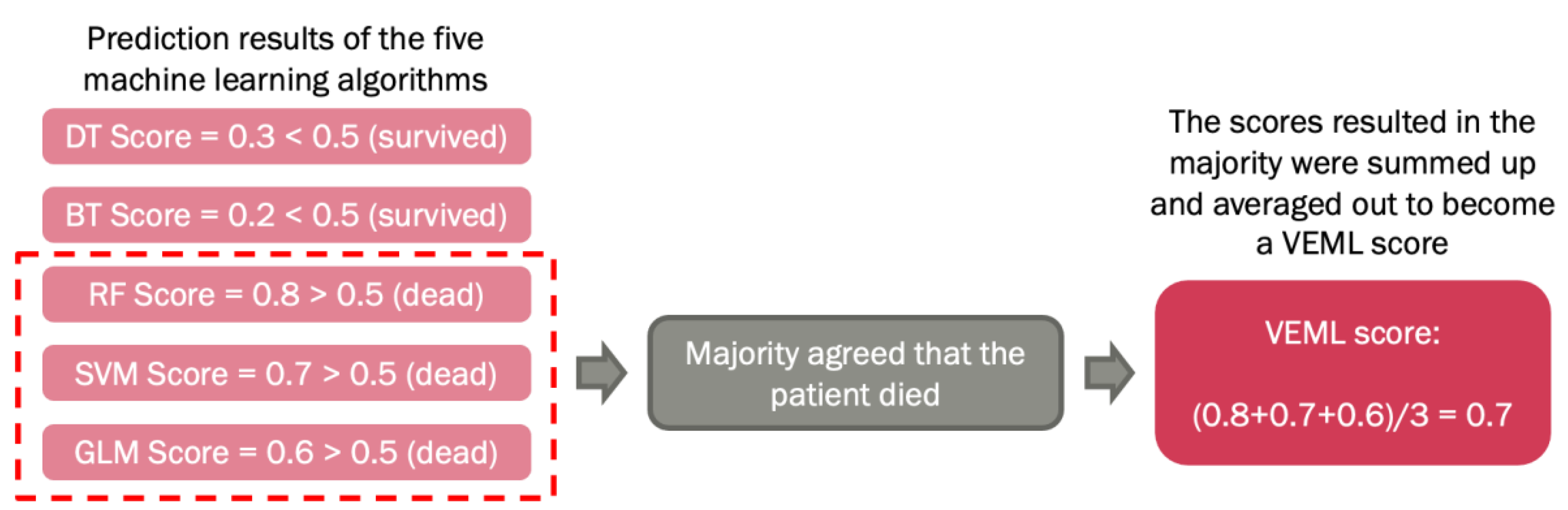
2.5. Machine Learning for Data Processing
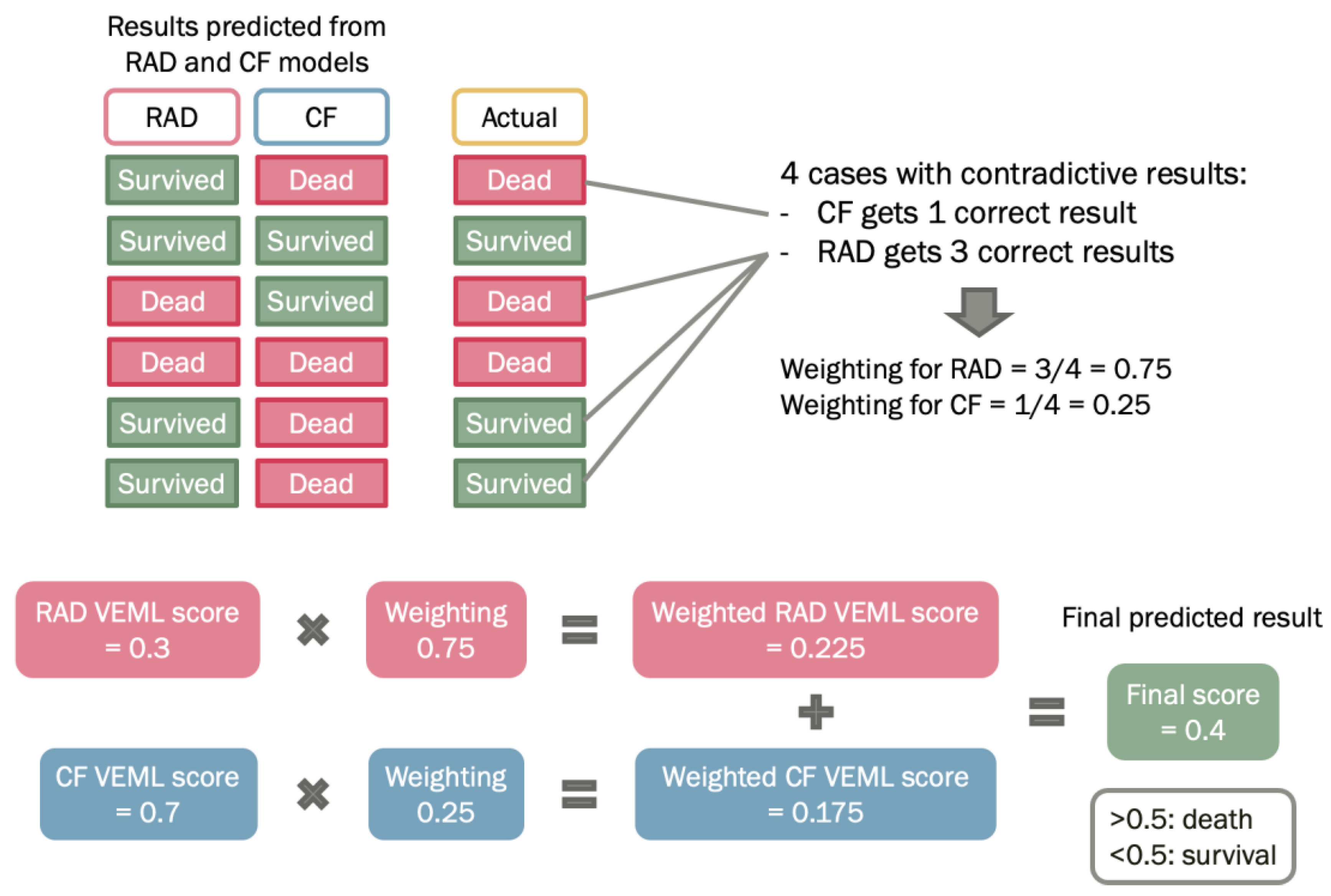
2.6. Probability Weighted Enhanced Model (PWEM)
3. Results
3.1. Patient Demographics and Tumor Characteristics
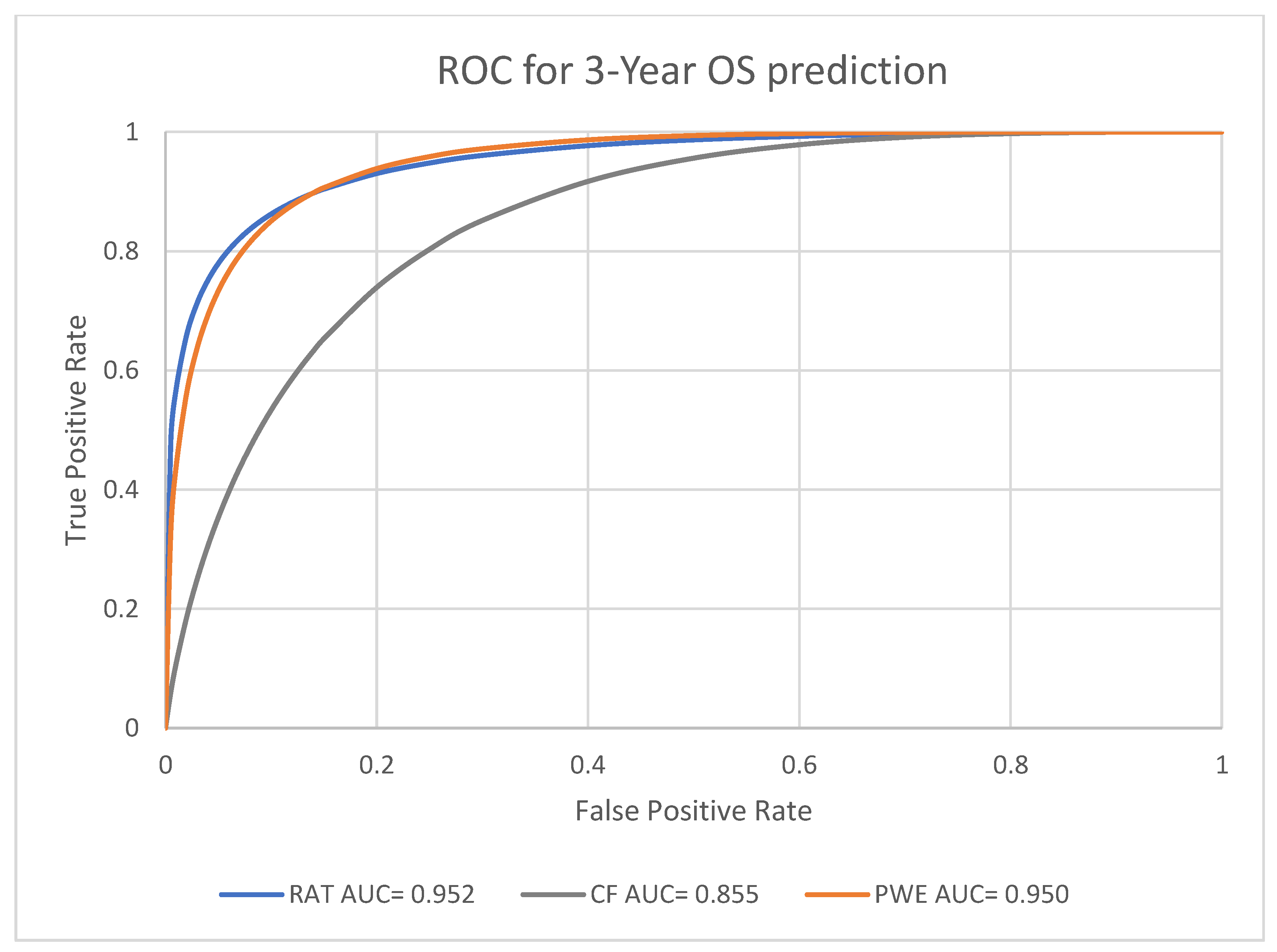
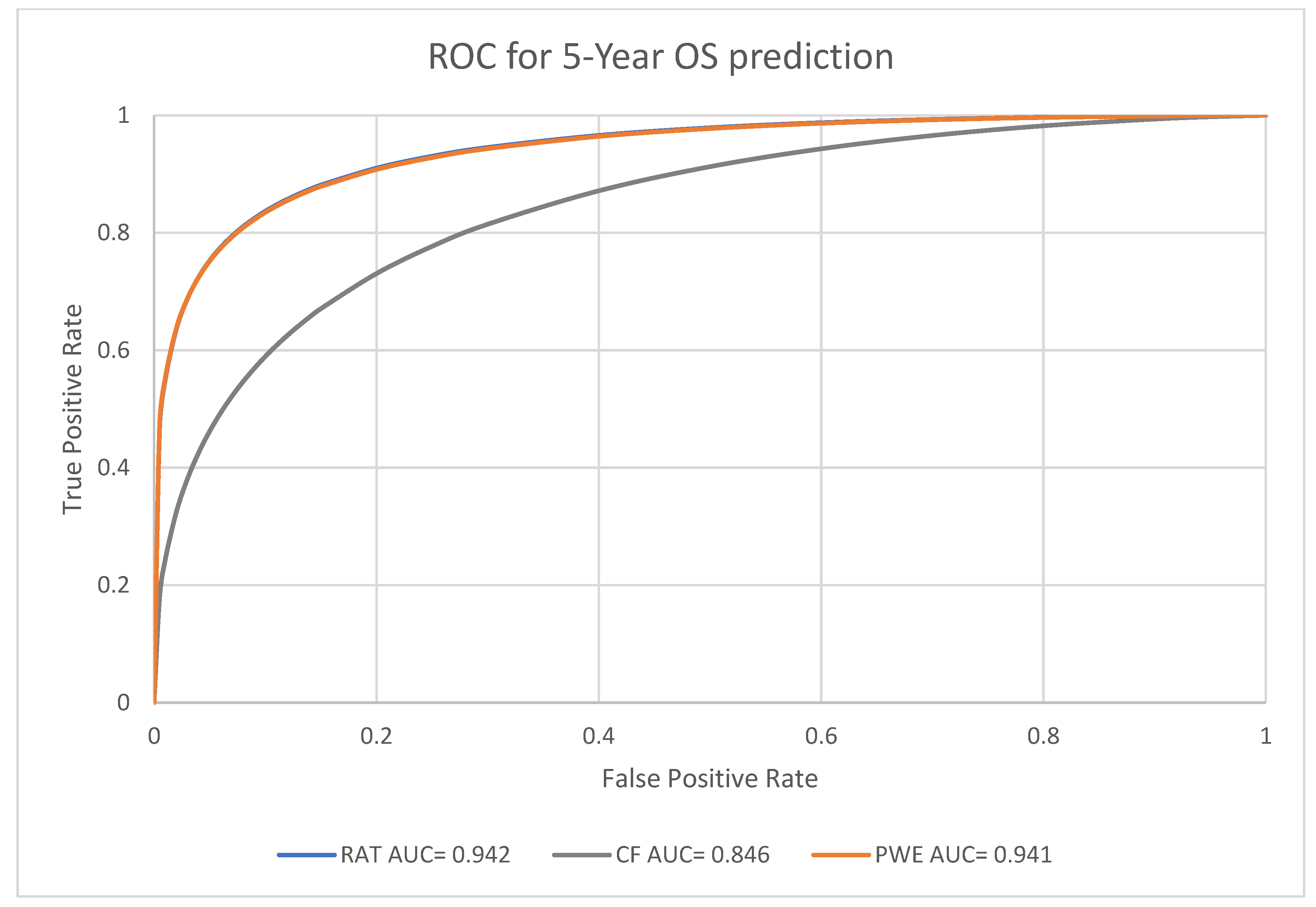
3.2. Prognosis Prediction Performance of the Models at Different Endpoints
3.3. Performance Analysis for Machine Learning Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Lung Cancer. 2023. Available online: https://www.cancer.org/cancer/lung-cancer.html (accessed on 2 March 2023).
- Huang, D.; Zhang, F.; Tao, H.; Zhang, S.; Ma, J.; Wang, J.; Liu, Z.; Cui, P.; Chen, S.; Huang, Z.; et al. Tumor Mutation Burden as a Potential Biomarker for PD-1/PD-L1 Inhibition in Advanced Non-small Cell Lung Cancer. Target. Oncol. 2020, 15, 93–100. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“How-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [Green Version]
- Staal, F.C.; Van Der Reijd, D.J.; Taghavi, M.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Maas, M. Radiomics for the Prediction of Treatment Outcome and Survival in Patients with Colorectal Cancer: A Systematic Review. Clin. Color. Cancer 2021, 20, 52–71. [Google Scholar] [CrossRef]
- Giraud, P.; Giraud, P.; Gasnier, A.; Ayachy, R.E.; Kreps, S.E.; Foy, J.; Durdux, C.; Huguet, F.; Burgun, A.; Bibault, J. Radiomics and Machine Learning for Radiotherapy in Head and Neck Cancers. Front. Oncol. 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.A.C.; Oliveira, B.C.; De Arimateia Batista Araujo-Filho, J.; Assuncao, A.N., Jr.; De MMachado, F.A.; Rocha, C.; Horvat, J.V.; Menezes, M.G.; Horvat, N. State-of-the-art in radiomics of hepatocellular carcinoma: A review of basic principles, applications, and limitations. Abdom. Imaging 2020, 45, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Walls, G.; Osman, S.O.; Brown, K.K.; Butterworth, K.T.; Hanna, G.B.; Hounsell, A.R.; McGarry, C.K.; Leijenaar, R.T.; Lambin, P.; Cole, A.A.; et al. Radiomics for Predicting Lung Cancer Outcomes Following Radiotherapy: A Systematic Review. Clin. Oncol. 2022, 34, e107–e122. [Google Scholar] [CrossRef]
- Parmar, C.; Grossmann, P.; Bussink, J.; Lambin, P.; Aerts, H.J. Machine Learning methods for Quantitative Radiomic Biomarkers. Sci. Rep. 2015, 5, 13087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaddad, A.; Desrosiers, C.; Toews, M.; Abdulkarim, B. Predicting survival time of lung cancer patients using radiomic analysis. Oncotarget 2017, 8, 104393–104407. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Kha, Q.H.; Hung, T.N.K.; Le, N.Q.K. Risk Score Generated from CT-Based Radiomics Signatures for Overall Survival Prediction in Non-Small Cell Lung Cancer. Cancers 2021, 13, 3616. [Google Scholar] [CrossRef]
- Ching, J.C.F.; Lam, S.; Lam, C.C.H.; Lui, A.O.Y.; Kwong, J.C.K.; Lo, A.Y.H.; Chan, J.W.H.; Cai, J.; Leung, W.S.; Lee, S.W.Y. Integrating CT-based radiomic model with clinical features improves long-term prognostication in high-risk prostate cancer. Front. Oncol. 2023, 13, 1060687. [Google Scholar] [CrossRef]
- Luna, J.; Barsky, A.; Shinohara, R.; Roshkovan, L.; Hershman, M.; Dreyfuss, A.; Horng, H.; Lou, C.; Noël, P.; Cengel, K.; et al. Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation. Cancers 2022, 14, 700. [Google Scholar] [CrossRef]
- The Cancer Imaging Archive (TCIA). 2020. Available online: https://www.cancerimagingarchive.net/ (accessed on 1 January 2023).
- R: The R Project for Statistical Computing. (n.d.). Available online: https://www.r-project.org/ (accessed on 5 January 2023).
- Tang, F.H.; Cheung, E.Y.W.; Wong, H.L.; Yuen, C.M.; Yu, M.H.; Ho, P.C. Radiomics from Various Tumour Volume Sizes for Prognosis Prediction of Head and Neck Squamous Cell Carcinoma: A Voted Ensemble Machine Learning Approach. Life 2022, 12, 1380. [Google Scholar] [CrossRef]
- Shin, T. Ensemble Learning, Bagging, and Boosting Explained in 3 Minutes. Medium. 2021. Available online: https://towardsdatascience.com/ensemble-learning-bagging-and-boosting-explained-in-3-minutes-2e6d2240ae21 (accessed on 6 April 2023).
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Shi, M.; Wang, Z.; Lu, H.; Li, C.M.; Tao, Y.; Chen, X.; Zhao, J. A molecular and staging model predicts survival in patients with resected non-small cell lung cancer. BMC Cancer 2018, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; She, Y.; Yang, Y.; Liu, X.; Chen, S.; Zhong, Y.; Deng, J.; Zhao, M.; Sun, X.; Xie, D.; et al. Radiomics for Survival Risk Stratification of Clinical and Pathologic Stage IA Pure-Solid Non–Small Cell Lung Cancer. Radiology 2021, 302, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, L.; Li, L.; Wang, Y.; Zhao, S.G.; Miao, L.; Gao, Y.; Liu, L.; Wu, N. Radiomics Signature to Predict Prognosis in Early-Stage Lung Adenocarcinoma (≤3 cm) Patients with No Lymph Node Metastasis. Diagnostics 2022, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Zhang, L.; Xu, K.; Wan, X.; Guo, Y. Prognostic Value of Pre-Treatment CT Radiomics and Clinical Factors for the Overall Survival of Advanced (IIIB–IV) Lung Adenocarcinoma Patients. Front. Oncol. 2021, 11, 628982. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Hou, Y.; Li, L.; Chang, L.; Li, Y.; Xie, K.; Qiu, L.; Mao, D.; Li, W.; et al. Combined Radiomics-Clinical Model to Predict Radiotherapy Response in Inoperable Stage III and IV Non-Small-Cell Lung Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221142400. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Zhang, J.; Jin, J.; Zhang, J.; Zhu, H.; Jin, X. Preoperative Prediction of Metastasis for Ovarian Cancer Based o Computed Tomography Radiomic Features and Clinical Factor. Front. Oncol. 2021, 11, 610742. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.H.; Ng, D.K.; Chow, D.H. An image feature approach for computer-aided detection of ischemic stroke. Comput. Biol. Med. 2011, 41, 529–536. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Sartor, G. Radiomics and deep learning in lung cancer. Strahlenther Onkol. 2020, 196, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Binczyk, F.; Prazuch, W.; Bozek, P.; Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1186–1199. [Google Scholar] [CrossRef]
- Cameron, A.; Khalvati, F.; Haider, M.A.; Wong, A. MAPS: A Quantitative Radiomics Approach for Prostate Cancer Detection. IEEE Trans. Biomed. Eng. 2016, 63, 1145–1156. [Google Scholar] [CrossRef]
- Zhou, M.; Scott, J.; Chaudhury, B.; Hall, L.; Goldgof, D.; Yeom, K.W.; Iv, M.; Ou, Y.; Kalpathy-Cramer, J.; Napel, S.; et al. Radiomics in Brain Tumor: Image Assessment, Quantitative Feature Descriptors, and Machine-Learning Approaches. AJNR Am. J. Neuroradiol. 2018, 39, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Obiols, M.H.; Jiao, Y.; Wang, Q. Can radiomics features boost the performance of deep learning upon histology images? In Proceedings of the 2019 International Conference on Medical Imaging Physics and Engineering (ICMIPE), Shenzhen, China, 22–24 November 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, F.F. Impact of image quality on radiomics applications. Phys. Med. Biol. 2022, 67, 15TR03. [Google Scholar] [CrossRef]
- Dai, H.; Lu, M.; Huang, B.; Tang, M.; Pang, T.; Liao, B.; Cai, H.; Huang, M.; Zhou, Y.; Chen, X.; et al. Considerable effects of imaging sequences, feature extraction, feature selection, and classifiers on radiomics-based prediction of microvascular invasion in hepatocellular carcinoma using magnetic resonance imaging. Quant. Imaging Med. Surg. 2021, 11, 1836–1853. [Google Scholar] [CrossRef]
- An, C.; Park, Y.W.; Ahn, S.S.; Han, K.; Kim, H.; Lee, S.-K. Radiomics machine learning study with a small sample size: Single random training-test set split may lead to unreliable results. PLoS ONE 2021, 16, e0256152. [Google Scholar] [CrossRef]
- Chetan, M.R.; Gleeson, F.V. Radiomics in predicting treatment response in non-small-cell lung cancer: Current status, challenges and future perspectives. Eur. Radiol. 2021, 31, 1049–1058. [Google Scholar] [CrossRef] [PubMed]

| Feature Group | Number of Features |
|---|---|
| Shape | 14 |
| First-order feature | 18 |
| Gray level co-occurrence matrix | 24 |
| Gray level dependence matrix | 14 |
| Gray level run length matrix | 16 |
| Gray level size zone matrix | 16 |
| Neighborhood gray tone difference matrix | 5 |
| Total | 107 |
| Endpoint | 1-Year Survival | 3-Year Survival | 5-Year Survival |
|---|---|---|---|
| 224 | Sample size | ||
| Balanced sample | 119 alive 119 dead | 112 alive 112 dead | 64 alive 64 dead |
| Patient Demographics | |||
|---|---|---|---|
| No. of Patients (%) | No. of Patients (%) | ||
| Gender | Age | ||
| Male | 237 (67%) | ≤65 y/o | 135 (38%) |
| Female | 115 (33%) | >65 y/o | 217 (62%) |
| Overall Stage | T Stage | ||
| I | 60 (17%) | T1 | 63 (18%) |
| II | 35 (10%) | T2 | 135 (38%) |
| IIIa | 103 (29%) | T3 | 49 (14%) |
| IIIb | 154 (44%) | T4 | 105 (30%) |
| Histology | N Stage | ||
| Adenocarcinoma | 48 (14%) | N0 | 131 (37%) |
| Large Cell Carcinoma | 105 (30%) | N1 | 20 (5%) |
| Squamous Cell Carcinoma | 142 (40%) | N2 | 125 (36%) |
| Not Otherwise Specified | 57 (16%) | N3 | 73 (21%) |
| N4 | 3 (1%) | ||
| Endpoint | Machine Learning Model | AUC [95% Confidence Interval] |
|---|---|---|
| Radiomic model | 0.931, [0.894, 0.956] | |
| 1-year survival | Clinical factors model | 0.869, [0.817, 0.909] |
| Probability weighted enhanced model | 0.955, [0.926, 0.974] | |
| Radiomic model | 0.952, [0.921, 0.973] | |
| 3-year survival | Clinical factors model | 0.855, [0.801, 0.898] |
| Probability weighted enhanced model | 0.950, [0.919, 0.971] | |
| Radiomic model | 0.942, [0.892, 0.971] | |
| 5-year survival | Clinical factors model | 0.846, [0.770, 0.903] |
| Probability weighted enhanced model | 0.941, [0.891, 0.971] |
| Survival Year(s) | RAT|CF | RAT|PWE | CF|PWE |
|---|---|---|---|
| 1 | 8.0667 | 10.5986 | 21.708 |
| (p < 0.05) | (p < 0.05) | (p < 0.05) | |
| 3 | 18.2596 | 2.2314 | 21.9264 |
| (p < 0.05) | (p > 0.05) | (p < 0.05) | |
| 5 | 10.1110 | 0.38 | 17.8133 |
| (p < 0.05) | (p > 0.05) | (p < 0.05) |
| Survival Year(s) | RAT | CF | PWE | |
|---|---|---|---|---|
| 1 | 0.9244 | 0.9076 | 0.9244 | |
| Sensitivity | 3 | 0.9107 | 0.8661 | 0.9196 |
| 5 | 0.7656 | 0.7969 | 0.7813 | |
| 1 | 0.8487 | 0.6723 | 0.8487 | |
| Specificity | 3 | 0.9018 | 0.7232 | 0.9018 |
| 5 | 0.9531 | 0.8594 | 0.9531 | |
| 1 | 0.8866 | 0.7899 | 0.8866 | |
| Accuracy | 3 | 0.9063 | 0.7946 | 0.9107 |
| 5 | 0.8594 | 0.8281 | 0.8672 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.-H.; Fong, Y.-W.; Yung, S.-H.; Wong, C.-K.; Tu, C.-L.; Chan, M.-T. Radiomics-Clinical AI Model with Probability Weighted Strategy for Prognosis Prediction in Non-Small Cell Lung Cancer. Biomedicines 2023, 11, 2093. https://doi.org/10.3390/biomedicines11082093
Tang F-H, Fong Y-W, Yung S-H, Wong C-K, Tu C-L, Chan M-T. Radiomics-Clinical AI Model with Probability Weighted Strategy for Prognosis Prediction in Non-Small Cell Lung Cancer. Biomedicines. 2023; 11(8):2093. https://doi.org/10.3390/biomedicines11082093
Chicago/Turabian StyleTang, Fuk-Hay, Yee-Wai Fong, Shing-Hei Yung, Chi-Kan Wong, Chak-Lap Tu, and Ming-To Chan. 2023. "Radiomics-Clinical AI Model with Probability Weighted Strategy for Prognosis Prediction in Non-Small Cell Lung Cancer" Biomedicines 11, no. 8: 2093. https://doi.org/10.3390/biomedicines11082093





