Glutathione: Lights and Shadows in Cancer Patients
Abstract
1. Introduction
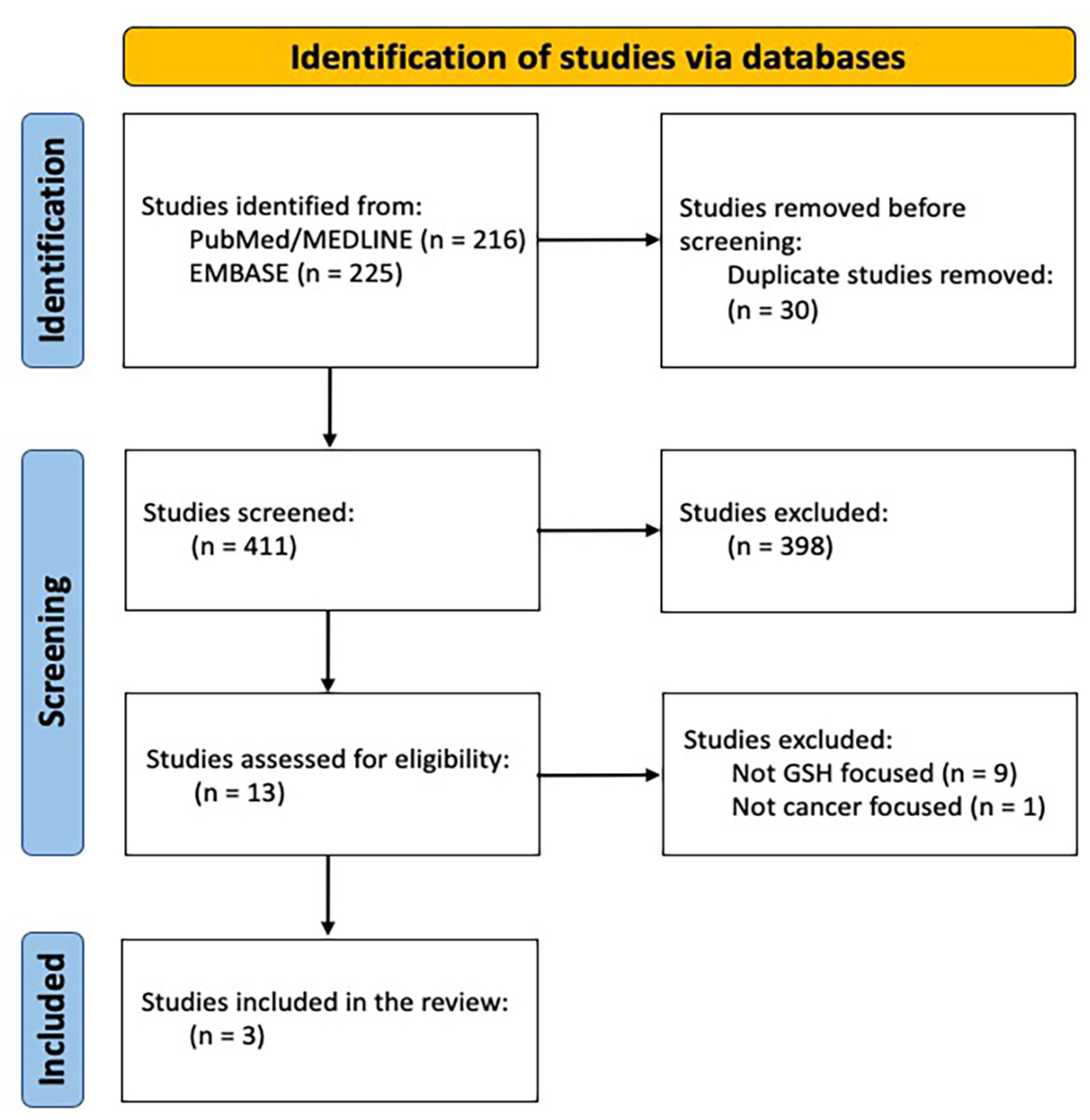
2. Materials and Methods
3. A Quick Overview of GSH Metabolic Processes
3.1. Genetic Findings of GSH
3.2. GSH Role in Cancer Cells
4. Glutathione, Nutrition and Nutraceuticals
4.1. Amino Acids
4.2. Omega-3 Fatty Acids
4.3. Selenium and Vitamins
4.4. Phytochemicals
4.5. Green Tea
4.6. Fruit and/or Vegetable Juices
4.7. Mediterranean Diet, Nutraceuticals and GSH: Perspectives and Challenges
5. Glutathione and Cancer
5.1. GSH and Platin-Compounds Related Toxicities
5.2. GSH Favourable Effects on Cancer Cells
6. Discussion
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, D.M.; Tew, K.D. The Role of Glutathione-S-Transferase in Anti-Cancer Drug Resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef]
- Zaric, B.L.; Macvanin, M.T.; Isenovic, E.R. Free Radicals Relationship to Human Diseases and Potential Therapeutic Applications. Int. J. Biochem. Cell. Biol. 2023, 154, 106346. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Micali, A.; Pallio, G.; Irrera, N.; Marini, H.; Trichilo, V.; Puzzolo, D.; Pisani, A.; Malta, C.; Santoro, G.; Laurà, R.; et al. Flavocoxid, a Natural Antioxidant, Protects Mouse Kidney from Cadmium-Induced Toxicity. Oxid. Med. Cell Longev. 2018, 2018, 9162946. [Google Scholar] [CrossRef]
- Checconi, P.; Limongi, D.; Baldelli, S.; Ciriolo, M.R.; Nencioni, L.; Palamara, A.T. Role of Glutathionylation in Infection and Inflammation. Nutrients 2019, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Dvash, E. Leukotrienes and Kidney Diseases. Curr. Opin. Nephrol. Hypertens. 2018, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a C. Nutrients 2020, 12, 3224. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Glutamate a Truly Functional Amino Acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Hansen, J.M.; Harris, C. Glutathione during Embryonic Development. Biochim. Biophys. Acta 2015, 1850, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Kim, E.S.; Koo, J.S. Amino Acid Transporters and Glutamine Metabolism in Breast Cancer. Int. J. Mol. Sci. 2018, 19, 907. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Console, L.; Losso, M.A.; Indiveri, C. The Human SLC1A5 (ASCT2) Amino Acid Transporter From Function to Structure and Role in Cell Biology. Front. Cell Dev. Biol. 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Daher, B.; Vučetić, M.; Pouysségur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Kassab, G.S. Glutathione A Samsonian Life-Sustaining Small Molecule That Protects against Oxidative Stress, Ageing and Damaging Inflammation. Front. Nutr. 2022, 9, 1007816. [Google Scholar] [CrossRef]
- Potęga, A. Glutathione-Mediated Conjugation of Anticancer Drugs An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules 2022, 27, 5252. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of Glutathione Depletion in Cancer Therapy Enhanced ROS-Based Therapy, Ferroptosis, and Chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Kumar, A.; Tikoo, S.; Maity, S.; Sengupta, S.; Sengupta, S.; Kaur, A.; Bachhawat, A.K. Mammalian Proapoptotic Factor ChaC1 and Its Homologues Function as γ-Glutamyl Cyclotransferases Acting Specifically on Glutathione. EMBO Rep. 2012, 13, 1095–1101. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The Glutathione Cycle Glutathione Metabolism beyond the γ-Glutamyl Cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P.; Deeley, R.G. Transport of Glutathione and Glutathione Conjugates by MRP1. Trends Pharmacol. Sci. 2006, 27, 438–446. [Google Scholar] [CrossRef]
- Gündüz, M.; Ünal, Ö.; Kavurt, S.; Türk, E.; Mungan, N.Ö. Clinical Findings and Effect of Sodium Hydrogen Carbonate in Patients with Glutathione Synthetase Deficiency. J. Pediatr. Endocrinol. Metab. 2016, 29, 481–485. [Google Scholar] [CrossRef]
- Ke, H.L.; Lin, J.; Ye, Y.; Wu, W.J.; Lin, H.H.; Wei, H.; Huang, M.; Chang, D.W.; Dinney, C.P.; Wu, X. Genetic Variations in Glutathione Pathway Genes Predict Cancer Recurrence in Patients Treated with Transurethral Resection and Bacillus Calmette-Guerin Instillation for Non-muscle Invasive Bladder Cancer. Ann. Surg. Oncol. 2015, 22, 4104–4110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varriale, E.; Ferrante, A.; Crocetto, F.; Cianciulli, M.R.; Palermo, E.; Vitale, A.; Benincasa, G. Assessment of Glutathione-S-Transferase (GSTP1) Methylation Status Is a Reliable Molecular Biomarker for Early Diagnosis of Prostatic Intraepithelial Neoplasia. WCRJ 2019, 6, 1384. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a New Form of Cell Death Opportunities and Challenges in Cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Y.; Chen, C.; Xu, Y.F.; Shen, J.J.; Guo, H.M.; Li, H.F.; Li, X.N.; Kang, D.; Shao, Y.H.; Zhu, Z.P.; et al. Is the combinational administration of doxorubicin and glutathione a reasonable proposal? Acta Pharmacol. Sin. 2019, 40, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Hu, X.; Xu, B.; Tong, T.; Jing, Y.; Xi, L.; Zhou, W.; Lu, J.; Wang, X.; Yang, X.; et al. Glutathione S-transferase isozyme alpha 1 is predominantly involved in the cisplatin resistance of common types of solid cancer. Oncol. Rep. 2019, 41, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Karpusas, M.; Axarli, I.; Chiniadis, L.; Papakyriakou, A.; Bethanis, K.; Scopelitou, K.; Clonis, Y.D.; Labrou, N.E. The Interaction of the Chemotherapeutic Drug Chlorambucil with Human Glutathione Transferase A1-1 k. PLoS ONE 2013, 8, 56337. [Google Scholar] [CrossRef]
- Sau, A.; Pellizzari Tregno, F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione Transferases and Development of New Principles to Overcome Drug Resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef]
- Katayanagi, S.; Katsumata, K.; Mori, Y.; Narahara, K.; Shigoka, M.; Matsudo, T.; Enomoto, M.; Suda, T.; Ishizaki, T.; Hisada, M.; et al. GSTP1 as a potential predictive factor for adverse events associated with platinum-based antitumor agent-induced peripheral neuropathy. Oncol. Lett. 2019, 17, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Zanna, C. Current Status and Future Prospects for the Treatment of Chemotherapy-Induced Peripheral Neurotoxicity. Eur. J. Cancer 2002, 38, 1832–1837. [Google Scholar] [CrossRef]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chéreau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of Hydrogen Peroxide Is an Early and Crucial Step for Paclitaxel-Induced Cancer Cell Death Both in Vitro and In Vivo. Int. J. Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef]
- Schwöbel, J.A.; Wondrousch, D.; Koleva, Y.K.; Madden, J.C.; Cronin, M.T.; Schüürmann, G. Prediction of Michael-Type Acceptor Reactivity toward Glutathione. Chem. Res. Toxicol. 2010, 23, 1576–1585. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pavadhgul, P.; Nunthanawanich, P.; Sirikanchanarod, A.; Adulbhan, A. Whey Protein Supplementation Improves Nutritional Status, Glutathione Levels, and Immune Function in Cancer Patients A Randomized, Double-Blind Controlled Trial. J. Med. Food 2018, 21, 612–616. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Zuo, S.; Zhang, Y.; Wan, D.; Long, C.; Huang, P.; Wu, X.; Wu, C.; Liu, G.; et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 488–498. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Wu, C.; Zhang, Y.; Wu, X.; Yin, Y. Serine Alleviates Oxidative Stress via Supporting Glutathione Synthesis and Methionine Cycle in Mice. Mol. Nutr. Food Res. 2017, 61, 1700262. [Google Scholar] [CrossRef]
- Gago-Dominguez, M.; Castelao, J.E.; Sun, C.-L.; Van Den Berg, D.; Koh, W.-P.; Lee, H.-P.; Yu, M.C. Marine n-3 Fatty Acid Intake, Glutathione S-Transferase Polymorphisms and Breast Cancer Risk in Post-Menopausal Chinese women in Singapore. Carcinogenesis 2004, 25, 2143–2147. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Akbari-Fakhrabadi, M.; Daneshzad, E.; Yavari, M.; Rezaeinejad, M.; Morvaridzadeh, M.; Heshmati, J. Effect of Omega-3 Fatty Acid plus Vitamin E Co-Supplementation on Oxidative Stress Parameters: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 1019–1025. [Google Scholar] [CrossRef]
- Minutoli, L.; Marini, H. Selenium: Sources, Functions and Health Effects; Aomori, C., Hokkaido, M., Eds.; Nova Publisher: Hauppage, NY, USA, 2012. [Google Scholar]
- Minutoli, L.; Squadrito, F.; Altavilla, D.; Marini, H. Selenium: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; RCS Publishing: Cambridge, UK, 2015. [Google Scholar]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A Critical Review of Selenium Biogeochemical Behavior in Soil-Plant System with an Inference to Human Health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Pfister, C.; Dawzcynski, H.; Schingale, F. Sodium Selenite and Cancer Related Lymphedema Biological and Pharmacological Effects. J. Trace Elem. Med. Bio. 2016, 37, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease-What Do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in Food and the Human Body: A Review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Frączek, A.; Pasternak, K. Selenium in Medicine and Treatment. J. Elem. 2013, 18, 145–163. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Selenium Significance, and Outlook for Supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Smrkolj, P.; Pograjc, L.; Hlastan-Ribič, C.; Stibilj, V. Selenium Content in Selected Slovenian Foodstuffs and Estimated Daily Intakes of Selenium. Food Chem. 2005, 90, 691–697. [Google Scholar] [CrossRef]
- Reilly, C. Selenium: A New Entrant into the Functional Food Arena. Trends Food Sci. Technol. 1998, 9, 114–118. [Google Scholar] [CrossRef]
- dos Santos, M.; da Silva Júnior, F.M.R.; Muccillo-Baisch, A.L. Selenium Content of Brazilian Foods: A Review of the Literature Values. J. Food Compost. Anal. 2017, 58, 10–15. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Wark, P.A.; Grubben, M.J.A.L.; Peters, W.H.M.; Nagengast, F.M.; Kampman, E.; Kok, F.J.; van’t Veer, P. Habitual Consumption of Fruits and Vegetables Associations with Human Rectal Glutathione S-Transferase. Carcinogenesis 2004, 25, 2135–2142. [Google Scholar] [CrossRef]
- Talalay, P.; Fahey, J.W.; Holtzclaw, W.D.; Prestera, T.; Zhang, Y. Chemoprotection against Cancer by Phase 2 Enzyme Induction. Toxicol. Lett. 1995, 82–83, 173–179. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, E1701000. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.O.; Mansour, D.F.; Hashad, I.M.; Bakeer, R.M. Effects of Sulforaphane on D-Galactose-Induced Liver Aging in Rats Role of Keap-1nrf-2 Pathway. Eur. J. Pharmacol. 2019, 855, 40–49. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; Konsue, N.; Ioannides, C. Isothiocyanates and Xenobiotic Detoxification. Mol. Nutr. Food Res. 2018, 62, E1700916. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, M.; Xie, X.; Jin, J.; Holman, C.A.J. Green Tea Consumption and Glutathione S-Transferases Genetic Polymorphisms on the Risk of Adult Leukemia. Eur. J. Nutr. 2017, 56, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Cordova, C.A.; Chew, W.M.; Xu, M.J.; Hsu, C.H.; Ranger-Moore, J.; Alberts, D.S. Effects of Repeated Green Tea Catechin Administration on Human Cytochrome P450 Activity. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 2473–2476. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef]
- Cho, M.R.; Han, J.H.; Lee, H.J.; Park, Y.K.; Kang, M.H. Purple Grape Juice Supplementation in Smokers and Antioxidant Status According to Different Types of GST Polymorphisms. J. Clin. Biochem. Nutr. 2015, 56, 49–56. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Cruz, F.A.; Da Silva, E.L.; Bordignon-Luiz, M.T. Acute Consumption of Organic and Conventional Tropical Grape Juices (Vitis labrusca L.) Increases Antioxidants in Plasma and Erythrocytes, but Not Glucose and uric acid levels, in healthy individuals. Nutr. Res. 2016, 36, 808–817. [Google Scholar] [CrossRef]
- Singletary, K.W.; Rokusek, J.T. Tissue-Specific Enhancement of Xenobiotic Detoxification Enzymes in Mice by Dietary Rosemary Extract. Plant Foods Hum. Nutr. 1997, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W. Rosemary Extract and Carnosol Stimulate Rat Liver Glutathione-S-Transferase and Quinone Reductase Activities. Cancer Lett. 1996, 100, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant Effects of Curcumin in Models of Neurodegeneration, Aging, Oxidative and Nitrosative Stress A Review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.; González-Rubio, M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Hatta, S.; Wada, K.; Ueda, N.; Yoshimura, T.; Endo, T.; Sakata, M.; Tanaka, T.; Haga, M. Effects of Extract of Ginkgo Biloba Leaves and Its Constituents on Carcinogen-Metabolizing Enzyme Activities and glutathione levels in mouse liver. Life Sci. 2002, 70, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, G.F.; Lee, K.; Lopez, R.; Previs, S.F.; Willard, B.; McCullough, A.; Kasumov, T.A. Western Diet Induced NAFLD in LDLR(-)(-) Mice Is Associated with Reduced Hepatic Glutathione Synthesis. Free Radic. Biol. Med. 2016, 96, 13–21. [Google Scholar] [CrossRef]
- Bettermann, E.L.; Hartman, T.J.; Easley, K.A.; Ferranti, E.P.; Jones, D.P.; Quyyumi, A.A.; Vaccarino, V.; Ziegler, T.R.; Alvarez, J.A. Higher Mediterranean Diet Quality Scores and Lower Body Mass Index Are Associated with a Less-Oxidized Plasma Glutathione and Cysteine Redox Status in Adults. J. Nutr. 2018, 148, 245–253. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
- Divella, R.; Daniele, A.; Savino, E.; Paradiso, A. Anticancer Effects of Nutraceuticals in the Mediterranean Diet An Epigenetic Diet Model. Cancer Genom. Proteom. 2020, 17, 335–350. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, A.A. Sustainable Diets The Interaction between Food Industry, Nutrition, Health and the Environment. Food Sci. Technol. Int. 2016, 22, 102–111. [Google Scholar] [CrossRef]
- Agaj, A.; Peršurić, Ž.; Pavelić, S.K. Mediterranean Food Industry By-Products as a Novel Source of Phytochemicals with a Promising Role in Cancer Prevention. Molecules 2022, 27, 8655. [Google Scholar] [CrossRef]
- Chasseaud, L.F. The Role of Glutathione and Glutathione S-Transferases in the Metabolism of Chemical Carcinogens and Other Electrophilic Agents. Adv. Cancer Res. 1979, 29, 175–274. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Glutathione: An Overview of Biosynthesis and Modulation. Chem. Biol. Interact. 1998, 111–112, 1–14. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The Importance of Glutathione in Human Disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Hanigan, M.H. Metabolism of Glutathione S-Conjugates Multiple Pathways. In Comprehensive Toxicology, 3rd ed.; McQueen, C.A., Ed.; Elsevier Ltd.: Oxford, UK, 2018; pp. 363–406. [Google Scholar]
- Van Bladeren, P.J. Glutathione Conjugation as a Bioactivation Reaction. Chem. Biol. Interact. 2000, 129, 61–76. [Google Scholar] [CrossRef]
- Armstrong, R.N. Structure, Catalytic Mechanism, and Evolution of the Glutathione Transferases. Chem. Res. Toxicol. 1997, 10, 2–18. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione Transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting Glutathione Metabolism Partner in Crime in Anticancer Therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef]
- Cazenave, L.A.; Moscow, J.A.; Myers, C.E.; Cowan, K.H. Glutathione S-Transferase and Drug Resistance. Cancer Treat. Res. 1989, 48, 171–187. [Google Scholar] [CrossRef]
- Desai, C. Busulfan. In Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions, 16th ed.; Aronson, J.K., Ed.; Elsevier Ltd.: Oxford, UK, 2016; pp. 1100–1103. [Google Scholar]
- Czerwinski, M.; Gibbs, J.P.; Slattery, J.T. Busulfan Conjugation by Glutathione S-Transferases Alpha, Mu, and Pi. Drug Metab. Dispos. 1996, 24, 1015–1019. [Google Scholar]
- Scian, M.; Atkins, W.M. The Busulfan Metabolite EdAG Irreversibly Glutathionylates Glutaredoxins. Arch. Biochem. Biophys. 2015, 583, 96–104. [Google Scholar] [CrossRef]
- Kurtovic, S.; Modén, O.; Shokeer, A.; Mannervik, B. Structural Determinants of Glutathione Transferases with Azathioprine Activity Identified by DNA Shuffling of Alpha Class Members. J. Mol. Biol. 2008, 375, 1365–1379. [Google Scholar] [CrossRef]
- Eklund, B.I.; Moberg, M.; Bergquist, J.; Mannervik, B. Divergent Activities of Human Glutathione Transferases in the Bioactivation of Azathioprine. Mol. Pharmacol. 2006, 70, 747–754. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [PubMed]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-Induced Peripheral Neurotoxicity (CIPN) An Update. Crit. Rev. Oncol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Roelofs, R.I.; Hrushesky, W.; Rogin, J.; Rosenberg, L. Peripheral Sensory Neuropathy and Cisplatin Chemotherapy. Neurology (Minneap) 1984, 34, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.; Chaudhry, V.; Cavaletti, G.; Donehower, R. Interventions for Preventing Neuropathy Caused by Cisplatin and Related Compounds. Cochrane Database Syst. Rev. 2007, CD005228, Update in: Cochrane Database Syst. Rev. 2011, CD005228. [Google Scholar] [CrossRef]
- Cascinu, S.; Cordella, L.; Del Ferro, E.; Fronzoni, M.; Catalano, G. Neuroprotective effect of reduced glutathione on cisplatin-based chemotherapy in advanced gastric cancer: A randomized double-blind placebo-controlled trial. J. Clin. Oncol. 1995, 13, 26–32. [Google Scholar] [CrossRef]
- Cascinu, S.; Catalano, V.; Cordella, L.; Labianca, R.; Giordani, P.; Baldelli, A.M.; Beretta, G.D.; Ubiali, E.; Catalano, G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: A randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2002, 20, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Schmidinger, M.; Budinsky, A.C.; Wenzel, C.; Piribauer, M.; Brix, R.; Kautzky, M.; Oder, W.; Locker, G.J.; Zielinski, C.C.; Steger, G.G. Glutathione in the Prevention of Cisplatin Induced Toxicities a Prospectively Randomized Pilot Trial in Patients with Head and Neck Cancer And Non Small Cell Lung Cancer. Wien. Klin. Wochenschr. 2000, 112, 617–623. [Google Scholar] [PubMed]
- Smyth, J.F.; Bowman, A.; Perren, T.; Wilkinson, P.; Prescott, R.J.; Quinn, K.J.; Tedeschi, M. Glutathione Reduces the Toxicity and Improves Quality of Life of Women Diagnosed with Ovarian Cancer Treated with Cisplatin: Results of a double-blind, randomised trial. Ann. Oncol. 1997, 8, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Milla, P.; Airoldi, M.; Weber, G.; Drescher, A.; Jaehde, U.; Cattel, L. Administration of Reduced Glutathione in FOLFOX4 Adjuvant Treatment for Colorectal Cancer Effect on Oxaliplatin Pharmacokinetics, Pt-DNA Adduct Formation, and neurotoxicity. Anticancer. Drugs 2009, 20, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Bini, S.; Miceli, D.; Bogliun, G.; Marzorati, L.; Cavaletti, G.; Parmigiani, F.; Venturino, P.; Tedeschi, M.; Frattola, L.; et al. Weekly Cisplatin +- Glutathione in Relapsed Ovarian Carcinoma. Int. J. Gynecol. Cancer 1995, 5, 81–86. [Google Scholar] [CrossRef]
- Bogliun, G.; Marzorati, L.; Marzola, M.; Miceli, M.D.; Cantu, M.G.; Cavaletti, G. Neurotoxicity of Cisplatin +/− Reduced Glutathione in the First-Line Treatment of Advanced Ovarian Cancer. Int. J. Gynecol. Cancer 1996, 6, 415–419. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakayama, J.; Sadakata, Y. Protective Effects of Exogenous Glutathione and Related Thiol Compounds against Drug-Induced Liver Injury. Biol. Pharm. Bull. 2011, 34, 366–370. [Google Scholar] [CrossRef]
- Ntamo, Y.; Ziqubu, K.; Chellan, N.; Nkambule, B.B.; Nyambuya, T.M.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Marcheggiani, F.; Tiano, L.; Dludla, P.V. Drug-Induced Liver Injury Clinical Evidence of N-Acetyl Cysteine Protective Effects. Oxid. Med. Cell Longev. 2021, 2021, 3320325. [Google Scholar] [CrossRef]
- Zhang, L.; Hanigan, M.H. Role of Cysteine S-Conjugate β-Lyase in the Metabolism of Cisplatin. J. Pharmacol. Exp. Ther. 2003, 306, 988–994. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Calvert, P.; Yao, K.S.; Hamilton, T.C.; O’Dwyer, P.J. Clinical Studies of Reversal of Drug Resistance Based on Glutathione. Chem. Biol. Interact. 1998, 111–112, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.L.; Tew, K.D. Glutathione and Related Enzymes in Multidrug Resistance. Eur. J. Cancer 1996, 32A, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; Israels, L.G.; Goldenberg, G.J.; Anhalt, C.D.; Verburg, L.; Mowat, M.R.; Begleiter, A. Glutathione S-Transferase Activity, Sulfhydryl Group and Glutathione Levels, and DNA Cross-Linking Activity with chlorambucil in chronic lymphocytic leukemia. J. Natl. Cancer Inst. 1990, 82, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiecka, K.; Dyląg, M.; Augustyniak, D.; Majkowska-Skrobek, G.; Cal-Bąkowska, M.; Ko, Y.H.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. Glutathione may have implications in the design of 3-bromopyruvate treatment protocols for both fungal and algal infections as well as multiple myeloma. Oncotarget 2016, 7, 65614–65626. [Google Scholar] [CrossRef]
- Dirven, H.A.; Van Ommen, B.; Van Bladeren, P.J. Involvement of Human Glutathione S-Transferase Isoenzymes in the Conjugation of Cyclophosphamide Metabolites with Glutathione. Cancer Res. 1994, 54, 6215–6220. [Google Scholar]
- Mena, S.; Benlloch, M.; Ortega, A.; Carretero, J.; Obrador, E.; Asensi, M.; Petschen, I.; Brown, B.D.; Estrela, J.M. Bcl-2 and Glutathione Depletion Sensitizes B16 Melanoma to Combination Therapy and Eliminates Metastatic Disease. Clin. Cancer Res. 2007, 13, 2658–2666. [Google Scholar] [CrossRef]
- Song, E.A.; Kim, H. Docosahexaenoic Acid Induces Oxidative DNA Damage and Apoptosis, and Enhances the Chemosensitivity of Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef]
- Messina, J.P.; Lawrence, D.A. Cell Cycle Progression of Glutathione-Depleted Human Peripheral Blood Mononuclear Cells Is Inhibited at S Phase. J. Immunol. 1989, 143, 1974–1981. [Google Scholar] [CrossRef]
- Lu, S.C.; Ge, J.L. Loss of Suppression of GSH Synthesis at Low Cell Density in Primary Cultures of Rat Hepatocytes. Am. J. Physiol. 1992, 263, C1181-9. [Google Scholar] [CrossRef]
- Carretero, J.; Obrador, E.; Anasagasti, M.J.; Martin, J.J.; Vidal-Vanaclocha, F.; Estrela, J.M. Growth-Associated Changes in Glutathione Content Correlate with Liver Metastatic Activity of B16 Melanoma Cells. Clin. Exp. Metastasis 1999, 17, 567–574. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants Can Increase Melanoma Metastasis in Mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Bekeschus, S. Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione. Int. J. Mol. Sci. 2022, 23, 14772. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Della Pepa, C.; Tralongo, P.; Fulvi, A.; Martellotta, F.; Lleshi, A.; Nasti, G.; Fisichella, R.; Romano, C.; De Divitiis, C.; et al. Use of Complementary and Alternative Medicine (CAM) in cancer patients: An Italian multicenter survey. Oncotarget 2017, 8, 24401–24414. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Rinaldi, L.; Taibi, R.; Tralongo, P.; Fulvi, A.; Montesarchio, V.; Madeddu, G.; Magistri, P.; Bimonte, S.; Trovò, M.; et al. Physician Attitudes and Perceptions of Complementary and Alternative Medicine (CAM): A Multicentre Italian Study. Front. Oncol. 2020, 10, 594. [Google Scholar] [CrossRef]
- Berretta, M.; Dal Lago, L.; Tinazzi, M.; Ronchi, A.; La Rocca, G.; Montella, L.; Di Francia, R.; Facchini, B.A.; Bignucolo, A.; Montopoli, M. Evaluation of Concomitant Use of Anticancer Drugs and Herbal Products: From Interactions to Synergic Activity. Cancers 2022, 14, 5203. [Google Scholar] [CrossRef]
- İnci, H.; İnci, F. Complementary and alternative medicine awareness in cancer patients receiving chemotherapy. WCRJ 2020, 7, e1752. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, H.R.; Facchini, B.A.; di Francia, R.; Freni, J.; Puzzolo, D.; Montella, L.; Facchini, G.; Ottaiano, A.; Berretta, M.; Minutoli, L. Glutathione: Lights and Shadows in Cancer Patients. Biomedicines 2023, 11, 2226. https://doi.org/10.3390/biomedicines11082226
Marini HR, Facchini BA, di Francia R, Freni J, Puzzolo D, Montella L, Facchini G, Ottaiano A, Berretta M, Minutoli L. Glutathione: Lights and Shadows in Cancer Patients. Biomedicines. 2023; 11(8):2226. https://doi.org/10.3390/biomedicines11082226
Chicago/Turabian StyleMarini, Herbert Ryan, Bianca Arianna Facchini, Raffaele di Francia, José Freni, Domenico Puzzolo, Liliana Montella, Gaetano Facchini, Alessandro Ottaiano, Massimiliano Berretta, and Letteria Minutoli. 2023. "Glutathione: Lights and Shadows in Cancer Patients" Biomedicines 11, no. 8: 2226. https://doi.org/10.3390/biomedicines11082226
APA StyleMarini, H. R., Facchini, B. A., di Francia, R., Freni, J., Puzzolo, D., Montella, L., Facchini, G., Ottaiano, A., Berretta, M., & Minutoli, L. (2023). Glutathione: Lights and Shadows in Cancer Patients. Biomedicines, 11(8), 2226. https://doi.org/10.3390/biomedicines11082226








