The Combined Effect of Western Diet Consumption and Diclofenac Administration Alters the Gut Microbiota and Promotes Anastomotic Leakage in the Distal Colon
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
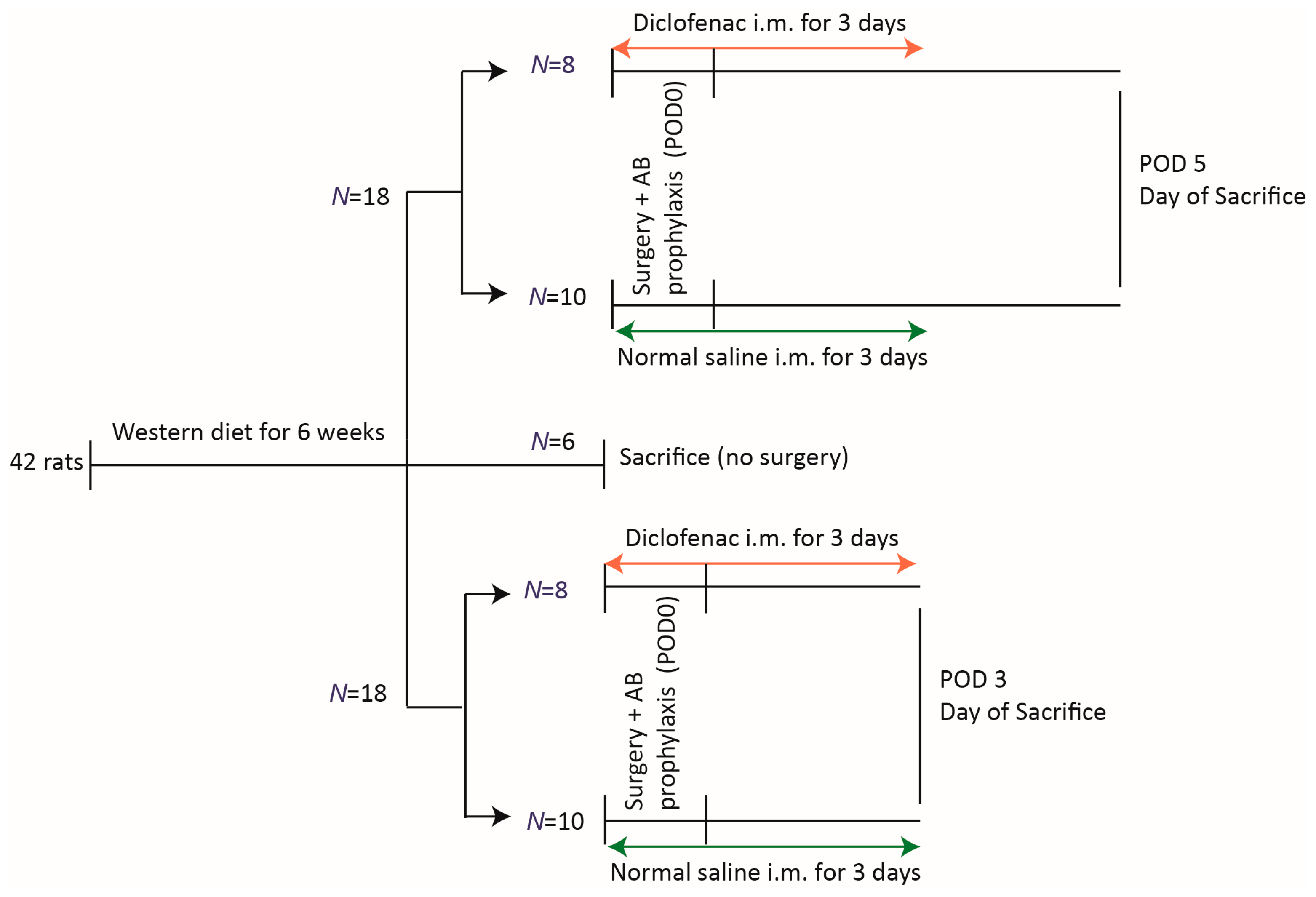
2.2. Groups
2.3. Surgical Technique and Diclofenac Administration
2.4. Outcome Parameters
2.4.1. Anastomotic Healing
2.4.2. Microbiota Analysis: Sample Collection
2.4.3. Microbiota Analysis: DNA Isolation
2.4.4. Microbiota Analysis: 16S Ribosomal RNA Marker Gene Library Preparation and Sequencing
2.4.5. Histology and Staining
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Animal Welfare
3.2. Anastomotic Healing
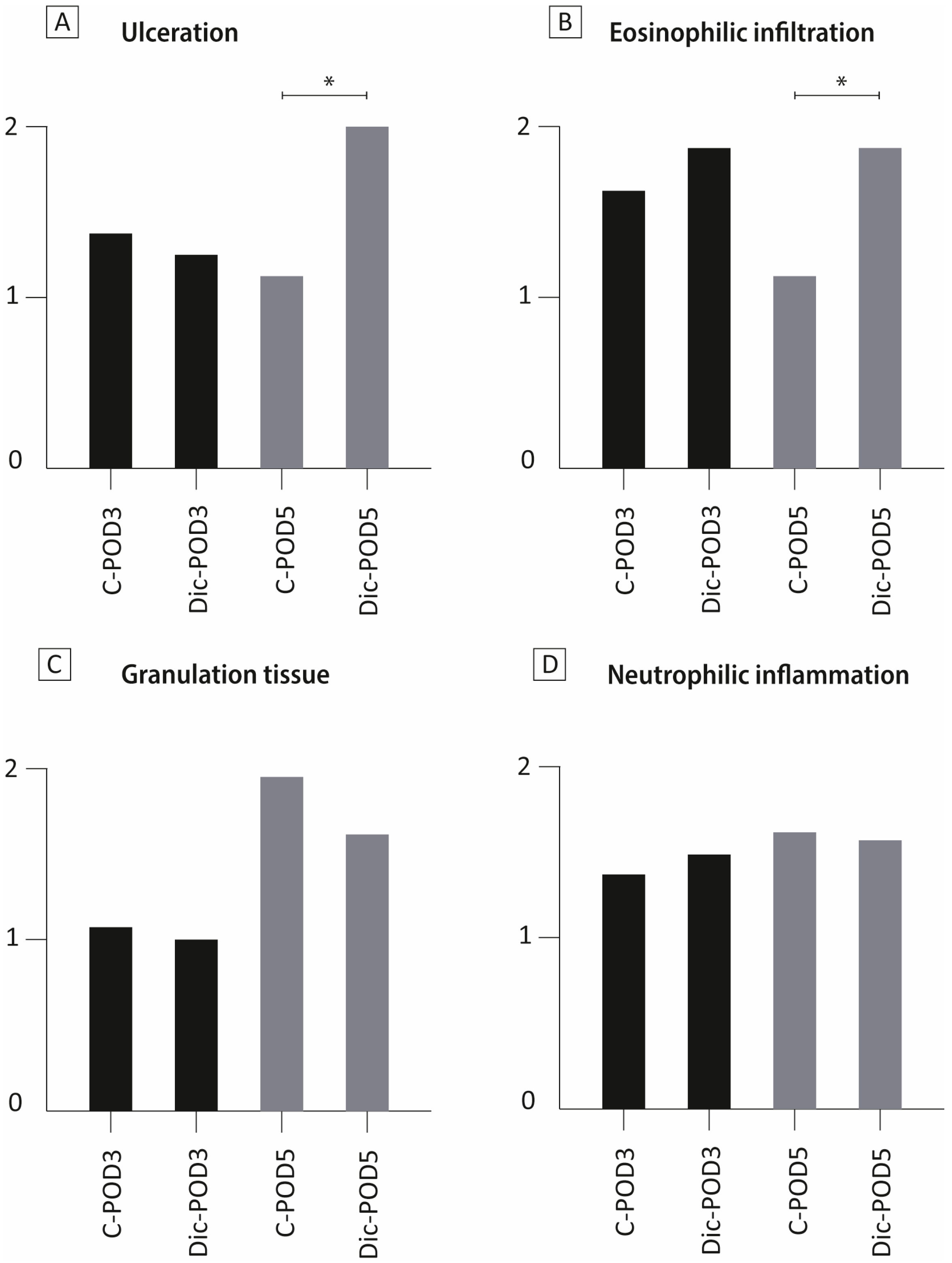
3.3. The Effect of Diclofenac on Colonic Mucosa
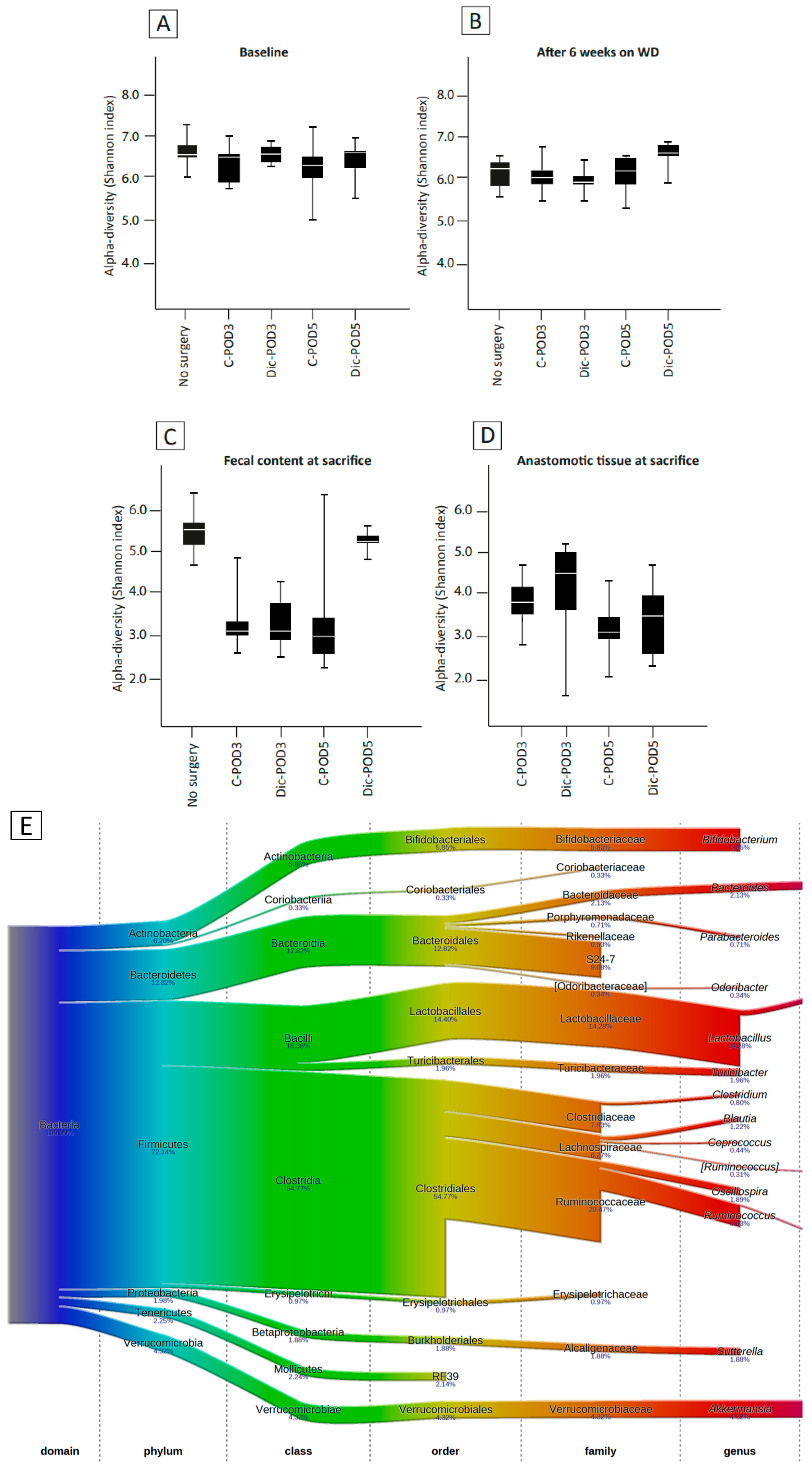
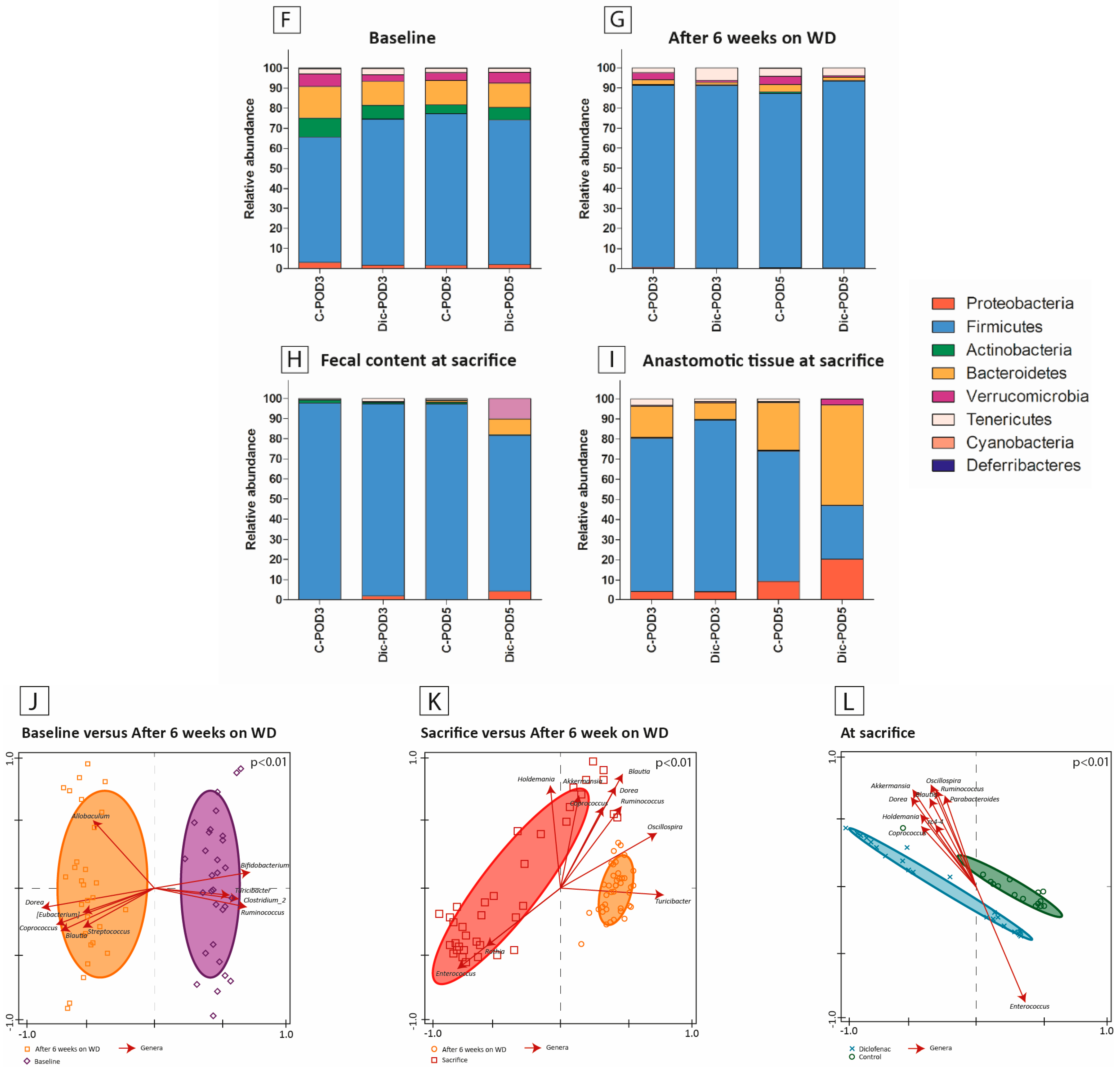
3.4. The Effect of WD and Surgery on Microbial Diversity and Composition (No Diclofenac)
3.5. The Effect of WD, Surgery and Diclofenac on Microbial Diversity and Composition
3.6. Microbiota Composition Differences between Anastomotic Tissues and Fecal Contents
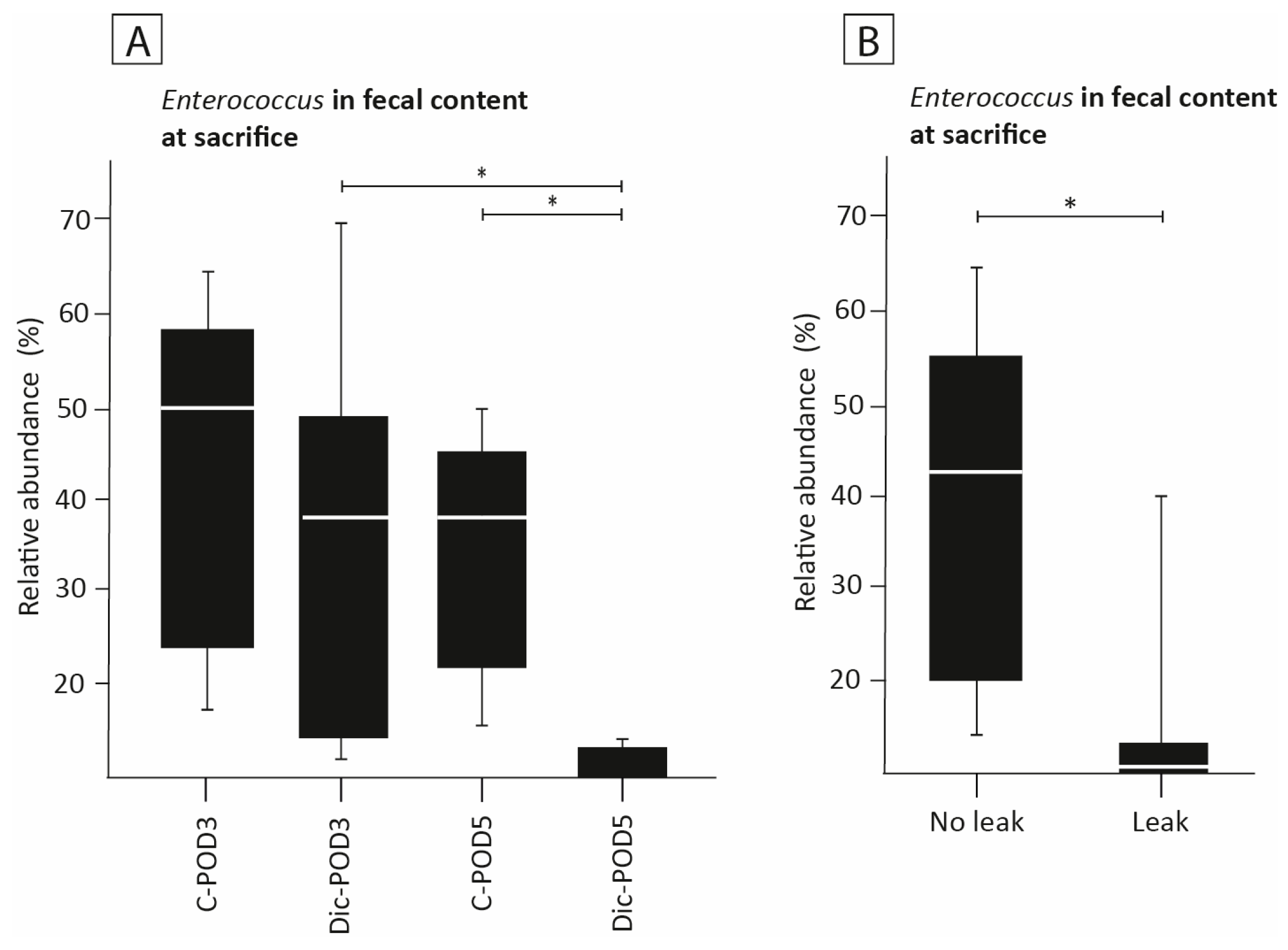
3.7. The Effect of Diclofenac on the Presence of Enterococcus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arron, M.N.N.; Greijdanus, N.G.; Broek, R.P.G.T.; Dekker, J.W.T.; van Workum, F.; van Goor, H.; Tanis, P.J.; de Wilt, J.H.W. Trends in risk factors of anastomotic leakage after colorectal cancer surgery (2011–2019): A Dutch population-based study. Color. Dis. 2021, 23, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Krarup, P.; Jorgensen, L.N.; Andreasen, A.H.; Harling, H.; on behalf of the Danish Colorectal Cancer Group. A nationwide study on anastomotic leakage after colonic cancer surgery. Color. Dis. 2012, 14, e661–e667. [Google Scholar] [CrossRef]
- Kelly, M.; Bhangu, A.; Singh, P.; Fitzgerald, J.E.F.; Tekkis, P.P. Systematic review and meta-analysis of trainee-versus expert surgeon-performed colorectal resection. Br. J. Surg. 2014, 101, 750–759. [Google Scholar] [CrossRef]
- Gaines, S.; Shao, C.; Hyman, N.; Alverdy, J.C. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br. J. Surg. 2018, 105, e131–e141. [Google Scholar] [CrossRef]
- Cohn, I.J.; Rives, J.D. Antibiotic Protection of Colon Anastomoses. Ann. Surg. 1955, 141, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Binda, C.; Cristofaro, L.; Sbrancia, M.; Coluccio, C.; Petraroli, C.; Jung, C.F.M.; Cucchetti, A.; Cavaliere, D.; Ercolani, G.; et al. Dysbiosis and Gastrointestinal Surgery: Current Insights and Future Research. Biomedicines 2022, 10, 2532. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Smith, D.P.; Christley, S.; Gilbert, J.A.; Zaborina, O.; Alverdy, J.C. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014, 2, 35. [Google Scholar] [CrossRef]
- Shakhsheer, B.A.; Versten, L.A.; Luo, J.N.; Defazio, J.R.; Klabbers, R.; Christley, S.; Zaborin, A.; Guyton, K.L.; Krezalek, M.; Smith, D.P.; et al. Morphine Promotes Colonization of Anastomotic Tissues with Collagenase—Producing Enterococcus faecalis and Causes Leak. J. Gastrointest. Surg. 2016, 20, 1744–1751. [Google Scholar] [CrossRef]
- van der Vijver, R.J.; van Laarhoven, C.J.H.M.; Lomme, R.M.L.M.; Hendriks, T. Diclofenac causes more leakage than naproxen in anastomoses in the small intestine of the rat. Int. J. Color. Dis. 2013, 28, 1209–1216. [Google Scholar] [CrossRef]
- Yauw, S.T.; Lomme, R.M.; van der Vijver, R.J.; Hendriks, T.; van Laarhoven, K.J.; van Goor, H. Diclofenac causes anastomotic leakage in the proximal colon but not in the distal colon of the rat. Am. J. Surg. 2015, 210, 382–388. [Google Scholar] [CrossRef]
- Nugent, T.S.; Kelly, M.E.; Donlon, N.E.; Fahy, M.R.; Larkin, J.O.; McCormick, P.H.; Mehigan, B.J. Obesity and anastomotic leak rates in colorectal cancer: A meta-analysis. Int. J. Color. Dis. 2021, 36, 1819–1829. [Google Scholar] [CrossRef]
- Hyoju, S.K.; Adriaansens, C.; Wienholts, K.; Sharma, A.; Keskey, R.; Arnold, W.; van Dalen, D.; Gottel, N.; Hyman, N.; Zaborin, A.; et al. Low-fat/high-fibre diet prehabilitation improves anastomotic healing via the microbiome: An experimental model. Br. J. Surg. 2019, 107, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G.; et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci. Transl. Med. 2015, 7, 286ra68. [Google Scholar] [CrossRef]
- Salem-Milani, A.; Balaei-Gajan, E.; Rahimi, S.; Moosavi, Z.; Abdollahi, A.; Zakeri-Milani, P.; Bolourian, M. Antibacterial effect of diclofenac sodium on Enterococcus faecalis. J. Dent. (Tehran) 2013, 10, 16–22. [Google Scholar] [PubMed]
- Hyoju, S.K.; Klabbers, R.E.; Aaron, M.; Krezalek, M.A.; Zaborin, A.; Wiegerinck, M.; Hyman, N.H.; Zaborina, O.; Van Goor, H.; Alverdy, J.C. Oral Polyphosphate Suppresses Bacterial Collagenase Production and Prevents Anastomotic Leak Due to Serratia marcescens and Pseudomonas aeruginosa. Ann. Surg. 2018, 267, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Yauw, S.T.K.; Lomme, R.M.L.M.; van den Broek, P.; Greupink, R.; Russel, F.G.M.; van Goor, H. Experimental study of diclofenac and its biliary metabolites on anastomotic healing. BJS Open 2018, 2, 220–228. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2013, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 22 May 2019).
- Xiao, X.; Nakatsu, G.; Jin, Y.; Wong, S.; Yu, J.; Lau, J.Y.W. Gut Microbiota Mediates Protection Against Enteropathy Induced by Indomethacin. Sci. Rep. 2017, 7, 40317. [Google Scholar] [CrossRef]
- Robert, A.; Asano, T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins 1977, 14, 333–341. [Google Scholar] [CrossRef]
- Butt, I.; Shrestha, B.M. Two-hit hypothesis and multiple organ dysfunction syndrome. J. Nepal Med. Assoc. 2008, 47. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Colucci, R.; Pellegrini, C.; Fornai, M.; Tirotta, E.; Antonioli, L.; Renzulli, C.; Ghelardi, E.; Piccoli, E.; Gentile, D.; Benvenuti, L.; et al. Pathophysiology of NSAID-associated intestinal lesions in the rat: Luminal bacteria and mucosal inflammation as targets for prevention. Front. Pharmacol. 2018, 9, 1340. [Google Scholar] [CrossRef]
- Reuter, B.; Davies, N.; Wallace, J. Nonsteroidal anti-inflammatory drug enteropathy in rats: Role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 1997, 112, 109–117. [Google Scholar] [CrossRef]
- Terán-Ventura, E.; Aguilera, M.; Vergara, P.; Martínez, V. Specific changes of gut commensal microbiota and TLRs during indomethacin-induced acute intestinal inflammation in rats. J. Crohn’s Colitis 2014, 8, 1043–1054. [Google Scholar] [CrossRef]
- Ferrie, S.; Webster, A.; Wu, B.; Tan, C.; Carey, S. Gastrointestinal surgery and the gut microbiome: A systematic literature review. Eur. J. Clin. Nutr. 2021, 75, 12–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Sun, Y.; Liu, Q.; Cao, Q.; Li, T.; Li, J. Risk Factors and Preventive Measures for Anastomotic Leak in Colorectal Cancer. Technol. Cancer Res. Treat. 2022, 21. [Google Scholar] [CrossRef]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: A pilot study. Surg. Endosc. 2015, 30, 2259–2265. [Google Scholar] [CrossRef]
- Zamorano, D.; Ivulic, D.; Viver, T.; Morales, F.; López-Kostner, F.; Vidal, R.M. Microbiota Phenotype Promotes Anastomotic Leakage in a Model of Rats with Ischemic Colon Resection. Microorganisms 2023, 11, 680. [Google Scholar] [CrossRef]
- Seitz, S.; Boelsterli, U.A. Diclofenac acyl glucuronide, a major biliary metabolite, is directly involved in small intestinal injury in rats. Gastroenterology 1998, 115, 1476–1482. [Google Scholar] [CrossRef]
- Wallace, J.L.; McKnight, G.W. Characterization of a simple animal model for nonsteroidal anti-inflammatory drug induced antral ulcer. Can. J. Physiol. Pharmacol. 1993, 71, 447–452. [Google Scholar] [CrossRef]
- Katsinelos, P.; Christodoulou, K.; Pilpilidis, I.; Xiarchos, P.; Papagiannis, A.; Dimiropoulos, S.; Amperiadis, P.; Vasiliadis, T.; Tarpagos, A.; Katsos, I.; et al. Colopathy associated with the systemic use of nonsteroidal anti-inflammatory medications. An underestimated entity. Hepatogastroenterology 2002, 49, 345–348. [Google Scholar] [PubMed]
- Davies, N.M. Toxicity of nonsteroidal anti-inflammatory drugs in the large intestine. Dis. Colon Rectum 1995, 38, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Holte, K.; Andersen, J.; Jakobsen, D.H.; Kehlet, H. Cyclo-oxygenase 2 inhibitors and the risk of anastomotic leakage after fast-track colonic surgery. Br. J. Surg. 2009, 96, 650–654. [Google Scholar] [CrossRef] [PubMed]
- de Hingh, I.H.J.T.; van Goor, H.; de Man, B.M.; Lomme, R.M.L.M.; Bleichrodt, R.P.; Hendriks, T. Selective cyclo-oxygenase 2 inhibition affects ileal but not colonic anastomotic healing in the early postoperative period. Br. J. Surg. 2006, 93, 489–497. [Google Scholar] [CrossRef]
- Morgan, R.B.; Shogan, B.D. The science of anastomotic healing. Semin. Colon Rectal Surg. 2022, 33. [Google Scholar] [CrossRef]
- Yu, Q.; Yuan, L.; Deng, J.; Yang, Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell. Infect. Microbiol. 2015, 5, 26. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol. Liver Physiol. 2009, 297, G506–G513. [Google Scholar] [CrossRef]
- Syer, S.D.; McKnight, W.; Aucouturier, A.; Martin, R.; Langella, P.; Wallace, J.L. Su1724 Bifidobacteria Exert a Protective Effect Against NSAID-Induced Enteropathy That is Dependent on Lactate Production. Gastroenterology 2012, 142, s0016–s5085. [Google Scholar] [CrossRef]
- Mortensen, B.; Murphy, C.; O’grady, J.; Lucey, M.; Elsafi, G.; Barry, L.; Westphal, V.; Wellejus, A.; Lukjancenko, O.; Eklund, A.C.; et al. Bifidobacterium breve Bif195 Protects Against Small-Intestinal Damage Caused by Acetylsalicylic Acid in Healthy Volunteers. Gastroenterology 2019, 157, 637–646.e4. [Google Scholar] [CrossRef]
- Boatman, S.; Kaiser, T.; Nalluri-Butz, H.; Khan, M.H.; Dietz, M.; Kohn, J.; Johnson, A.J.; Gaertner, W.B.; Staley, C.; Jahansouz, C. Diet-induced shifts in the gut microbiota influence anastomotic healing in a murine model of colonic surgery. Gut Microbes 2023, 15, 2283147. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Yang, C.; Mai, J.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L. ggpicrust2: An R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arron, M.N.N.; Bluiminck, S.; ten Broek, R.P.G.; Ederveen, T.H.A.; Alpert, L.; Zaborina, O.; Alverdy, J.C.; van Goor, H. The Combined Effect of Western Diet Consumption and Diclofenac Administration Alters the Gut Microbiota and Promotes Anastomotic Leakage in the Distal Colon. Biomedicines 2024, 12, 2170. https://doi.org/10.3390/biomedicines12102170
Arron MNN, Bluiminck S, ten Broek RPG, Ederveen THA, Alpert L, Zaborina O, Alverdy JC, van Goor H. The Combined Effect of Western Diet Consumption and Diclofenac Administration Alters the Gut Microbiota and Promotes Anastomotic Leakage in the Distal Colon. Biomedicines. 2024; 12(10):2170. https://doi.org/10.3390/biomedicines12102170
Chicago/Turabian StyleArron, Melissa N. N., Stijn Bluiminck, Richard P. G. ten Broek, Thomas H. A. Ederveen, Lindsay Alpert, Olga Zaborina, John C. Alverdy, and Harry van Goor. 2024. "The Combined Effect of Western Diet Consumption and Diclofenac Administration Alters the Gut Microbiota and Promotes Anastomotic Leakage in the Distal Colon" Biomedicines 12, no. 10: 2170. https://doi.org/10.3390/biomedicines12102170
APA StyleArron, M. N. N., Bluiminck, S., ten Broek, R. P. G., Ederveen, T. H. A., Alpert, L., Zaborina, O., Alverdy, J. C., & van Goor, H. (2024). The Combined Effect of Western Diet Consumption and Diclofenac Administration Alters the Gut Microbiota and Promotes Anastomotic Leakage in the Distal Colon. Biomedicines, 12(10), 2170. https://doi.org/10.3390/biomedicines12102170





