Locoregional Therapies for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
1.1. NAFLD
1.2. NAFLD-HCC Treatment Overview
2. Percutaneous Ablation
2.1. Radiofrequency Ablation
2.1.1. Overview
| Modality | Indications | Contraindications | Treatment Outcomes in HCC | Treatment Outcomes in NAFLD-HCC |
|---|---|---|---|---|
| Percutaneous ablation | NAFLD-HCC with BCLC stage 0 and A, nonsurgical candidates [31] | Vascular invasion, intrahepatic biliary tree dilation, exophytic tumor location, uncorrectable coagulopathy, tumor is surgically resectable [2] | 1- and 3-year OS was 95.8% and 71.4% 1- and 3-year DFS was 85.9% and 64.1% [35] mRECIST-CR 47% mRECIST-PR 39% [36] | Similar [37] |
| TAE | HCC with BCLC stage B and C; less acute toxicity [38] | Decompensated cirrhosis, reduced portal vein flow, PVT creatinine clearance < 30 mL/min, high tumor burden, untreated esophageal varices, elevated LFT marker [39] | 1- and 3-year OS was 84.8% and 38.3% [40] Median PFS was 7.2 months [41] mRECIST-CR was 18.4% mRECIST-PR was 28.8% [40] | No direct studies |
| TACE | Intermediate, unresectable NAFLD-HCC; downstaging for OLT [42] | Decompensated cirrhosis, reduced portal vein flow, creatinine clearance < 30 mL/min, bi-lobar tumor involvement [3] | 1- and 3-year OS was 89.9% and, 66.3% [43] Median PFS was 13.5 months [44] mRECIST-CR was 47.3% mRECIST-PR was 67.4% [41] | Similar [45] |
| TARE | Intermediate, unresectable NAFLD-HCC; no limitations on PVT; downstaging for OLT [46,47] | Decompensated cirrhosis, creatinine clearance < 30 mL/min, bi-lobar tumor involvement [48] | 1- and 3-year OS was 63% and 27% [49] Median PFS was 14.5 months [50] mRECIST-CR was 13.7% mRECIST-PR was 43.1% [51] | Similar [52] Similar [53] |
2.1.2. Indications and Contraindications
2.1.3. Outcomes
| Modality | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Percutaneous ablation | Radiofrequency current, microwaves, or cycles of freezing and thawing which cause cell death. | Able to function as monotherapy for early-stage disease; fewer complications compared with transarterial therapies; potentially curative. | PAS, bleeding, iatrogenic injury, and cryoshock (cryoablation) [2,57]. |
| TAE | Micro-embolic particles causing tumor ischemia. | Avoids ionizing radiation or systemic chemotherapy exposure; inexpensive. | PES, liver failure, abscess formation, and biloma [39]. |
| TACE | Micro-embolic particles infused with chemotherapy causing a combination of tissue ischemia and chemotoxicity. | Higher radiologic response than TAE; well studied; first-line treatment for intermediate-stage HCC. | PES, liver failure, abscess formation, biloma, and systemic chemotherapy exposure [3]. |
| TARE | Yttrium-90 beta-degradation causing focal cell death with ionizing radiation. | May be used early in disease with curative intent; higher quality of life compared with other transarterial therapies. | Radiation pneumonitis, fibrotic lung disease, RILD, liver failure, and abscess formation [63]. |
2.2. Microwave Ablation
2.2.1. Overview
2.2.2. Indications and Complications
2.2.3. Outcomes
2.3. Cryoablation
2.3.1. Overview
2.3.2. Indications and Complications
2.3.3. Outcomes
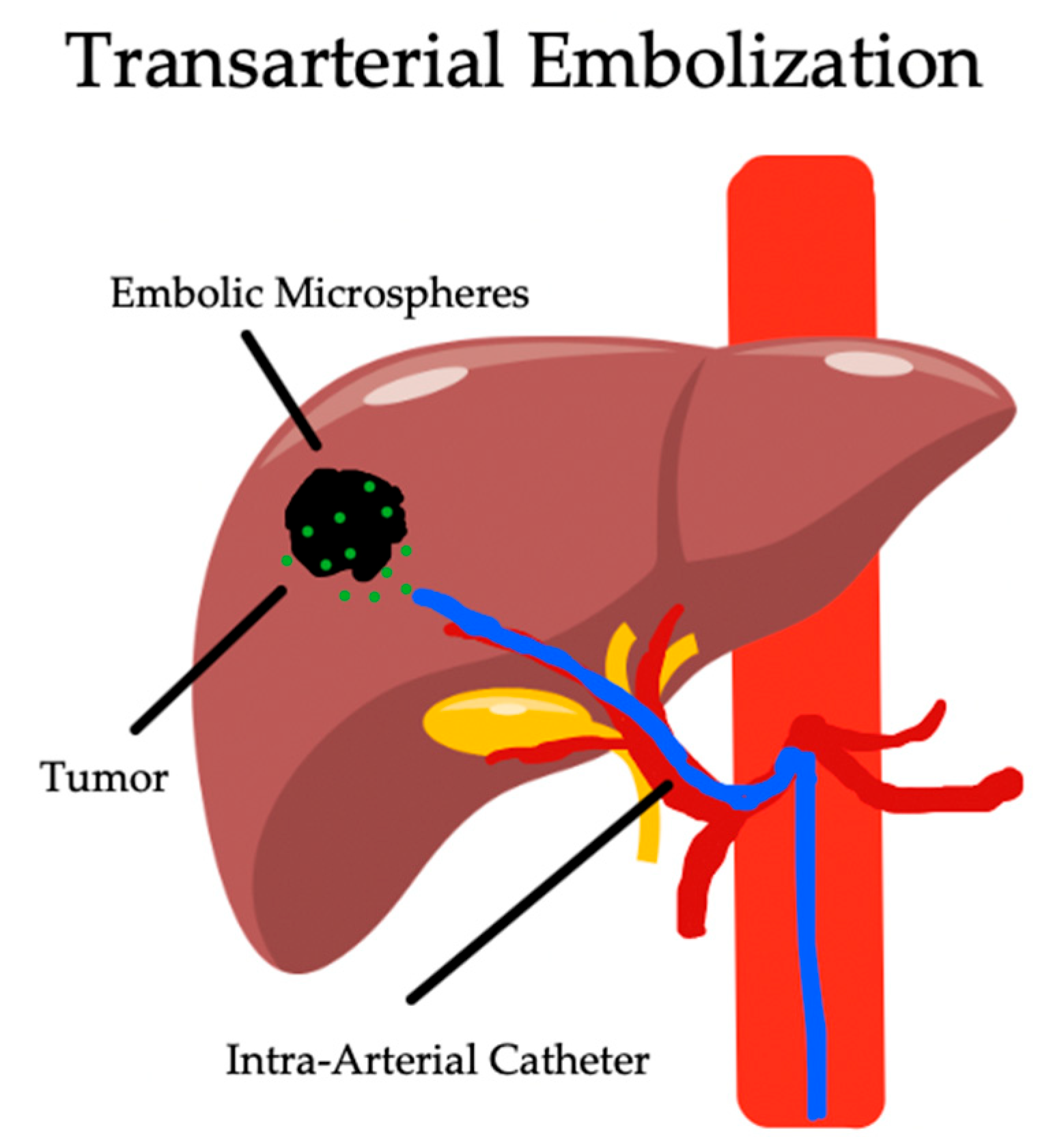
3. Transarterial Embolization
3.1. Overview
3.2. Indications and Complications
3.3. Outcomes
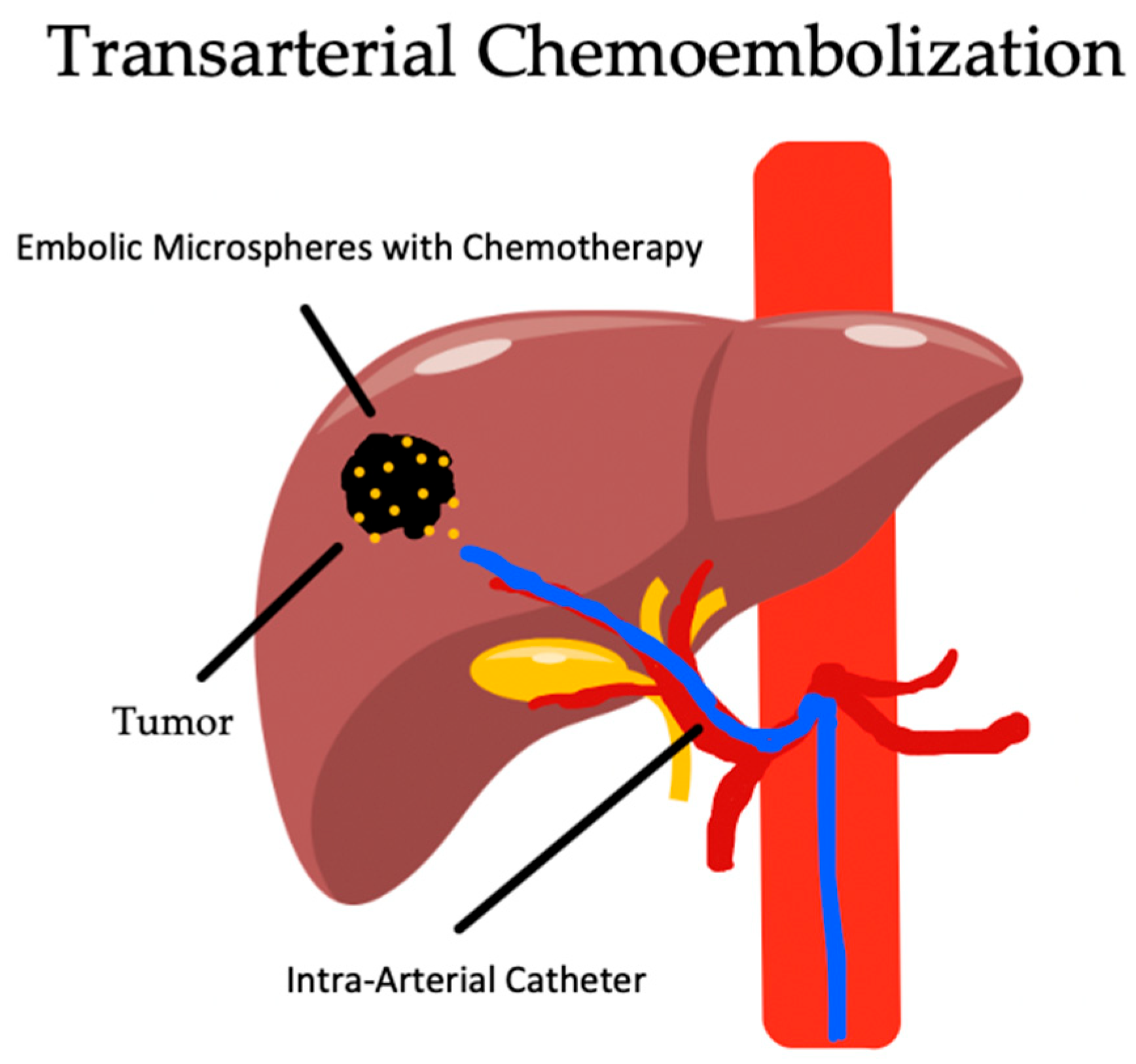
4. Transarterial Chemoembolization
4.1. Overview
4.2. Indications and Complications
4.3. Outcomes
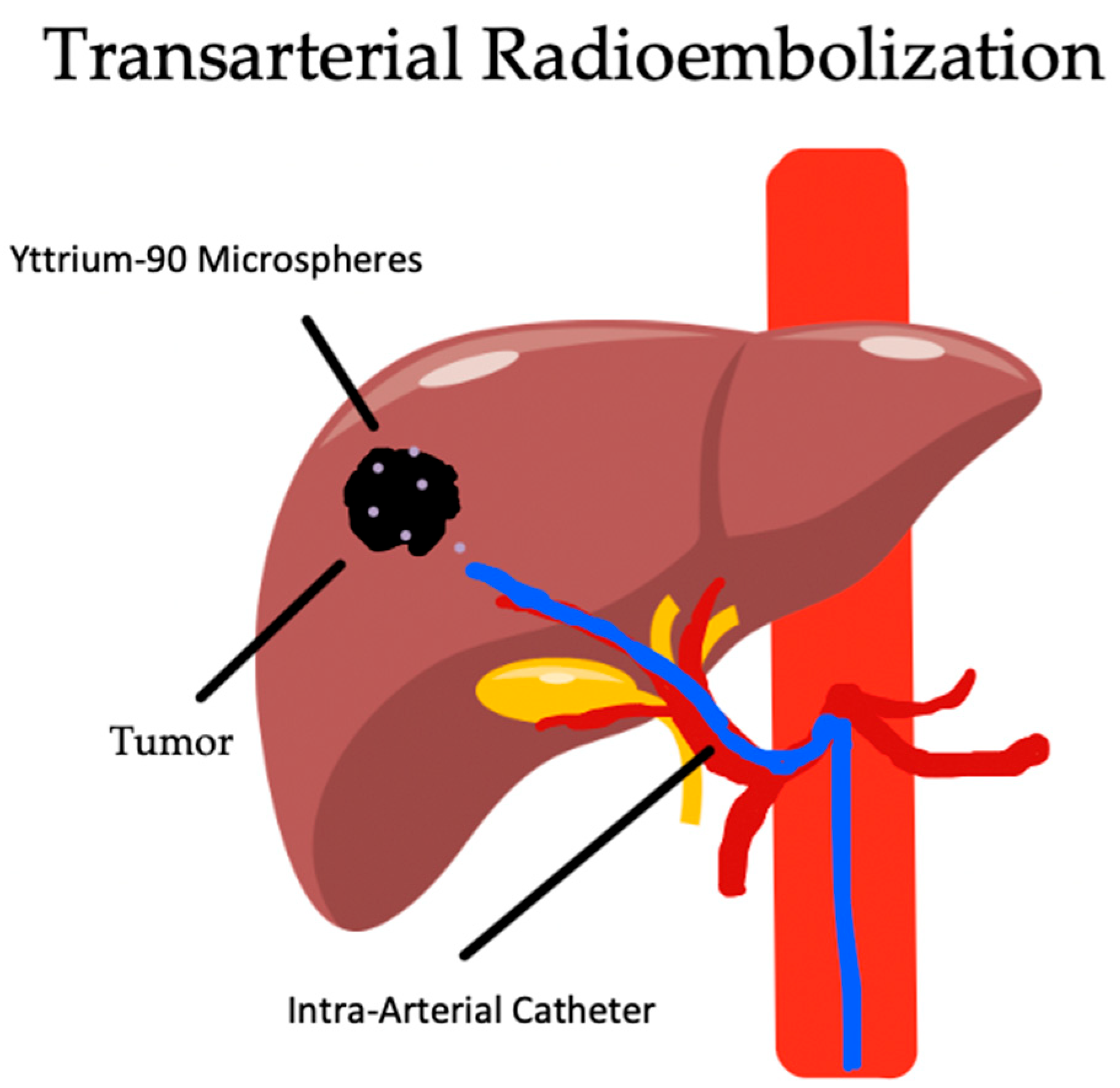
5. Transarterial Radioembolization
5.1. Overview
5.2. Indications and Complications
5.3. Outcomes
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Fite, E.L.; Makary, M.S. Transarterial Chemoembolization Treatment Paradigms for Hepatocellular Carcinoma. Cancers 2024, 16, 2430. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL–EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Wieckowska, A.; Lopez, A.R.; Liu, Y.C.; Zein, N.N.; McCullough, A.J. Cytokeratin-18 Fragment Levels as Noninvasive Biomarkers for Nonalcoholic Steatohepatitis: A Multicenter Validation Study. Hepatology 2009, 50, 1072–1078. [Google Scholar] [CrossRef]
- Ioannou, G.N. Epidemiology and Risk-Stratification of NAFLD-Associated HCC. J. Hepatol. 2021, 75, 1476–1484. [Google Scholar] [CrossRef]
- Castellana, M.; Donghia, R.; Lampignano, L.; Castellana, F.; Zupo, R.; Sardone, R.; De Pergola, G.; Giannelli, G. Prevalence of the Absence of Cirrhosis in Subjects with NAFLD-Associated Hepatocellular Carcinoma. J. Clin. Med. 2021, 10, 4638. [Google Scholar] [CrossRef]
- Behari, J.; Gougol, A.; Wang, R.; Luu, H.N.; Paragomi, P.; Yu, Y.-C.; Molinari, M.; Chopra, K.; Malik, S.M.; Geller, D.; et al. Incidence of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease without Cirrhosis or Advanced Liver Fibrosis. Hepatol. Commun. 2023, 7, e00183. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the Epidemic of Nonalcoholic Fatty Liver Disease Demonstrates an Exponential Increase in Burden of Disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current Concepts and Future Challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Allemann, P.; Demartines, N.; Bouzourene, H.; Tempia, A.; Halkic, N. Long-Term Outcome after Liver Resection for Hepatocellular Carcinoma Larger than 10 cm. World J. Surg. 2013, 37, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The Role of Hepatic Resection in the Treatment of Hepatocellular Cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zane, K.E.; Nagib, P.B.; Jalil, S.; Mumtaz, K.; Makary, M.S. Emerging Curative-Intent Minimally-Invasive Therapies for Hepatocellular Carcinoma. World J. Hepatol. 2022, 14, 885–895. [Google Scholar] [CrossRef]
- Wong, R.; Frenette, C. Updates in the Management of Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2011, 7, 16–24. [Google Scholar]
- Doyle, M.M.B.; Vachharajani, N.; Maynard, E.; Shenoy, S.; Anderson, C.; Wellen, J.R.; Lowell, J.A.; Chapman, W.C. Liver Transplantation for Hepatocellular Carcinoma: Long-Term Results Suggest Excellent Outcomes. J. Am. Coll. Surg. 2012, 215, 19–28. [Google Scholar] [CrossRef]
- Figueras, J.; Jaurrieta, E.; Valls, C.; Benasco, C.; Rafecas, A.; Xiol, X.; Fabregat, J.; Casanovas, T.; Torras, J.; Baliellas, C.; et al. Survival after Liver Transplantation in Cirrhotic Patients with and without Hepatocellular Carcinoma: A Comparative Study. Hepatology 1997, 25, 1485–1489. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of Nonalcoholic Fatty Liver Disease (NAFLD) with Hepatocellular Carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Elshaarawy, O.; Han, G.; Chong, C.C.N.; Serra, C.; O’Rourke, J.M.; Crew, R.; Felicani, C.; Ercolani, G.; Shah, T.; et al. ‘Potentially Curative Therapies’ for Hepatocellular Carcinoma: How Many Patients Can Actually Be Cured? Br. J. Cancer 2023, 128, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Glass, L.M.; Hunt, C.M.; Fuchs, M.; Su, G.L. Comorbidities and Nonalcoholic Fatty Liver Disease: The Chicken, the Egg, or Both? Fed. Pract. 2019, 36, 64–71. [Google Scholar]
- Manikat, R.; Nguyen, M.H. Nonalcoholic Fatty Liver Disease and Non-Liver Co-Morbidities. Clin. Mol. Hepatol. 2023, 29, S86–S102. [Google Scholar] [CrossRef] [PubMed]
- Bhayani, N.H.; Hyder, O.; Frederick, W.; Schulick, R.D.; Wolgang, C.L.; Hirose, K.; Edil, B.; Herman, J.M.; Choti, M.A.; Pawlik, T.M. Effect of Metabolic Syndrome on Perioperative Outcomes after Liver Surgery: A National Surgical Quality Improvement Program (NSQIP) Analysis. Surgery 2012, 152, 218–226. [Google Scholar] [CrossRef]
- Chin, K.M.; Prieto, M.; Cheong, C.K.; Di Martino, M.; Ielpo, B.; Goh, B.K.P.; Koh, Y.X. Outcomes after Curative Therapy for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis and Review of Current Literature. HPB 2021, 23, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Pinto, E.; Meneghel, P.; Farinati, F.; Russo, F.P.; Pelizzaro, F.; Gambato, M. Efficacy of Immunotherapy in Hepatocellular Carcinoma: Does Liver Disease Etiology Have a Role? Dig. Liver Dis. 2024, 56, 579–588. [Google Scholar] [CrossRef]
- Kulik, L.M.; Atassi, B.; van Holsbeeck, L.; Souman, T.; Lewandowski, R.J.; Mulcahy, M.F.; Hunter, R.D.; Nemcek, A.A., Jr.; Abecassis, M.M.; Haines, K.G., III; et al. Yttrium-90 Microspheres (TheraSphere®) Treatment of Unresectable Hepatocellular Carcinoma: Downstaging to Resection, RFA and Bridge to Transplantation. J. Surg. Oncol. 2006, 94, 572–586. [Google Scholar] [CrossRef]
- Campbell, W.A.; Makary, M.S. Advances in Image-Guided Ablation Therapies for Solid Tumors. Cancers 2024, 16, 2560. [Google Scholar] [CrossRef]
- Ahmed, M.; Brace, C.L.; Lee, F.T.; Goldberg, S.N. Principles of and Advances in Percutaneous Ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Sainani, N.I.; Gervais, D.A.; Mueller, P.R.; Arellano, R.S. Imaging After Percutaneous Radiofrequency Ablation of Hepatic Tumors: Part 1, Normal Findings. Am. J. Roentgenol. 2013, 200, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L. Microwave Tissue Ablation: Biophysics, Technology and Applications. Crit. Rev. Biomed. Eng. 2010, 38, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yan, K.; Wu, G.X.; Wu, W.; Fu, Y.; Lee, J.C.; Zhang, Z.Y.; Wang, S.; Chen, M.H. Radiofrequency Ablation of Hepatocellular Carcinoma in Difficult Locations: Strategies and Long-Term Outcomes. World J. Gastroenterol. 2015, 21, 1554–1566. [Google Scholar] [CrossRef]
- Chen, M.-S.; Li, J.-Q.; Zheng, Y.; Guo, R.-P.; Liang, H.-H.; Zhang, Y.-Q.; Lin, X.-J.; Lau, W.Y. A Prospective Randomized Trial Comparing Percutaneous Local Ablative Therapy and Partial Hepatectomy for Small Hepatocellular Carcinoma. Ann. Surg. 2006, 243, 321–328. [Google Scholar] [CrossRef]
- Vouche, M.; Habib, A.; Ward, T.J.; Kim, E.; Kulik, L.; Ganger, D.; Mulcahy, M.; Baker, T.; Abecassis, M.; Sato, K.T.; et al. Unresectable Solitary Hepatocellular Carcinoma Not Amenable to Radiofrequency Ablation: Multicenter Radiology-Pathology Correlation and Survival of Radiation Segmentectomy. Hepatology 2014, 60, 192–201. [Google Scholar] [CrossRef]
- Wong, C.R.; Njei, B.; Nguyen, M.H.; Nguyen, A.; Lim, J.K. Survival after Treatment with Curative Intent for Hepatocellular Carcinoma among Patients with vs. without Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2017, 46, 1061–1069. [Google Scholar] [CrossRef]
- Kishore, S.; Friedman, T.; Madoff, D.C. Update on Embolization Therapies for Hepatocellular Carcinoma. Curr. Oncol. Rep. 2017, 19, 40. [Google Scholar] [CrossRef]
- Agrawal, R.; Majeed, M.; Aqeel, S.B.; Wang, Y.; Haque, Z.; Omar, Y.A.; Upadhyay, S.B.; Gast, T.; Attar, B.M.; Gandhi, S. Identifying Predictors and Evaluating the Role of Steroids in the Prevention of Post-Embolization Syndrome after Transarterial Chemoembolization and Bland Embolization. Ann. Gastroenterol. 2021, 34, 241–246. [Google Scholar] [CrossRef]
- Lanza, E.; Muglia, R.; Bolengo, I.; Poretti, D.; D’Antuono, F.; Ceriani, R.; Torzilli, G.; Pedicini, V. Survival Analysis of 230 Patients with Unresectable Hepatocellular Carcinoma Treated with Bland Transarterial Embolization. PLoS ONE 2020, 15, e0227711. [Google Scholar] [CrossRef]
- Meyer, T.; Kirkwood, A.; Roughton, M.; Beare, S.; Tsochatzis, E.; Yu, D.; Davies, N.; Williams, E.; Pereira, S.P.; Hochhauser, D.; et al. A Randomised Phase II/III Trial of 3-Weekly Cisplatin-Based Sequential Transarterial Chemoembolisation vs. Embolisation Alone for Hepatocellular Carcinoma. Br. J. Cancer 2013, 108, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Hernandez-Gea, V.; Llovet, J.M. Medical Therapies for Hepatocellular Carcinoma: A Critical View of the Evidence. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Burrel, M.; Reig, M.; Forner, A.; Barrufet, M.; de Lope, C.R.; Tremosini, S.; Ayuso, C.; Llovet, J.M.; Real, M.I.; Bruix, J. Survival of Patients with Hepatocellular Carcinoma Treated by Transarterial Chemoembolisation (TACE) Using Drug Eluting Beads. Implications for Clinical Practice and Trial Design. J. Hepatol. 2012, 56, 1330–1335. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Sanghvi, T.; Rubin, N.; Hall, D.; Roller, L.; Charaf, Y.; Golzarian, J. Transarterial Chemoembolization of Hepatocellular Carcinoma: Propensity Score Matching Study Comparing Survival and Complications in Patients with Nonalcoholic Steatohepatitis Versus Other Causes Cirrhosis. Cardiovasc Intervent Radiol 2020, 43, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Gabr, A.; Abouchaleh, N.; Ali, R.; Baker, T.; Caicedo, J.; Katariya, N.; Abecassis, M.; Riaz, A.; Lewandowski, R.J.; Salem, R. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 1502–1510.e1. [Google Scholar] [CrossRef]
- Salem, R.; Mazzaferro, V.; Sangro, B. Yttrium 90 Radioembolization for the Treatment of Hepatocellular Carcinoma: Biological Lessons, Current Challenges, and Clinical Perspectives. Hepatology 2013, 58, 2188–2197. [Google Scholar] [CrossRef]
- Gordon, C.; Ciani, O.; Sommariva, S.; Facciorusso, A.; Tarricone, R.; Bhoori, S.; Mazzaferro, V. Trans-Arterial Radioembolization in Intermediate-Advanced Hepatocellular Carcinoma: Systematic Review and Meta-Analyses. Oncotarget 2016, 7, 72343–72355. [Google Scholar]
- Gordon, A.; Lewandowski, R.; Hickey, R.; Kallini, J.; Gabr, A.; Sato, K.; Desai, K.; Thornburg, B.; Gates, V.; Ganger, D.; et al. Prospective Randomized Phase 2 Study of Chemoembolization versus Radioembolization in Hepatocellular Carcinoma: Results from the PREMIERE Trial. J. Vasc. Interv. Radiol. 2016, 27, S61–S62. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, S.U. Predictors of Complete Response in Patients with Hepatocellular Carcinoma Treated with Trans-Arterial Radioembolization. Curr. Oncol. 2021, 28, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Brunson, C.; Struycken, L.; Schaub, D.; Ref, J.; Goldberg, D.; Hannallah, J.; Woodhead, G.; Young, S. Comparative Outcomes of Trans-Arterial Radioembolization in Patients with Non-Alcoholic Steatohepatitis/Non-Alcoholic Fatty Liver Disease-Induced HCC: A Retrospective Analysis. Abdom. Radiol. 2024, 49, 2714–2725. [Google Scholar] [CrossRef] [PubMed]
- Schotten, C.; Bechmann, L.P.; Manka, P.; Theysohn, J.; Dechêne, A.; El Fouly, A.; Barbato, F.; Neumann, U.; Radünz, S.; Sydor, S.; et al. NAFLD-Associated Comorbidities in Advanced Stage HCC Do Not Alter the Safety and Efficacy of Yttrium-90 Radioembolization. Liver Cancer 2019, 8, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Zane, K.E.; Makary, M.S. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers 2021, 13, 5430. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, H.; Zhou, L.; Chen, X.; Zheng, S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 8619096. [Google Scholar] [CrossRef]
- Sheta, E.; El-Kalla, F.; El-Gharib, M.; Kobtan, A.; Elhendawy, M.; Abd-Elsalam, S.; Mansour, L.; Amer, I. Comparison of Single-Session Transarterial Chemoembolization Combined with Microwave Ablation or Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma: A Randomized-Controlled Study. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1198–1203. [Google Scholar] [CrossRef]
- De Muzio, F.; Cutolo, C.; Dell’Aversana, F.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef]
- Li, J.K.; Liu, X.H.; Cui, H.; Xie, X.H. Radiofrequency Ablation vs. Surgical Resection for Resectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Mol. Clin. Oncol. 2020, 12, 15–22. [Google Scholar] [CrossRef]
- Donadon, V.; Balbi, M.; Mas, M.D.; Casarin, P.; Zanette, G. Metformin and Reduced Risk of Hepatocellular Carcinoma in Diabetic Patients with Chronic Liver Disease. Liver Int. 2010, 30, 750–758. [Google Scholar] [CrossRef]
- Chen, T.-M.; Lin, C.-C.; Huang, P.-T.; Wen, C.-F. Metformin Associated with Lower Mortality in Diabetic Patients with Early Stage Hepatocellular Carcinoma after Radiofrequency Ablation. J. Gastroenterol. Hepatol. 2011, 26, 858–865. [Google Scholar] [CrossRef]
- Ohki, T.; Tateishi, R.; Shiina, S.; Sato, T.; Masuzaki, R.; Yoshida, H.; Kanai, F.; Obi, S.; Yoshida, H.; Omata, M. Obesity Did Not Diminish the Efficacy of Percutaneous Ablation for Hepatocellular Carcinoma. Liver Int. 2007, 27, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.R.; Makary, M.S. Recent Advances in Image-Guided Locoregional Therapies for Primary Liver Tumors. Biology 2023, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional Treatments for Hepatocellular Carcinoma: Current Evidence and Future Directions. World J. Gastroenterol. 2019, 25, 4614–4628. [Google Scholar] [CrossRef]
- Strickland, A.D.; Clegg, P.J.; Cronin, N.J.; Swift, B.; Festing, M.; West, K.P.; Robertson, G.S.M.; Lloyd, D.M. Experimental Study of Large-Volume Microwave Ablation in the Liver. Br. J. Surg. 2002, 89, 1003–1007. [Google Scholar] [CrossRef]
- Mansur, A.; Garg, T.; Shrigiriwar, A.; Etezadi, V.; Georgiades, C.; Habibollahi, P.; Huber, T.C.; Camacho, J.C.; Nour, S.G.; Sag, A.A.; et al. Image-Guided Percutaneous Ablation for Primary and Metastatic Tumors. Diagnostics 2022, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Poulou, L.S.; Botsa, E.; Thanou, I.; Ziakas, P.D.; Thanos, L. Percutaneous Microwave Ablation vs Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma. World J. Hepatol. 2015, 7, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Ramsell, S.; Miller, E.; Beal, E.W.; Dowell, J.D. Hepatocellular Carcinoma Locoregional Therapies: Outcomes and Future Horizons. World J. Gastroenterol. 2021, 27, 7462–7479. [Google Scholar] [CrossRef]
- Radosevic, A.; Quesada, R.; Serlavos, C.; Sánchez, J.; Zugazaga, A.; Sierra, A.; Coll, S.; Busto, M.; Aguilar, G.; Flores, D.; et al. Microwave versus Radiofrequency Ablation for the Treatment of Liver Malignancies: A Randomized Controlled Phase 2 Trial. Sci. Rep. 2022, 12, 316. [Google Scholar] [CrossRef]
- Bajestani, N.; Wu, G.; Hussein, A.; Makary, M.S. Examining the Efficacy and Safety of Combined Locoregional Therapy and Immunotherapy in Treating Hepatocellular Carcinoma. Biomedicines 2024, 12, 1432. [Google Scholar] [CrossRef]
- Vietti Violi, N.; Duran, R.; Guiu, B.; Cercueil, J.-P.; Aubé, C.; Digklia, A.; Pache, I.; Deltenre, P.; Knebel, J.-F.; Denys, A. Efficacy of Microwave Ablation versus Radiofrequency Ablation for the Treatment of Hepatocellular Carcinoma in Patients with Chronic Liver Disease: A Randomised Controlled Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2018, 3, 317–325. [Google Scholar] [CrossRef]
- Erinjeri, J.P.; Clark, T.W.I. Cryoablation: Mechanism of Action and Devices. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. S8), S187–S191. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Lu, Y.; Bai, W.; An, L.; Qu, J.; Gao, X.; Chen, Y.; Zhou, L.; Wu, Y.; et al. Outcomes of Ultrasound-Guided Percutaneous Argon-Helium Cryoablation of Hepatocellular Carcinoma. J. Hepatobiliary Pancreat. Sci. 2012, 19, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter Randomized Controlled Trial of Percutaneous Cryoablation versus Radiofrequency Ablation in Hepatocellular Carcinoma. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef]
- Glazer, D.I.; Tatli, S.; Shyn, P.B.; Vangel, M.G.; Tuncali, K.; Silverman, S.G. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience With Intermediate to Long-Term Outcomes. Am. J. Roentgenol. 2017, 209, 1381–1389. [Google Scholar] [CrossRef]
- Yovwg, G. The Blood Supply of Neoplasms in the Liver. Am. J. Pathol. 1954, 30, 969–985. [Google Scholar]
- Shah, R.P.; Brown, K.T.; Sofocleous, C.T. Arterially Directed Therapies for Hepatocellular Carcinoma. Am. J. Roentgenol. 2011, 197, W590–W602. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Fatourou, E.; O’Beirne, J.; Meyer, T.; Burroughs, A.K. Transarterial Chemoembolization and Bland Embolization for Hepatocellular Carcinoma. World J. Gastroenterol. 2014, 20, 3069–3077. [Google Scholar] [CrossRef]
- Gaba, R.C.; Lokken, R.P.; Hickey, R.M.; Lipnik, A.J.; Lewandowski, R.J.; Salem, R.; Brown, D.B.; Walker, T.G.; Silberzweig, J.E.; Baerlocher, M.O.; et al. Quality Improvement Guidelines for Transarterial Chemoembolization and Embolization of Hepatic Malignancy. J. Vasc. Interv. Radiol. 2017, 28, 1210–1223.e3. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; Schacht, M.A.; Beckley, E.W.; LaRoche, T.P.; O’Neil, B.H.; Pyko, M. Locoregional and Systemic Therapy for Hepatocellular Carcinoma. J. Gastrointest. Oncol. 2017, 8, 215–228. [Google Scholar] [CrossRef]
- Hodavance, M.S.; Vikingstad, E.M.; Griffin, A.S.; Pabon-Ramos, W.M.; Berg, C.L.; Suhocki, P.V.; Kim, C.Y. Effectiveness of Transarterial Embolization of Hepatocellular Carcinoma as a Bridge to Transplantation. J. Vasc. Interv. Radiol. 2016, 27, 39–45. [Google Scholar] [CrossRef]
- Raoul, J.-L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving Strategies for the Management of Intermediate-Stage Hepatocellular Carcinoma: Available Evidence and Expert Opinion on the Use of Transarterial Chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Berti, S.; Bartorelli, A.L.; Koni, E.; Giordano, A.; Petronio, A.S.; Iadanza, A.; Bedogni, F.; Reimers, B.; Spaccarotella, C.; Trani, C.; et al. Impact of High Body Mass Index on Vascular and Bleeding Complications after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 155, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, G.G.; Gates, J.; Stuart, K.; Underhill, J.; Brophy, D.P. Hepatic Chemoembolization: Effect of Intraarterial Lidocaine on Pain and Postprocedure Recovery. Cardiovasc. Intervent Radiol. 1999, 22, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Sullivan, K.L.; McCann, J.W.; Gonsalves, C.F.; Sato, T.; Eschelman, D.J.; Brown, D.B. Moxifloxacin Prophylaxis for Chemoembolization or Embolization in Patients With Previous Biliary Interventions: A Pilot Study. Am. J. Roentgenol. 2011, 197, W343–W345. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial Embolisation or Chemoembolisation versus Symptomatic Treatment in Patients with Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Roth, G.S.; Benhamou, M.; Teyssier, Y.; Seigneurin, A.; Abousalihac, M.; Sengel, C.; Seror, O.; Ghelfi, J.; Ganne-Carrié, N.; Blaise, L.; et al. Comparison of Trans-Arterial Chemoembolization and Bland Embolization for the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers 2021, 13, 812. [Google Scholar] [CrossRef]
- Malagari, K.; Pomoni, M.; Kelekis, A.; Pomoni, A.; Dourakis, S.; Spyridopoulos, T.; Moschouris, H.; Emmanouil, E.; Rizos, S.; Kelekis, D. Prospective Randomized Comparison of Chemoembolization with Doxorubicin-Eluting Beads and Bland Embolization with BeadBlock for Hepatocellular Carcinoma. Cardiovasc. Intervent Radiol. 2010, 33, 541–551. [Google Scholar] [CrossRef]
- Brown, K.T.; Do, R.K.; Gonen, M.; Covey, A.M.; Getrajdman, G.I.; Sofocleous, C.T.; Jarnagin, W.R.; D’Angelica, M.I.; Allen, P.J.; Erinjeri, J.P.; et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared with Embolization with Microspheres Alone. J. Clin. Oncol. 2016, 34, 2046–2053. [Google Scholar] [CrossRef]
- Kluger, M.D.; Halazun, K.J.; Barroso, R.T.; Fox, A.N.; Olsen, S.K.; Madoff, D.C.; Siegel, A.B.; Weintraub, J.L.; Sussman, J.; Brown, R.S., Jr.; et al. Bland Embolization versus Chemoembolization of Hepatocellular Carcinoma before Transplantation. Liver Transplant. 2014, 20, 536–543. [Google Scholar] [CrossRef]
- Chang, J.-M.; Tzeng, W.-S.; Pan, H.-B.; Yang, C.-F.; Lai, K.-H. Transcatheter Arterial Embolization with or without Cisplatin Treatment of Hepatocellular Carcinoma. A Randomized Controlled Study. Cancer 1994, 74, 2449–2453. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Fatourou, E.M.; Triantos, C.K.; Burroughs, A.K. Transarterial Therapies for Hepatocellular Carcinoma. In Multidisciplinary Treatment of Hepatocellular Carcinoma; Vauthey, J.-N., Brouquet, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–206. [Google Scholar] [CrossRef]
- Marelli, L.; Stigliano, R.; Triantos, C.; Senzolo, M.; Cholongitas, E.; Davies, N.; Tibballs, J.; Meyer, T.; Patch, D.W.; Burroughs, A.K. Transarterial Therapy for Hepatocellular Carcinoma: Which Technique Is More Effective? A Systematic Review of Cohort and Randomized Studies. Cardiovasc. Intervent Radiol. 2007, 30, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Konno, T. Targeting Cancer Chemotherapeutic Agents by Use of Lipiodol Contrast Medium. Cancer 1990, 66, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and Biological Properties of the Vascular Endothelial Growth Factor Family of Proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc. Intervent Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of Hepatocellular Carcinoma with Drug Eluting Beads: Efficacy and Doxorubicin Pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef]
- Jipa, A.M.; Makary, M.S. Locoregional Therapies for Hepatobiliary Tumors: Contemporary Strategies and Novel Applications. Cancers 2024, 16, 1271. [Google Scholar] [CrossRef] [PubMed]
- May, B.J.; Madoff, D.C. Portal Vein Embolization: Rationale, Technique, and Current Application. Semin. Interv. Radiol. 2012, 29, 81–89. [Google Scholar] [CrossRef]
- Tjeertes, E.E.; Hoeks, S.S.; Beks, S.S.; Valentijn, T.T.; Hoofwijk, A.A.; Stolker, R.J.R. Obesity—A Risk Factor for Postoperative Complications in General Surgery? BMC Anesthesiol. 2015, 15, 112. [Google Scholar] [CrossRef]
- Luo, J.; Guo, R.-P.; Lai, E.C.H.; Zhang, Y.-J.; Lau, W.Y.; Chen, M.-S.; Shi, M. Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: A Prospective Comparative Study. Ann. Surg. Oncol. 2011, 18, 413–420. [Google Scholar] [CrossRef]
- Clark, T.W.I. Complications of Hepatic Chemoembolization. Semin. Interv. Radiol. 2006, 23, 119–125. [Google Scholar] [CrossRef]
- Lo, C.-M.; Ngan, H.; Tso, W.-K.; Liu, C.-L.; Lam, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Wong, J. Randomized Controlled Trial of Transarterial Lipiodol Chemoembolization for Unresectable Hepatocellular Carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Systematic Review of Randomized Trials for Unresectable Hepatocellular Carcinoma: Chemoembolization Improves Survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.E.; Charles, H.W.; Park, J.S.; Goldenberg, A.S.; Deipolyi, A.R. Obesity Conveys Poor Outcome in Patients with Hepatocellular Carcinoma Treated by Transarterial Chemoembolization. Diagn. Interv. Imaging 2017, 98, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised Controlled Trial of Doxorubicin-Eluting Beads vs Conventional Chemoembolisation for Hepatocellular Carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mariani, L.; Sposito, C.; Spreafico, C.; Bongini, M.; Morosi, C.; Cascella, T.; Marchianò, A.; Camerini, T.; Bhoori, S.; et al. Drug-Eluting Beads versus Conventional Chemoembolization for the Treatment of Unresectable Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 645–653. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Sato, K.T.; Atassi, B.; Ryu, R.K.; Ibrahim, S.; Nemcek, A.A., Jr.; Omary, R.A.; Madoff, D.C.; Murthy, R. Technical Aspects of Radioembolization with 90Y Microspheres. Tech. Vasc. Interv. Radiol. 2007, 10, 12–29. [Google Scholar] [CrossRef]
- Mosconi, C.; Cappelli, A.; Pettinato, C.; Golfieri, R. Radioembolization with Yttrium-90 Microspheres in Hepatocellular Carcinoma: Role and Perspectives. World J. Hepatol. 2015, 7, 738–752. [Google Scholar] [CrossRef]
- Makary, M.S.; Krishner, L.S.; Wuthrick, E.J.; Dowell, J.D.; Bloomston, M.P. Yttrium-90 Microsphere Selective Internal Radiation Therapy for Liver Metastases Following Systemic Chemotherapy and Surgical Resection for Metastatic Adrenocortical Carcinoma. World J. Clin. Oncol. 2018, 9, 20–25. [Google Scholar] [CrossRef]
- Moir, J.A.G.; Burns, J.; Barnes, J.; Colgan, F.; White, S.A.; Littler, P.; Manas, D.M.; French, J.J. Selective Internal Radiation Therapy for Liver Malignancies. Br. J. Surg. 2015, 102, 1533–1540. [Google Scholar] [CrossRef]
- Kokabi, N.; Camacho, J.C.; Xing, M.; El-Rayes, B.F.; Spivey, J.R.; Knechtle, S.J.; Kim, H.S. Open-Label Prospective Study of the Safety and Efficacy of Glass-Based Yttrium 90 Radioembolization for Infiltrative Hepatocellular Carcinoma with Portal Vein Thrombosis. Cancer 2015, 121, 2164–2174. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for Hepatocellular Carcinoma Using Yttrium-90 Microspheres: A Comprehensive Report of Long-Term Outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gilbertsen, M.; Butt, Z.; Memon, K.; Vouche, M.; Hickey, R.; Baker, T.; Abecassis, M.M.; Atassi, R.; Riaz, A.; et al. Increased Quality of Life Among Hepatocellular Carcinoma Patients Treated with Radioembolization, Compared with Chemoembolization. Clin. Gastroenterol. Hepatol. 2013, 11, 1358–1365.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Radiation-Induced Liver Disease: Current Understanding and Future Perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional Decision to Adopt Y90 as Primary Treatment for Hepatocellular Carcinoma Informed by a 1000-patient 15-year Experience. Hepatology 2018, 68, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Toom, S.; Avula, A.; Kumar, V.; Rahma, O.E. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2020, 7, 11–17. [Google Scholar] [CrossRef]
- Lobo, L.; Yakoub, D.; Picado, O.; Ripat, C.; Pendola, F.; Sharma, R.; ElTawil, R.; Kwon, D.; Venkat, S.; Portelance, L.; et al. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2016, 39, 1580–1588. [Google Scholar] [CrossRef]
- Kolligs, F.T.; Bilbao, J.I.; Jakobs, T.; Iñarrairaegui, M.; Nagel, J.M.; Rodriguez, M.; Haug, A.; D’Avola, D.; op den Winkel, M.; Martinez-Cuesta, A.; et al. Pilot Randomized Trial of Selective Internal Radiation Therapy vs. Chemoembolization in Unresectable Hepatocellular Carcinoma. Liver Int. 2015, 35, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- De Toni, E.N. Immune Checkpoint Inhibitors: Use Them Early, Combined and Instead of TACE? Gut 2020, 69, 1887. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Lencioni, R.; Kudo, M.; Erinjeri, J.; Qin, S.; Ren, Z.; Chan, S.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. EMERALD-1: A Phase 3, Randomized, Placebo-Controlled Study of Transarterial Chemoembolization Combined with Durvalumab with or without Bevacizumab in Participants with Unresectable Hepatocellular Carcinoma Eligible for Embolization. J. Clin. Oncol. 2024, 42, LBA432. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Müller, L.; Galle, P.R. NAFLD-Driven HCC: Safety and Efficacy of Current and Emerging Treatment Options. J. Hepatol. 2022, 76, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Yoneda, M.; Kobayashi, T.; Asako, N.; Iwaki, M.; Saito, S.; Nakajima, A. Pan-Peroxisome Proliferator-Activated Receptor Agonist Lanifibranor as a Dominant Candidate Pharmacological Therapy for Nonalcoholic Fatty Liver Disease. Hepatobiliary Surg. Nutr. 2022, 11, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Dendy, M.S.; Ludwig, J.M.; Stein, S.M.; Kim, H.S. Locoregional Therapy, Immunotherapy and the Combination in Hepatocellular Carcinoma: Future Directions. Liver Cancer 2019, 8, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.; Duran, R.; Denys, A.; Bize, P.E.; Borchard, G.; Jordan, O. Drug-Eluting Embolic Microspheres for Local Drug Delivery–State of the Art. J. Control. Release 2017, 262, 127–138. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-Man Histotripsy of Hepatic Tumors: The THERESA Trial, a Feasibility Study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Créixams, X.; White, S.B.; Davis, C.; Ahmed, O.; Parikh, N.D.; Planert, M.; Thormann, M.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors. Radiology 2024, 312, e233051. [Google Scholar] [CrossRef]

| Stage | BCLC 0 | BCLC A | BCLC B | BCLC C | BCLC D |
|---|---|---|---|---|---|
| Severity | Very early stage | Early stage | Intermediate stage | Advanced stage | Terminal stage |
| Definition | Single < 2 cm, Child–Pugh A/B | Less than 3 nodules of <3 cm, Child–Pugh A/B | Multinodular, Child–Pugh A/B | Portal invasion and/or extrahepatic spread, Child–Pugh A/B | Any tumor burden if Child–Pugh C |
| Treatment | Resection; if nonsurgical candidate, ablation | Resection/OLT; if nonsurgical candidate, ablation | TACE/TARE/TAE | Systemic therapy; Possible TACE/TARE/TAE | Supportive care |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susman, S.; Santoso, B.; Makary, M.S. Locoregional Therapies for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Biomedicines 2024, 12, 2226. https://doi.org/10.3390/biomedicines12102226
Susman S, Santoso B, Makary MS. Locoregional Therapies for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Biomedicines. 2024; 12(10):2226. https://doi.org/10.3390/biomedicines12102226
Chicago/Turabian StyleSusman, Stephen, Breanna Santoso, and Mina S. Makary. 2024. "Locoregional Therapies for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease" Biomedicines 12, no. 10: 2226. https://doi.org/10.3390/biomedicines12102226






