Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer’s Disease In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. PC12 Cell Cultivation
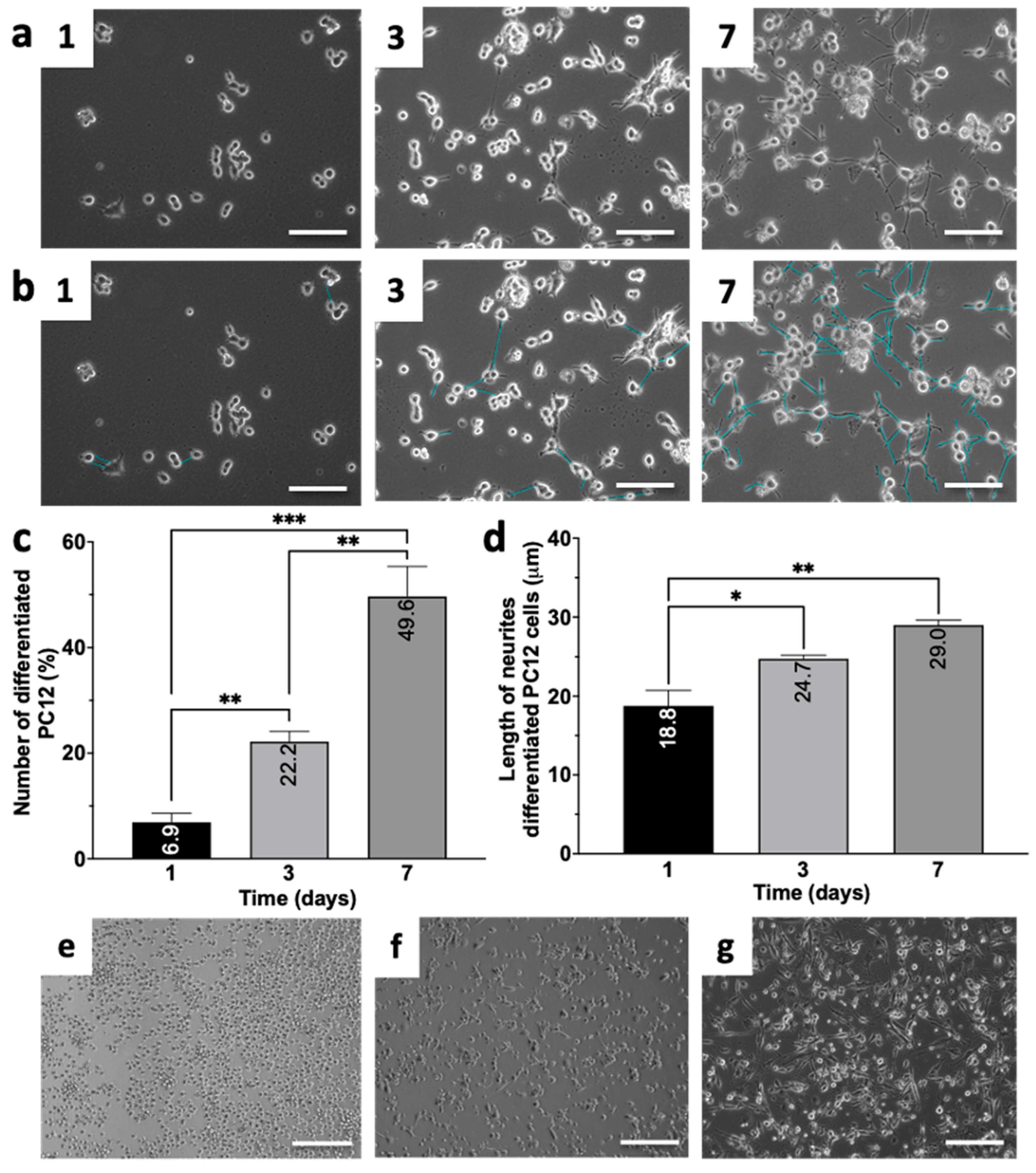
2.2. PC12 Cell Differentiation into Neuron-like Cells
2.3. Protocol of H2O2 Treatment
2.4. THP-1 Cell Cultivation and Differentiation into M0 Macrophages
2.5. MSC Expansion
2.6. MSC Differentiation
2.7. Immunophenotype Characterization of MSCs
2.8. MSCs Priming Protocols
2.9. Collection of MSC-Derived CM
2.10. Treatment of the PC12 Cell Line with CM
2.11. Evaluation of CM Neurotrophic Potential
2.12. Investigation of the Neuroprotective Properties of CM (MTT Assay)
2.13. Assessment of ROS Levels in trPC12 Cells
2.14. Effect of CM on M1/M2 Phenotypes Macrophage Differentiation
2.15. qRT‒PCR
2.16. ELISA
2.17. Statistical Analysis
3. Results
3.1. Differentiation of PC12 and THP-1 Cell Lines
3.2. Confirmation of the MSC Phenotype and Assessment of Preconditioning Effects
3.3. MSC-Derived CMs Exhibit Neurotrophic Effects
3.4. MSC-Derived CMs Exhibit Neuroprotective Properties
3.5. MSC-Derived CM Leads to Reduced LPS-Induced Polarization of M1 Macrophages
3.6. MSC-Derived CMs Induce the Polarization of M0 Macrophages toward the M2 Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alidoust, L.; Akhoondian, M.; Atefi, A.H.; Keivanlou, M.-H.; Hedayati Ch, M.; Jafari, A. Stem cell-conditioned medium is a promising treatment for Alzheimer’s disease. Behav. Brain Res. 2023, 452, 114543. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots?: Miniperspective. J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal Stem Cell Therapy and Alzheimer’s Disease: Current Status and Future Perspectives. J. Alzheimer’s Dis. 2020, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, K.; Iwahara, N.; Hisahara, S.; Emoto, M.C.; Saito, T.; Suzuki, H.; Manabe, T.; Matsumura, A.; Matsushita, T.; Suzuki, S.; et al. Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress. J. Alzheimer’s Dis. 2019, 72, 867–884. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Park, H.J.; Kim, H.N.; Oh, S.H.; Bae, J.-S.; Ha, H.-J.; Lee, P.H. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy 2014, 10, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sheng, H.; Liao, L.; Xu, C.; Zhang, A.; Yang, Y.; Zhao, L.; Duan, L.; Chen, H.; Zhang, B. Mesenchymal Stem Cell-Conditioned Medium Improves Mitochondrial Dysfunction and Suppresses Apoptosis in Okadaic Acid-Treated SH-SY5Y Cells by Extracellular Vesicle Mitochondrial Transfer. J. Alzheimer’s Dis. 2020, 78, 1161–1176. [Google Scholar] [CrossRef]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press. Res. 2016, 41, 119–128. [Google Scholar] [CrossRef]
- Wen, C.; Huang, C.; Yang, M.; Fan, C.; Li, Q.; Zhao, J.; Gan, D.; Li, A.; Zhu, L.; Lu, D. The Secretion from Bone Marrow Mesenchymal Stem Cells Pretreated with Berberine Rescues Neurons with Oxidative Damage Through Activation of the Keap1-Nrf2-HO-1 Signaling Pathway. Neurotox. Res. 2020, 38, 59–73. [Google Scholar] [CrossRef]
- Park, H.S.; Pang, Q.Q.; Kim, Y.S.; Kim, J.H.; Cho, E.J. Neuroprotective Effect of Membrane-Free Stem Cell Extract against Amyloid Beta25–35-Induced Neurotoxicity in SH-SY5Y Cells. Appl. Sci. 2021, 11, 2219. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Fraga, J.S.; Grãos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Asgari Taei, A.; Dargahi, L.; Khodabakhsh, P.; Kadivar, M.; Farahmandfar, M. Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke. CNS Neurosci. Ther. 2022, 28, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Rbia, N.; Bulstra, L.F.; Lewallen, E.A.; Hovius, S.E.R.; Van Wijnen, A.J.; Shin, A.Y. Seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells: An in vitro analysis of the gene expression and growth factors produced. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.Y.; Dheen, S.T.; Sam Wah Tay, S. Paracrine Effects of Mesenchymal Stem Cells-Conditioned Medium on Microglial Cytokines Expression and Nitric Oxide Production. Neuroimmunomodulation 2015, 22, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Furukawa-Hibi, Y.; Takeuchi, H.; Hattori, H.; Yamada, K.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 293, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Giannasi, C.; Della Morte, E.; Cadelano, F.; Valenza, A.; Casati, S.; Dei Cas, M.; Niada, S.; Brini, A.T. Boosting the therapeutic potential of cell secretome against osteoarthritis: Comparison of cytokine-based priming strategies. Biomed. Pharmacother. 2024, 170, 115970. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, A.; Zahavi, E.; Netzer, N.; Ofir, R.; Pinzur, L.; Raveh, S.; Arien-Zakay, H.; Yavin, E.; Lazarovici, P. Human PLacental eXpanded (PLX) mesenchymal-like adherent stromal cells confer neuroprotection to nerve growth factor (NGF)-differentiated PC12 cells exposed to ischemia by secretion of IL-6 and VEGF. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Bi, Y.; Gong, M.; Jiang, W.; Wei, X.; Li, T.; Chen, J. Mesenchymal stromal cell neuroprotection of hydrogen peroxide -challenged pheochromocytoma cells through reducing apoptosis and releasing cytokines. Cytotherapy 2012, 14, 954–966. [Google Scholar] [CrossRef]
- Evangelista, A.F.; Vannier-Santos, M.A.; De Assis Silva, G.S.; Silva, D.N.; Juiz, P.J.L.; Nonaka, C.K.V.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J. Neuroinflam. 2018, 15, 189. [Google Scholar] [CrossRef]
- Mead, B.; Chamling, X.; Zack, D.J.; Ahmed, Z.; Tomarev, S. TNFα-Mediated Priming of Mesenchymal Stem Cells Enhances Their Neuroprotective Effect on Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, C.; Yang, Y.; Xi, C.; Yin, Y.; Wu, H.; Qian, C. Therapeutic potential of HUC-MSC-exos primed with IFN-γ against LPS-induced acute lung injury. Iran J. Basic Med. Sci. 2023, 27, 375–382. [Google Scholar] [CrossRef]
- Tolstova, T.; Dotsenko, E.; Kozhin, P.; Novikova, S.; Zgoda, V.; Rusanov, A.; Luzgina, N. The effect of TLR3 priming conditions on MSC immunosuppressive properties. Stem Cell Res. Ther. 2023, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Mastri, M.; Shah, Z.; McLaughlin, T.; Greene, C.J.; Baum, L.; Suzuki, G.; Lee, T. Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. Am. J. Physiol.-Cell Physiol. 2012, 303, C1021–C1033. [Google Scholar] [CrossRef] [PubMed]
- Shoshina, O.O.; Kozhin, P.M.; Shadrin, V.S.; Romashin, D.D.; Rusanov, A.L.; Luzgina, N.G. Phenotypic Features of Mesenchymal Stem Cell Subpopulations Obtained under the Influence of Various Toll-Like Receptors Ligands. Bull. Exp. Biol. Med. 2021, 170, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, L.; Su, Y.; Su, L.; Lan, X.; Wu, D.; Han, S.; Li, J.; Kvederis, L.; Corey, S.; et al. Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Res. 2019, 1725, 146432. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, S.; Motevaseli, E.; Sadr, S.S.; Moradbeygi, K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR)2 and TLR4 expression in model of Alzheimer’s disease rats. Behav. Brain Res. 2020, 379, 112362. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R. Hypoxia and hyperbaric oxygen therapy: A review. Int. J. Gen. Med. 2018, 11, 431–442. [Google Scholar] [CrossRef]
- Isildar, B.; Ozkan, S.; Sahin, H.; Ercin, M.; Gezginci-Oktayoglu, S.; Koyuturk, M. Preconditioning of human umbilical cord mesenchymal stem cells with deferoxamine potentiates the capacity of the secretome released from the cells and promotes immunomodulation and beta cell regeneration in a rat model of type 1 diabetes. Int. Immunopharmacol. 2024, 129, 111662. [Google Scholar] [CrossRef]
- Nowak-Stępniowska, A.; Osuchowska, P.N.; Fiedorowicz, H.; Trafny, E.A. Insight in Hypoxia-Mimetic Agents as Potential Tools for Mesenchymal Stem Cell Priming in Regenerative Medicine. Stem Cells Int. 2022, 2022, 8775591. [Google Scholar] [CrossRef] [PubMed]
- Taheem, D.K.; Foyt, D.A.; Loaiza, S.; Ferreira, S.A.; Ilic, D.; Auner, H.W.; Grigoriadis, A.E.; Jell, G.; Gentleman, E. Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase Inhibitors. Stem Cells 2018, 36, 1380–1392. [Google Scholar] [CrossRef]
- Arfianti, A.; Ulfah, U.; Hutabarat, L.S.; Ivana, G.A.; Budiarti, A.D.; Sahara, N.S.; Saputra, N.P.K. Hipoxia modulates the secretion of growth factors in of human umbilical cord-derived mesenchymal stem cells. BioMedicine 2023, 13, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Balon, K.; Wiatrak, B. PC12 and THP-1 Cell Lines as Neuronal and Microglia Model in Neurobiological Research. Appl. Sci. 2021, 11, 3729. [Google Scholar] [CrossRef]
- Xie, D.; Deng, T.; Zhai, Z.; Sun, T.; Xu, Y. The cellular model for Alzheimer’s disease research: PC12 cells. Front. Mol. Neurosci. 2023, 15, 1016559. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A.; Bissonnette, C.J.; McGeer, P.L. Modulation of human microglia and THP-1 cell toxicity by cytokines endogenous to the nervous system. Neurobiol. Aging 2005, 26, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, A.L.; Kozhin, P.M.; Tikhonova, O.V.; Zgoda, V.G.; Loginov, D.S.; Chlastáková, A.; Selinger, M.; Sterba, J.; Grubhoffer, L.; Luzgina, N.G. Proteome Profiling of PMJ2-R and Primary Peritoneal Macrophages. Int. J. Mol. Sci. 2021, 22, 6323. [Google Scholar] [CrossRef]
- Ramalingam, M.; Jang, S.; Jeong, H.-S. Neural-Induced Human Adipose Tissue-Derived Stem Cells Conditioned Medium Ameliorates Rotenone-Induced Toxicity in SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 2322. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zafranskaya, M.; Nizheharodava, D.; Yurkevich, M.; Ivanchik, G.; Demidchik, Y.; Kozhukh, H.; Fedulov, A. PGE2 Contributes to In vitro MSC-Mediated Inhibition of Non-Specific and Antigen-Specific T Cell Proliferation in MS Patients. Scand. J. Immunol. 2013, 78, 455–462. [Google Scholar] [CrossRef]
- Kota, D.J.; Wiggins, L.L.; Yoon, N.; Lee, R.H. TSG-6 Produced by hMSCs Delays the Onset of Autoimmune Diabetes by Suppressing Th1 Development and Enhancing Tolerogenicity. Diabetes 2013, 62, 2048–2058. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, J.; Mao, R.; Chao, K.; Chen, B.; He, Y.; Zeng, Z.; Zhang, S.; Chen, M. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal. Immunol. 2017, 10, 727–742. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC Suppression Correlates with Cytokine Induction of Indoleamine 2,3-Dioxygenase and Bystander M2 Macrophage Differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fu, F.; Li, X.; Zhang, S. Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int. J. Neurosci. 2017, 127, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.-B.; Lim, J.-Y.; Lee, S.-E.; Park, G.; Min, C.-K. Induction of Indoleamine 2,3-dioxygenase by Pre-treatment with Poly(I:C) May Enhance the Efficacy of MSC Treatment in DSS-induced Colitis. Immune Netw. 2016, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, X.; Huang, Y.; Li, W.; Li, J.; Cao, K.; Cao, G.; Zhang, L.; Li, F.; Roberts, A.I.; et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014, 21, 388–396. [Google Scholar] [CrossRef]
- Behm, C.; Blufstein, A.; Gahn, J.; Kubin, B.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Continuing Effect of Cytokines and Toll-Like Receptor Agonists on Indoleamine-2,3-Dioxygenase-1 in Human Periodontal Ligament Stem/Stromal Cells. Cells 2020, 9, 2696. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Lim, S.M.; Oh, K.-W.; Cho, K.-A.; Park, J.; Kim, K.-S.; Lee, S.-J.; Kwon, M.-S.; Kim, S.H. Mesenchymal Stem Cells Modulate the Functional Properties of Microglia via TGF-β Secretion. Stem Cells Transl. Med. 2016, 5, 1538–1549. [Google Scholar] [CrossRef]
- Wilkins, A.; Kemp, K.; Ginty, M.; Hares, K.; Mallam, E.; Scolding, N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009, 3, 63–70. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the Neurotrophic and Angiogenic Properties of Human Adipose-Derived Stem Cells Enhances Nerve Repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rawat, S.; Krishnakumar, V.; Rao, E.P.; Mohanty, S. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res. 2022, 388, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.d.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Noh, M.Y.; Cho, K.A.; Kim, H.; Kwon, M.-S.; Kim, K.S.; Kim, J.; Koh, S.-H.; Kim, S.H. Hypoxia/Reoxygenation-Preconditioned Human Bone Marrow-Derived Mesenchymal Stromal Cells Rescue Ischemic Rat Cortical Neurons by Enhancing Trophic Factor Release. Mol. Neurobiol. 2015, 52, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Q.; Feng, J.-Q.; Chen, J.; Chen, P.-X.; Zhi, J.-L.; Cui, Y.; Guo, R.-X.; Yu, H.-M. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005, 1057, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lam, P.K.; Tong, C.S.W.; Lo, K.K.Y.; Wong, G.K.C.; Poon, W.S. The neuroprotection of hypoxic adipose tissue-derived mesenchymal stem cells in experimental traumatic brain injury. Cell Transplant. 2019, 28, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Lee, J.K.; Jin, H.K.; Bae, J. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-β deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci. Lett. 2009, 450, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lu, C.; Han, Q.; Li, J.; Du, Z.; Liao, L.; Zhao, R.C. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology 2011, 279, 189–195. [Google Scholar] [CrossRef]
- Naaldijk, Y.; Jäger, C.; Fabian, C.; Leovsky, C.; Blüher, A.; Rudolph, L.; Hinze, A.; Stolzing, A. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS 1 Alzheimer mice. Neuropathol. Appl. Neurobiol. 2017, 43, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, R.; Wang, Y.; Huang, W.; Hu, B.; Zhu, G.; Zhang, R.; Li, F.; Han, J.; Li, Y. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res. 2019, 1724, 146422. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.C.; Ribeiro, D.E.; Hashiguchi, D.; Hukuda, D.Y.; Gimenes, C.; Romariz, S.A.A.; Ye, Q.; Tang, Y.; Ulrich, H.; Longo, B.M. Distinct Effects of the Hippocampal Transplantation of Neural and Mesenchymal Stem Cells in a Transgenic Model of Alzheimer’s Disease. Stem Cell Rev. Rep. 2022, 18, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.N.; Villemagne, V.; Sohrabi, H.R.; Chatterjee, P.; Shah, T.M.; Verdile, G.; Fraser, P.; Taddei, K.; Gupta, V.B.; Rainey-Smith, S.R.; et al. Alzheimer’s Disease: A Journey from Amyloid Peptides and Oxidative Stress, to Biomarker Technologies and Disease Prevention Strategies—Gains from AIBL and DIAN Cohort Studies. J. Alzheimer’s Dis. 2018, 62, 965–992. [Google Scholar] [CrossRef] [PubMed]
- Bona, D.; Scapagnini, G.; Candore, G.; Castiglia, L.; Colonna-Romano, G.; Duro, G.; Nuzzo, D.; Iemolo, F.; Lio, D.; Pellicano, M.; et al. Immune-Inflammatory Responses and Oxidative Stress in Alzheimer’s Disease: Therapeutic Implications. Curr. Pharm. Des. 2010, 16, 684–691. [Google Scholar] [CrossRef]
- Walters, A.; Phillips, E.; Zheng, R.; Biju, M.; Kuruvilla, T. Evidence for neuroinflammation in Alzheimer’s disease: Neuroinflammation in Alzheimer’s. Prog. Neurol. Psychiatry 2016, 20, 25–31. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, S.; Wang, Q.; Shi, Y.; Liu, G.; Zhi, J.; Wang, F. Saikosaponin-D Reduces H2O2-Induced PC12 Cell Apoptosis by Removing ROS and Blocking MAPK-Dependent Oxidative Damage. Cell. Mol. Neurobiol. 2016, 36, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-L.; Zhang, Q.; Liu, J.; Sun, J.; He, L.-Y.; Duan, H.-X.; Peng, W.; Wu, C.-J. Hydroxy-α-sanshool Possesses Protective Potentials on H2O2 -Stimulated PC12 Cells by Suppression of Oxidative Stress-Induced Apoptosis through Regulation of PI3K/Akt Signal Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sapiéns, M.A.; Reza-Zaldívar, E.E.; Cevallos, R.R.; Márquez-Aguirre, A.L.; Gazarian, K.; Canales-Aguirre, A.A. A Three-Dimensional Alzheimer’s Disease Cell Culture Model Using iPSC-Derived Neurons Carrying A246E Mutation in PSEN1. Front. Cell. Neurosci. 2020, 14, 151. [Google Scholar] [CrossRef]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Tichauer, J.E.; Eugenín, J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 2010, 112, 1099–1114. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Tsai, M.-J.; Hsieh, N.; Lo, M.-J.; Lee, M.-J.; Cheng, H.; Huang, W.-C. The superiority of conditioned medium derived from rapidly expanded mesenchymal stem cells for neural repair. Stem Cell Res. Ther. 2019, 10, 390. [Google Scholar] [CrossRef]
- Amodeo, G.; Niada, S.; Moschetti, G.; Franchi, S.; Savadori, P.; Brini, A.T.; Sacerdote, P. Secretome of human adipose-derived mesenchymal stem cell relieves pain and neuroinflammation independently of the route of administration in experimental osteoarthritis. Brain. Behav. Immun. 2021, 94, 29–40. [Google Scholar] [CrossRef]
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control. Release 2020, 327, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Mah, D.; Simtchouk, S.; Kluftinger, A.; Little, J. Human Adipose Tissue Conditioned Media from Lean Subjects Is Protective against H2O2 Induced Neurotoxicity in Human SH-SY5Y Neuronal Cells. Int. J. Mol. Sci. 2015, 16, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wu, J.; Mou, F.; Xie, W.; Wang, F.; Wang, Q.; Fang, J.; Xu, Y.; Dong, Y.; Liu, J.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Reza-Zaldivar, E.; Hernández-Sapiéns, M.; Gutiérrez-Mercado, Y.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Osama, A.; Zhang, J.; Yao, J.; Yao, X.; Fang, J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020, 64, 101206. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Xian, P.; Wang, T.; Wu, S.; Sun, T.; Wang, W.; Wang, B.; Yang, H.; Yang, Y.; Wang, H.; et al. Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics 2021, 11, 5986–6005. [Google Scholar] [CrossRef] [PubMed]
- Farfán, N.; Carril, J.; Redel, M.; Zamorano, M.; Araya, M.; Monzón, E.; Alvarado, R.; Contreras, N.; Tapia-Bustos, A.; Quintanilla, M.E.; et al. Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia. Int. J. Mol. Sci. 2020, 21, 7800. [Google Scholar] [CrossRef]
- Jin, Q.-H.; Kim, H.-K.; Na, J.-Y.; Jin, C.; Seon, J.-K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, A.; Fa, Z.; Ding, Z.; Xie, J.; Sun, Y.; Zhang, R.; Wang, Q. Adipose-Derived Stem Cells Modulate BV2 Microglial M1/M2 Polarization by Producing GDNF. Stem Cells Dev. 2020, 29, 714–727. [Google Scholar] [CrossRef]
- Sart, S.; Liu, Y.; Ma, T.; Li, Y. Microenvironment Regulation of Pluripotent Stem Cell-Derived Neural Progenitor Aggregates by Human Mesenchymal Stem Cell Secretome. Tissue Eng. Part A 2014, 20, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R.; Vasandan, A.B. Pro-Inflammatory Cytokines, IFNγ and TNFα, Influence Immune Properties of Human Bone Marrow and Wharton Jelly Mesenchymal Stem Cells Differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Lee, B.-C.; Lee, J.Y.; Kim, J.-J.; Lee, S.-E.; Shin, N.; Choi, S.W.; Kang, K.-S. Interferon-γ-mediated secretion of tryptophanyl-tRNA synthetases has a role in protection of human umbilical cord blood-derived mesenchymal stem cells against experimental colitis. BMB Rep. 2019, 52, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, Y.; Xu, Z.; Wang, H.; Zhao, Z.; Li, Y.; Yang, P.; Wei, X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J. Cell. Mol. Med. 2012, 16, 1840–1855. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Roddy, G.W.; Choi, H.; Lee, R.H.; Ylöstalo, J.H.; Rosa, R.H.; Prockop, D.J. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. USA 2010, 107, 16875–16880. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Fraser, J.L.; Lu, Z.-Y.; Hu, X.; Yu, S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 2012, 46, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Iohara, K.; Watanabe, H.; Ishikawa, M.; Tominaga, M.; Nakashima, M. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res. Ther. 2021, 12, 302. [Google Scholar] [CrossRef]
- Sheng, L.; Mao, X.; Yu, Q.; Yu, D. Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Exp. Ther. Med. 2017, 13, 55–62. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, Y.; Fu, Y.; Liu, J.; He, Z.; Huang, Z. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest–induced global cerebral ischemia in rats. J. Neurosci. Res. 2017, 95, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Golovicheva, V.V.; Varlamova, E.G.; Danilina, T.I.; Goryunov, K.V.; Shevtsova, Y.A.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Evtushenko, E.A.; et al. Mesenchymal stromal cell-derived extracellular vesicles afford neuroprotection by modulating PI3K/AKT pathway and calcium oscillations. Int. J. Biol. Sci. 2022, 18, 5345–5368. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, D.H.; Kim, D.-S.; Kim, J.H.; Jeong, S.Y.; Jeon, H.B.; Lee, E.H.; Yang, Y.S.; Oh, W.; Chang, J.W. Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces amyloid-β42 neurotoxicity in vitro. FEBS Lett. 2010, 584, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Humphrey, A.; Roy, D.; Sheridan, M.-E.; Versey, Z.; Jaworski, A.; Edwards, A.; Donner, J.; Abizaid, A.; Willmore, W.; et al. HIF-1α Regulation of Cytokine Production following TLR3 Engagement in Murine Bone Marrow-Derived Macrophages Is Dependent on Viral Nucleic Acid Length and Glucose Availability. J. Immunol. Baltim. Md 1950 2021, 207, 2813–2827. [Google Scholar] [CrossRef] [PubMed]
- Leonov, G.; Salikhova, D.; Shedenkova, M.; Bukharova, T.; Fatkhudinov, T.; Goldshtein, D. Comparative Study of the Protective and Neurotrophic Effects of Neuronal and Glial Progenitor Cells-Derived Conditioned Media in a Model of Glutamate Toxicity In Vitro. Biomolecules 2023, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G. Genetic risk factors and candidate biomarkers for Alzheimer’s disease. Front. Biosci. 2010, S2, 616–622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scapagnini, G.; D’Agata, V.; Calabrese, V.; Pascale, A.; Colombrita, C.; Alkon, D.; Cavallaro, S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002, 954, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M. Heme oxygenase-1: Role in brain aging and neurodegeneration. Exp. Gerontol. 2000, 35, 821–830. [Google Scholar] [CrossRef]
- Li, M.; Xu, T.; Zhou, F.; Wang, M.; Song, H.; Xiao, X.; Lu, B. Neuroprotective Effects of Four Phenylethanoid Glycosides on H2O2-Induced Apoptosis on PC12 Cells via the Nrf2/ARE Pathway. Int. J. Mol. Sci. 2018, 19, 1135. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, G.; Chen, D.; Yang, J.; Wang, J.; Sun, Y.; Zhang, Q.; Liu, Y. NQO1 regulates expression and alternative splicing of apoptotic genes associated with Alzheimer’s disease in PC12 cells. Brain Behav. 2023, 13, e2917. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Mohamed Asik, R.; Suganthy, N.; Aarifa, M.A.; Kumar, A.; Szigeti, K.; Mathe, D.; Gulyás, B.; Archunan, G.; Padmanabhan, P. Alzheimer’s Disease: A Molecular View of β-Amyloid Induced Morbific Events. Biomedicines 2021, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tuo, P.; Zhang, W.; Wang, S. Inhibition of the TLR4/NF-κB pathway promotes the polarization of LPS-induced BV2 microglia toward the M2 phenotype. NeuroReport 2023, 34, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Ozen, I.; Ruscher, K.; Nilsson, R.; Flygt, J.; Clausen, F.; Marklund, N. Interleukin-1 Beta Neutralization Attenuates Traumatic Brain Injury-Induced Microglia Activation and Neuronal Changes in the Globus Pallidus. Int. J. Mol. Sci. 2020, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Sung, D.K.; Kim, Y.E.; Yang, M.; Ahn, S.Y.; Sung, S.I.; Chang, Y.S. Mesenchymal Stromal Cells Primed by Toll-like Receptors 3 and 4 Enhanced Anti-Inflammatory Effects against LPS-Induced Macrophages via Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 16264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, J.; Lin, Y.; Liu, F.; Tao, J.; Zhang, W.; Xu, J.; Zhang, M. Extracellular vesicles derived from human ESC–MSCs target macrophage and promote anti-inflammation process, angiogenesis, and functional recovery in ACS-induced severe skeletal muscle injury. Stem Cell Res. Ther. 2023, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Jansen-West, K.; Beccard, A.; Ceballos-Diaz, C.; Levites, Y.; Verbeeck, C.; Zubair, A.C.; Dickson, D.; Golde, T.E.; Das, P. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010, 24, 548–559. [Google Scholar] [CrossRef]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Eskes, C.; Honegger, P.; Juillerat-Jeanneret, L.; Monnet-Tschudi, F. Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release. Glia 2002, 37, 43–52. [Google Scholar] [CrossRef]
- Yang, M.; Sun, W.; Xiao, L.; He, M.; Gu, Y.; Yang, T.; Chen, J.; Liang, X. Mesenchymal Stromal Cells Suppress Hippocampal Neuron Autophagy Stress Induced by Hypoxic-Ischemic Brain Damage: The Possible Role of Endogenous IL-6 Secretion. Neural Plast. 2020, 2020, 8822579. [Google Scholar] [CrossRef]
- Lin, H.; Levison, S.W. Context-dependent IL-6 potentiation of interferon-gamma-induced IL-12 secretion and CD40 expression in murine microglia. J. Neurochem. 2009, 111, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Wahyuningtyas, R.; Aui, S.; Chang, K. Autocrine VEGF signalling on M2 macrophages regulates PD -L1 expression for immunomodulation of T cells. J. Cell. Mol. Med. 2019, 23, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Lee, D.Y.; Joe, E.H.; Kim, S.U.; Jin, B.K. Neuroprotective role of microglia expressing interleukin-4. J. Neurosci. Res. 2005, 81, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Enam, S.F.; Kader, S.R.; Bodkin, N.; Lyon, J.G.; Calhoun, M.; Azrak, C.; Tiwari, P.M.; Vanover, D.; Wang, H.; Santangelo, P.J.; et al. Evaluation of M2-like macrophage enrichment after diffuse traumatic brain injury through transient interleukin-4 expression from engineered mesenchymal stromal cells. J. Neuroinflammation 2020, 17, 197. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, T.; Okuyama, S.; Swan, R.J.; Jacobsen, M.T.; Gendelman, H.E.; Ikezu, T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010, 24, 3093–3102. [Google Scholar] [CrossRef]
- Papadimitriou, C.; Celikkaya, H.; Cosacak, M.I.; Mashkaryan, V.; Bray, L.; Bhattarai, P.; Brandt, K.; Hollak, H.; Chen, X.; He, S.; et al. 3D Culture Method for Alzheimer’s Disease Modeling Reveals Interleukin-4 Rescues Aβ42-Induced Loss of Human Neural Stem Cell Plasticity. Dev. Cell 2018, 46, 85–101.e8. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-S.; Peshavariya, H.M.; Higuchi, M.; Chan, E.C.; Dusting, G.J.; Jiang, F. Pharmacological priming of adipose-derived stem cells for paracrine VEGF production with deferoxamine: Priming adipose-derived stem cells with deferoxamine. J. Tissue Eng. Regen. Med. 2016, 10, E167–E176. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.I.; Moon, Y.H.; Kim, M.S. Effects of CoCl2 on multi-lineage differentiation of C3H/10T1/2 mesenchymal stem cells. Korean J. Physiol. Pharmacol. 2016, 20, 53. [Google Scholar] [CrossRef]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.-D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, J.; Lan, L.; Zhang, H.; Liu, F.; Wu, Z.; Ni, H.; Wang, Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol. Ther. 2015, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Jhong, J.-H.; Chen, Q.; Huang, K.-Y.; Strittmatter, K.; Kreuzer, J.; DeRan, M.; Wu, X.; Lee, T.-Y.; Slavov, N.; et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021, 37, 109955. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-K.; Llewellyn, O.P.C.; Bates, D.O.; Nicholson, L.B.; Dick, A.D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 2010, 215, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Xu, Y.; Liu, Q.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 681171. [Google Scholar] [CrossRef] [PubMed]





| Culture Medium | Components | Concentration |
|---|---|---|
| Osteogenic | Dexamethasone | 100 nM |
| β-Glycerol phosphate | 10 mM | |
| Ascorbate-2-phosphate | 0.5 mM | |
| Adipogenic | Insulin | 1 μg/mL |
| Isobutyl-1-methylxanthine | 0.5 mM | |
| Dexamethasone | 0.5 μM |
| Cell Types | Genes | Forward Sequences | Reverse Sequences |
|---|---|---|---|
| MSCs | ACTB | TCAGAAGGATTCCTATGTGGGCGA | CACGCAGCTCATTGTAGAAGGTGT |
| GAPDH | TCGACAGTCAGCCGCATCTTCTTT | ACCAAATCCGTTGACTCCGACCTT | |
| IDO1 | CCCTTCAAGTGTTTCACCAAATC | GTCTTCCCAGAACCCTTCATAC | |
| TNFAIP6 | AAGATGGGATGCCTATTGCTAC | ATTTGGGAAGCCTGGAGATTTA | |
| PTGES2 | CAGCACTTCACGCATCAGTT | GTCTAGCCAGAGTTTCACCGTA | |
| THP-1 | MRC1 | GCAAAGTGGATTACGTGTCTTG | CTGTTATGTCGCTGGCAAATG |
| IL10 | TCAGGCTGAGGCTACGG | AGATGTCAAACTCACTCATGGC | |
| TGFB1 | CGTGGAGCTGTACCAGAAATAC | CACAACTCCGGTGACATCAA | |
| PC12 | GAPDH | CCATCAACGACCCCTTCATT | GACCAGCTTCCCATTCTCAG |
| HMOX1 | CCAACATTGCCGTGCCAC | GCTCCTGCAACTCCTCAAAGAG | |
| NQO1 | TGCAGCGGCTTTGAAGAAGA | AGGGTCCTTCAGTTTACCTGTG | |
| BCL2 | GATGACTGAGTACCTGAACCG | CAGAGACAGCCAGGAGAAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolstova, T.; Dotsenko, E.; Luzgina, N.; Rusanov, A. Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer’s Disease In Vitro Model. Biomedicines 2024, 12, 2243. https://doi.org/10.3390/biomedicines12102243
Tolstova T, Dotsenko E, Luzgina N, Rusanov A. Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer’s Disease In Vitro Model. Biomedicines. 2024; 12(10):2243. https://doi.org/10.3390/biomedicines12102243
Chicago/Turabian StyleTolstova, Tatiana, Ekaterina Dotsenko, Natalia Luzgina, and Alexander Rusanov. 2024. "Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer’s Disease In Vitro Model" Biomedicines 12, no. 10: 2243. https://doi.org/10.3390/biomedicines12102243
APA StyleTolstova, T., Dotsenko, E., Luzgina, N., & Rusanov, A. (2024). Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer’s Disease In Vitro Model. Biomedicines, 12(10), 2243. https://doi.org/10.3390/biomedicines12102243






