Novel Insights into the Cardioprotective Effects of the Peptides of the Counter-Regulatory Renin–Angiotensin System
Abstract
:1. Introduction
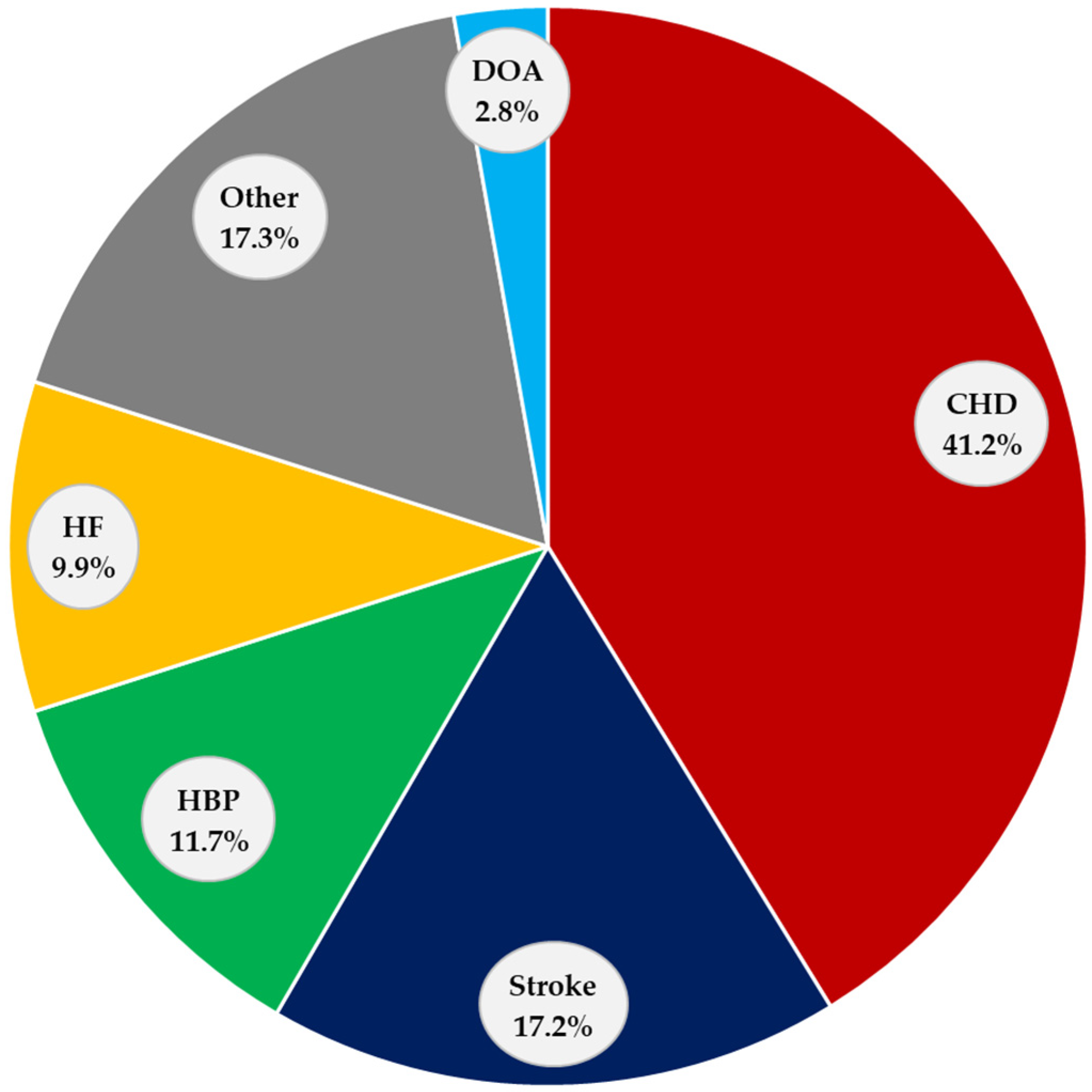
2. Cardiovascular Diseases
2.1. Coronary Heart Disease (CHD)
2.2. Stroke
2.3. High Blood Pressure (HBP)
2.4. Heart Failure (HF)
2.5. Other CVDs
3. Renin–Angiotensin System (RAS)
3.1. Classical RAS
3.2. Counter-Regulatory RAS
3.2.1. Ang-(1-7)
3.2.2. Ang-(1-9)
3.2.3. Alamandine
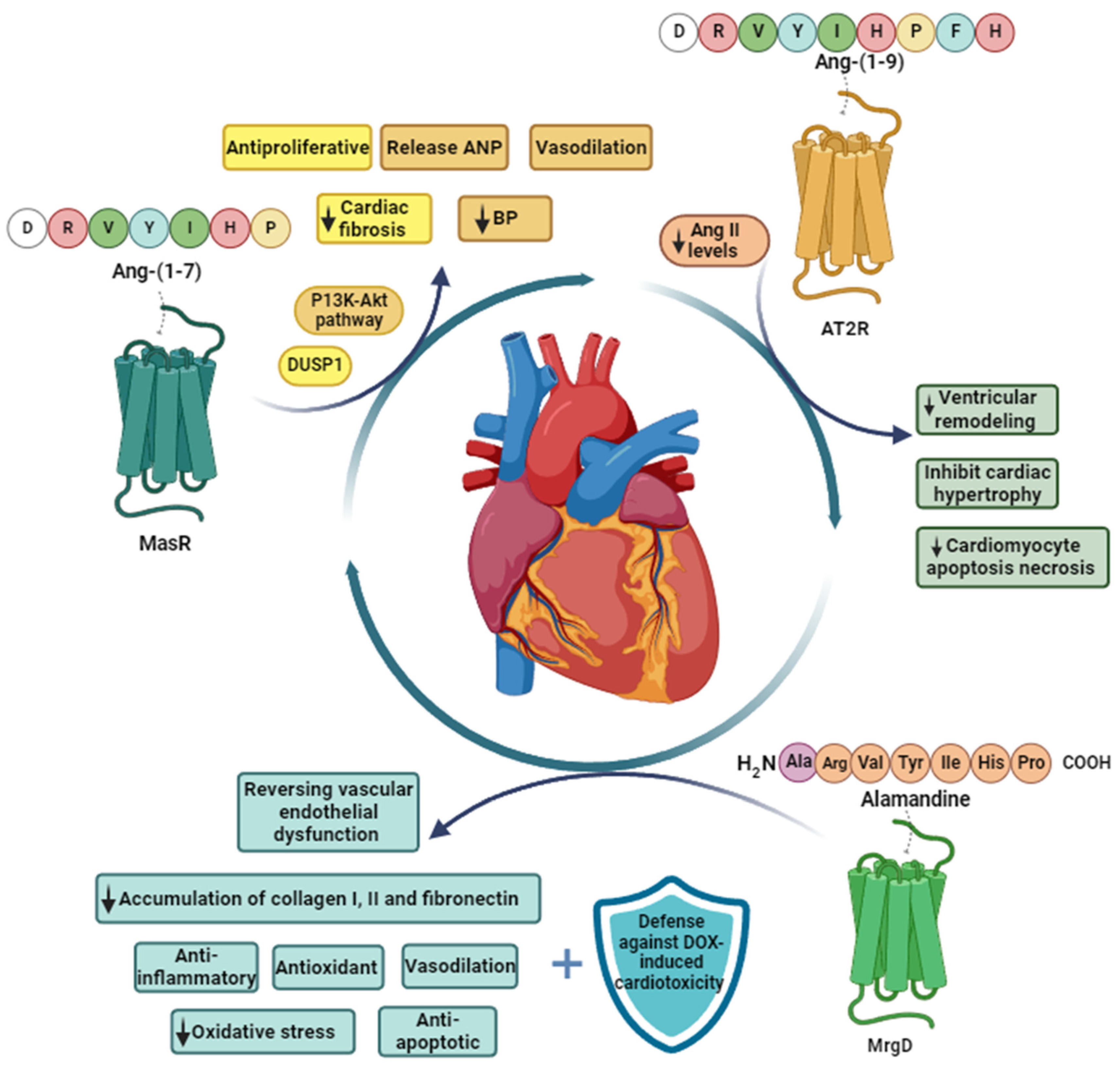
4. Cardioprotective Effects of the Peptides of the Counter-Regulatory RAS
4.1. Cardioprotective Effects of Ang-(1-7)
4.2. Cardioprotective Effects of Ang-(1-9)
4.3. Cardioprotective Effects of Alamandine
5. Clinical Trials with the Peptides of the Counter-Regulatory RAS
6. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [PubMed]
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The Economic Burden of Cardiovascular Disease and Hypertension in Low- and Middle-Income Countries: A Systematic Review. BMC Public Health 2018, 18, 975. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Campbell, W.W.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; et al. Physical Activity, All-Cause and Cardiovascular Mortality, and Cardiovascular Disease. Med. Sci. Sport. Exerc. 2019, 51, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Zeng, X.; Wang, H.; Yin, P.; Wang, L.; Liu, Y.; Liu, J.; Qi, J.; Ran, S.; et al. Burden of Cardiovascular Diseases in China, 1990–2016. JAMA Cardiol. 2019, 4, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 8 July 2023).
- Defunciones Registradas en México 2020. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2021/EstSociodemo/DefuncionesRegistradas2020_Pre_07.pdf (accessed on 28 July 2023).
- Te Riet, L.; Van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Defunciones Fetales y No Fetales—Dane. Available online: https://www.dane.gov.co/files/investigaciones/poblacion/bt_estadisticasvitales_defunciones_Itrim_2022pr.pdf (accessed on 25 July 2023).
- Miller, A.J.; Arnold, A.C. The Renin–Angiotensin System in Cardiovascular Autonomic Control: Recent Developments and Clinical Implications. Clin. Auton. Res. 2018, 29, 231–243. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Santos, R.A.; Oudit, G.Y.; Verano-Braga, T.; Canta, G.; Steckelings, U.M.; Bader, M. The Renin-Angiotensin System: Going beyond the Classical Paradigms. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, 958–970. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. 2017, 28, 1040–1049. [Google Scholar] [CrossRef]
- Chaszczewska-Markowska, M.; Sagan, M.; Bogunia-Kubik, K. The Renin-Angiotensin-Aldosterone System (RAAS)—Physiology and Molecular Mechanisms of Functioning. Postep. Hyg. Med. Dosw. (Online) 2016, 70, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Kobori, H. Independent Regulation of Renin–Angiotensin–Aldosterone System in the Kidney. Clin. Exp. Nephrol. 2018, 22, 1231–1239. [Google Scholar] [CrossRef]
- Bitker, L.; Burrell, L.M. Classic and Nonclassic Renin-Angiotensin Systems in the Critically Ill. Crit. Care Clin. 2019, 35, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.; Lavandero, S. Counter-Regulatory Renin–Angiotensin System in Cardiovascular Disease. Nat. Rev. Cardiol. 2019, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Zhang, J.; Zhuo, J.L. The Vasoprotective Axes of the Renin-Angiotensin System: Physiological Relevance and Therapeutic Implications in Cardiovascular, Hypertensive and Kidney Diseases. Pharmacol. Res. 2017, 125, 21–38. [Google Scholar] [CrossRef]
- Karnik, S.S.; Singh, K.D.; Tirupula, K.; Unal, H. Significance of Angiotensin 1–7 Coupling with MAS1 Receptor and Other GPCRs to the Renin-angiotensin System: IUPHAR Review 22. Br. J. Pharmacol. 2017, 174, 737–753. [Google Scholar] [CrossRef]
- Kangussu, L.M.; Guimaraes, P.S.; Nadu, A.P.; Melo, M.B.; Santos, R.A.S.; Campagnole-Santos, M.J. Activation of Angiotensin-(1–7)/Mas Axis in the Brain Lowers Blood Pressure and Attenuates Cardiac Remodeling in Hypertensive Transgenic (MREN2)27 Rats. Neuropharmacology 2015, 97, 58–66. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Aguiar, J.E. Preventing Premature Mortality from Cardiovascular Disease: A Prime Goal. Rev. Port. Cardiol. 2020, 39, 35–36. [Google Scholar] [CrossRef]
- Tsao, C.; Aday, A. Correction to: Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 146, 141. [Google Scholar]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K. Consenso ESC 2018 Sobre la Cuarta Definición Universal del Infarto. Rev. Esp. Cardiol. 2019, 72, 72. [Google Scholar]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [PubMed]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Ferry, A.V.; Anand, A.; Strachan, F.E.; Mooney, L.; Stewart, S.D.; Marshall, L. Presenting Symptoms in Men and Women Diagnosed with Myocardial Infarction Using Sex-Specific Criteria. J. Am. Heart Assoc. 2019, 8, e012307. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The Coronary Circulation in Acute Myocardial Ischaemia/Reperfusion Injury: A Target for Cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation Following Acute Myocardial Infarction: Multiple Players, Dynamic Roles, and Novel Therapeutic Opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Hao, K.; Lei, W.; Wu, H.; Wu, J.; Yang, Z.; Yan, S.; Lu, X.A.; Li, J.; Xia, X.; Han, X.; et al. Lncrna-Safe Contributes to Cardiac Fibrosis through Safe-Sfrp2-HuR Complex in Mouse Myocardial Infarction. Theranostics 2019, 9, 7282–7297. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Al Ghorani, H.; Götzinger, F.; Böhm, M.; Mahfoud, F. Arterial hypertension—Clinical trials update 2021. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 21–31. [Google Scholar] [CrossRef]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition from Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Aging and Heart Failure: Changing Demographics and Implications for Therapy in the Elderly. Heart Fail. Rev. 2010, 15, 401–405. [Google Scholar] [CrossRef]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K. Acute Heart Failure. Nat. Rev. Dis. Prim. 2020, 6, 16. [Google Scholar] [CrossRef]
- Kurmani, S.; Squire, I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef]
- Long, B.; Koyfman, A.; Gottlieb, M. Diagnosis of Acute Heart Failure in the Emergency Department: An Evidence-Based Review. West. J. Emerg. Med. 2019, 20, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, L.; Cai, H.L.; Lu, H.H. Relationship of the ORBIT and HAS-BLED Scores with Killip Class 3-4 in Patients with ST-Segment Elevation Myocardial Infarction. Medicine 2019, 98, e14578. [Google Scholar] [CrossRef] [PubMed]
- Curley, D.; Lavin Plaza, B.; Shah, A.M.; Botnar, R.M. Molecular Imaging of Cardiac Remodelling after Myocardial Infarction. Basic Res. Cardiol. 2018, 113, 10. [Google Scholar] [CrossRef] [PubMed]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac Monocytes and Macrophages after Myocardial Infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]
- Gabriel-Costa, D. The Pathophysiology of Myocardial Infarction-Induced Heart Failure. Pathophysiology 2018, 25, 277–284. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart Failure after Myocardial Infarction: Incidence and Predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Bostan, M.M.; Stătescu, C.; Anghel, L.; Șerban, I.L.; Cojocaru, E.; Sascău, R. Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic. Biomolecules 2020, 10, 1587. [Google Scholar] [CrossRef]
- Frampton, J.; Ortengren, A.R.; Zeitler, E.P. Arrhythmias after Acute Myocardial Infarction. Yale J. Biol. Med. 2023, 96, 83–94. [Google Scholar] [CrossRef]
- Liakos, C.I.; Grassos, C.A.; Papadopoulos, D.P.; Dimitriadis, K.S.; Tsioufis, C.P.; Tousoulis, D. Arterial hypertension and aortic valve stenosis: Shedding light on a common “liaison”. Hell. J. Cardiol. 2017, 58, 261–266. [Google Scholar] [CrossRef]
- Morales Olivas, F.J.; Estañ Yago, L. Conceptos Nuevos Sobre el Sistema Renina Angiotensina. Hipertens. Riesgo Vasc. 2010, 27, 211–217. [Google Scholar] [CrossRef]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The Renin-Angiotensin-Aldosterone System and Its Suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Bruce, E.B.; de Kloet, A.D. The Intricacies of the Renin-Angiotensin-System in Metabolic Regulation. Physiol. Behav. 2017, 178, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Hanff, T.C.; Bress, A.P.; South, A.M. Relationship between ACE2 and Other Components of the Renin-Angiotensin System. Curr. Hypertens. Rep. 2020, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Lelis, D.F.; Freitas, D.F.; Machado, A.S.; Crespo, T.S.; Santos, S.H.S. Angiotensin-(1-7), Adipokines and Inflammation. Metabolism 2019, 95, 36–45. [Google Scholar] [CrossRef]
- Santos, R.A.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takeshita, H.; Rakugi, H. ACE2, Angiotensin 1-7 and Skeletal Muscle: Review in the Era of COVID-19. Clin. Sci. 2020, 134, 3047–3062. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Medina, D.; Arnold, A.C. Angiotensin-(1-7): Translational Avenues in Cardiovascular Control. Am. J. Hypertens. 2019, 32, 1133–1142. [Google Scholar] [CrossRef]
- Freitas, R.A.; Junior, R.R.P.; Justina, V.D.; Bressan, A.F.M.; Bomfim, G.F.; Carneiro, F.S.; Giachini, F.R.; Lima, V.V. Angiotensin (1-7)-Attenuated Vasoconstriction is Associated with the Interleukin-10 Signaling Pathway. Life Sci. 2020, 262, 118552. [Google Scholar] [CrossRef]
- Miller, A.J.; Bingaman, S.S.; Mehay, D.; Medina, D.; Arnold, A.C. Angiotensin-(1-7) Improves Integrated Cardiometabolic Function in Aged Mice. Int. J. Mol. Sci. 2020, 21, 5131. [Google Scholar] [CrossRef] [PubMed]
- Kittana, N. Angiotensin-Converting Enzyme 2-Angiotensin 1-7/1-9 System: Novel Promising Targets for Heart Failure Treatment. Fundam. Clin. Pharmacol. 2018, 32, 14–25. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.A.; Fattah, C.; Loughrey, C.M.; Milligan, G.; Nicklin, S.A. Angiotensin-(1-7) and Angiotensin-(1-9): Function in Cardiac and Vascular Remodelling. Clin. Sci. 2014, 126, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Varagic, J.; Ahmad, S.; Nagata, S.; Ferrario, C.M. ACE2: Angiotensin II/Angiotensin-(1-7) Balance in Cardiac and Renal Injury. Curr. Hypertens. Rep. 2014, 16, 420. [Google Scholar] [CrossRef]
- Higuchi, S.; Ohtsu, H.; Suzuki, H.; Shirai, H.; Frank, G.D.; Eguchi, S. Angiotensin II Signal Transduction through the AT1 Receptor: Novel Insights into Mechanisms and Pathophysiology. Clin. Sci. 2007, 112, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Novoa, U.; Moya, J.; Gabrielli, L.; Jalil, J.E.; García, L.; Chiong, M.; Lavandero, S.; Ocaranza, M.P. Angiotensin-(1-9) Reduces Cardiovascular and Renal Inflammation in Experimental Renin-Independent Hypertension. Biochem. Pharmacol. 2018, 156, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Flores-Muñoz, M.; Smith, N.J.; Haggerty, C.; Milligan, G.; Nicklin, S.A. Angiotensin1-9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J. Physiol. 2011, 589, 939–951. [Google Scholar] [CrossRef]
- Kramkowski, K.; Mogielnicki, A.; Leszczynska, A.; Buczko, W. Angiotensin-(1-9), the Product of Angiotensin I Conversion in Platelets, Enhances Arterial Thrombosis in Rats. J. Physiol. Pharmacol. 2010, 61, 317–324. [Google Scholar]
- Schleifenbaum, J. Alamandine and its Receptor MrgD Pair up to Join the Protective Arm of the Renin-Angiotensin System. Front. Med. 2019, 6, 107. [Google Scholar] [CrossRef]
- Park, B.M.; Phuong, H.T.A.; Yu, L.; Kim, S.H. Alamandine Protects the Heart against Reperfusion Injury via the MrgD Receptor. Circ. J. 2018, 82, 2584–2593. [Google Scholar] [CrossRef]
- Jesus, I.C.G.; Mesquita, T.R.R.; Monteiro, A.L.L.; Parreira, A.B.; Santos, A.K.; Coelho, E.L.X.; Silva, M.M.; Souza, L.A.C.; Campagnole-Santos, M.J.; Santos, R.S.; et al. Alamandine Enhances Cardiomyocyte Contractility in Hypertensive Rats through a Nitric Oxide-Dependent Activation of Camkii. Am. J. Physiol. Cell 2020, 318, 740–750. [Google Scholar] [CrossRef]
- Jesus, I.C.G.; Scalzo, S.; Alves, F.; Marques, K.; Rocha-Resende, C.; Bader, M.; Santos, R.A.S.; Guatimosim, S. Alamandine Acts via MrgD to Induce AMPK/NO Activation against ANG II Hypertrophy in Cardiomyocytes. Am. J. Physiol. Cell Physiol. 2018, 314, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Matsoukas, M.T.; Hayes, A.; Rybalka, E.; Caprnda, M.; Rimarova, K.; Sepsi, M.; Büsselberg, D.; Kruzliak, P.; Matsoukas, J.; et al. Alamandine Reverses Hyperhomocysteinemia-Induced Vascular Dysfunction via PKA-Dependent Mechanisms. Cardiovasc. Ther. 2017, 35, e12306. [Google Scholar] [CrossRef] [PubMed]
- Lautner, R.Q.; Villela, D.C.; Fraga-Silva, R.A.; Silva, N.; Verano-Braga, T.; Costa-Fraga, F. Discovery and Characterization of Alamandine: A Novel Component of the Renin-Angiotensin System. Circ. Res. 2013, 112, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Song, X.D.; Feng, J.P.; Yang, R.X. Alamandine Protects Rat from Myocardial Ischemia-Reperfusion Injury by Activating JNK and Inhibiting NF-κB. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6718–6726. [Google Scholar]
- Mendoza-Torres, E.; Oyarzún, A.; Mondaca-Ruff, D.; Azocar, A.; Castro, P.F.; Jalil, J.E.; Chiong, M.; Lavandero, S.; Ocaranza, M.P. ACE2 and Vasoactive Peptides: Novel Players in Cardiovascular/Renal Remodeling and Hypertension. Ther. Adv. Cardiovasc. Dis. 2015, 9, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The PI3k/Akt Pathway is Associated with Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischemia. Physiol. Res. 2019, 68, 131–138. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, H.; Sun, S.; Luo, Y.; Ren, X.; Chen, A. Angiotensin-(1-7) induced vascular relaxation in spontaneously hypertensive rats. Nitric Oxide 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Carver, K.A.; Smith, T.L.; Gallagher, P.E.; Tallant, E.A. Angiotensin-(1-7) Prevents Angiotensin II-Induced Fibrosis in Cremaster Microvessels. Microcirculation 2015, 22, 19–27. [Google Scholar] [CrossRef]
- Wang, L.P.; Fan, S.J.; Li, S.M.; Wang, X.J.; Gao, J.L.; Yang, X.H. Protective Role of ACE2-Ang-(1–7)-Mas in Myocardial Fibrosis by Downregulating Kca3.1 Channel via ERK1/2 Pathway. Pflug. Arch. 2016, 468, 2041–2051. [Google Scholar] [CrossRef]
- Shah, A.; Oh, Y.B.; Lee, S.H.; Lim, J.M.; Kim, S.H. Angiotensin-(1-7) Attenuates Hypertension in Exercise-Trained Renal Hypertensive Rats. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Gava, E.; de Castro, C.H.; Ferreira, A.J.; Colleta, H.; Melo, M.B.; Alenina, N.; Bader, M.; Oliveira, L.A.; Santos, R.A.; Kitten, G.T. Angiotensin-(1-7) Receptor Mas Is An Essential Modulator of Extracellular Matrix Protein Expression in the Heart. Regul. Pept. 2012, 175, 30–42. [Google Scholar] [CrossRef]
- Iwata, M.; Cowling, R.T.; Gurantz, D.; Moore, C.; Zhang, S.; Yuan, J.X.; Greenberg, B.H. Angiotensin-(1-7) Binds to Specific Receptors on Cardiac Fibroblasts to Initiate Antifibrotic and Antitrophic Effects. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 2356–2363. [Google Scholar] [CrossRef]
- Grobe, J.L.; Mecca, A.P.; Lingis, M.; Shenoy, V.; Bolton, T.A.; Machado, J.M.; Speth, R.C.; Raizada, M.K.; Katovich, M.J. Prevention of Angiotensin II-Induced Cardiac Remodeling by Angiotensin-(1-7). Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Chen, W.; Leng, X.; He, J.G.; Ma, H. Chronic Angiotensin-(1-7) Administration Improves Vascular Remodeling after Angioplasty through the Regulation of the TGF-Beta/Smad Signaling Pathway in Rabbits. Biochem. Biophys. Res. Commun. 2009, 389, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.B.; Lima, W.G.; Silva, M.E.; Souza Santos, R.A.; Campagnole-Santos, M.J.; Alzamora, A.C. The Nonpeptide ANG-(1-7) Mimic AVE 0991 Attenuates Cardiac Remodeling and Improves Baroreflex Sensitivity in Renovascular Hypertensive Rats. Life Sci. 2013, 92, 266–275. [Google Scholar] [CrossRef] [PubMed]
- McCollum, L.T.; Gallagher, P.E.; Tallant, E.A. Angiotensin-(1-7) Abrogates Mitogen-Stimulated Proliferation of Cardiac Fibroblasts. Peptides 2012, 34, 380–388. [Google Scholar] [CrossRef]
- Marques, F.D.; Melo, M.B.; Souza, L.E.; Irigoyen, M.C.C.; Sinisterra, R.D.; de Sousa, F.B.; Savergnini, S.Q.; Braga, V.B.; Ferreira, A.J.; Santos, R.A. Beneficial Effects of Long-Term Administration of an Oral Formulation of Angiotensin-(1-7) in Infarcted Rats. Int. J. Hypertens. 2012, 2012, 795452. [Google Scholar] [CrossRef]
- Romero, A.; San Hipólito-Luengo, Á.; Villalobos, L.A.; Vallejo, S.; Valencia, I.; Michalska, P.; Pajuelo-Lozano, N.; Sánchez-Pérez, I.; León, R.; Bartha, J.L.; et al. The Angiotensin-(1-7)/Mas Receptor Axis Protects from Endothelial Cell Senescence via Klotho and Nrf2 Activation. Aging Cell 2019, 18, e12913. [Google Scholar] [CrossRef]
- Zhang, B.N.; Xu, H.; Gao, X.M.; Zhang, G.Z.; Zhang, X.; Yang, F. Protective Effect of Angiotensin (1-7) on Silicotic Fibrosis in Rats. Biomed. Environ. Sci. 2019, 32, 419–426. [Google Scholar]
- Kangussu, L.M.; Melo-Braga, M.N.; de Souza Lima, B.S.; Santos, R.A.S.; de Andrade, H.M.; Campagnole-Santos, M.J. Angiotensin-(1-7) Central Mechanisms after ICV Infusion in Hypertensive Transgenic (Mren2)27 Rats. Front. Neurosci. 2021, 15, 624249. [Google Scholar] [CrossRef]
- Stoyell-Conti, F.F.; Chabbra, A.; Puthentharayil, J.; Rigatto, K.; Speth, R.C. Chronic Administration of Pharmacological Doses of Angiotensin 1-7 and Iodoangiotensin 1-7 Has Minimal Effects on Blood Pressure, Heart Rate, and Cognitive Function of Spontaneously Hypertensive Rats. Physiol. Rep. 2021, 9, e14812. [Google Scholar] [CrossRef] [PubMed]
- Flores-Munoz, M.; Work, L.M.; Douglas, K.; Denby, L.; Dominiczak, A.F.; Graham, D.; Nicklin, S.A. Angiotensin-(1-9) Attenuates Cardiac Fibrosis in the Stroke-Prone Spontaneously Hypertensive Rat via the Angiotensin Type 2 Receptor. Hypertension 2012, 59, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Flores, C.; Rivera-Mejías, P.; Vásquez-Trincado, C.; López-Crisosto, C.; Morales, P.E.; Pennanen, C.; Polakovicova, I.; Aliaga-Tobar, V.; García, L.; Roa, J.C.; et al. Angiotensin-(1-9) Prevents Cardiomyocyte Hypertrophy by Controlling Mitochondrial Dynamics via Mir-129-3p/PKIA Pathway. Cell Death Differ. 2020, 27, 2586–2604. [Google Scholar] [CrossRef] [PubMed]
- Norambuena-Soto, I.; Ocaranza, M.P.; Cancino-Arenas, N.; Sanhueza-Olivares, F.; Villar-Fincheira, P.; Leiva-Navarrete, S.; Mancilla-Medina, C.; Moya, J.; Novoa, U.; Jalil, J.E.; et al. Angiotensin-(1-9) Prevents Vascular Remodeling by Decreasing Vascular Smooth Muscle Cell Dedifferentiation through a Foxo1-Dependent Mechanism. Biochem. Pharmacol. 2020, 180, 114190. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Torres, E.; Riquelme, J.A.; Vielma, A.; Sagredo, A.R.; Gabrielli, L.; Bravo-Sagua, R.; Jalil, J.E.; Rothermel, B.A.; Sanchez, G.; Ocaranza, M.P.; et al. Protection of the Myocardium against Ischemia/Reperfusion Injury by Angiotensin-(1-9) through an AT2R and Akt-Dependent Mechanism. Pharmacol. Res. 2018, 135, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P. Cardioplegic Solution Challenges. Ital. Heart J. 2000, 1, S40–S42. [Google Scholar] [PubMed]
- Reichert, K.; Carmo, H.R.; Lima, F.; Torina, A.G.; Vilarinho, K.A.; Oliveira, P.P.; Silveira Filho, L.M.; Severino, E.S.; Petrucci, O. Development of Cardioplegic Solution without Potassium: Experimental Study in Rat. Rev. Bras. Cir. Cardiovasc. 2013, 28, 524–530. [Google Scholar]
- Ocaranza, M.P.; Lavandero, S.; Jalil, J.E.; Moya, J.; Pinto, M.; Novoa, U. Angiotensin-(1-9) Regulates Cardiac Hypertrophy In Vivo and In Vitro. J. Hypertens. 2010, 28, 1054–1064. [Google Scholar] [CrossRef]
- Gomes, E.R.M.; Lara, A.A.; Almeida, P.W.M.; Guimarães, D.; Resende, R.R.; Campagnole-Santos, M.J. Angiotensin-(1-7) Prevents Cardiomyocyte Pathological Remodeling through a Nitric Oxide/Guanosine 3′,5′-Cyclic Monophosphate-Dependent Pathway. Hypertension 2010, 55, 153–160. [Google Scholar] [CrossRef]
- Oparil, S.; Sanders, C.A.; Haber, E. In-Vivo and In-Vitro Conversion of Angiotensin I to Angiotensin II in Dog Blood. Circ. Res. 1970, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.I.; Thomas, D.A.; Grant, P.J.; Turner, A.J.; Hooper, N.M. Evaluation of Angiotensin-Converting Enzyme (ACE), Its Homologue ACE2 and Neprilysin in Angiotensin Peptide Metabolism. Biochem. J. 2004, 383, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.P.; Jalil, J.E. Protective Role of the ACE2/Ang-(1-9) Axis in Cardiovascular Remodeling. Int. J. Hypertens. 2012, 2012, 594361. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q. PI3K/Akt Signaling Transduction Pathway, Erythropoiesis and Glycolysis in Hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Weeks, K.L.; Bernardo, B.C.; Ooi, J.Y.Y.; Patterson, N.L.; McMullen, J.R. The IGF1-PI3K-Akt Signaling Pathway in Mediating Exercise-Induced Cardiac Hypertrophy and Protection. Adv. Exp. Med. Biol. 2017, 1000, 187–210. [Google Scholar]
- Yang, C.; Wu, X.; Shen, Y.; Liu, C.; Kong, X.; Li, P. Alamandine Attenuates Angiotensin II-Induced Vascular Fibrosis via Inhibiting P38 MAPK Pathway. Eur. J. Pharmacol. 2020, 883, 173384. [Google Scholar] [CrossRef]
- Hekmat, A.S.; Navabi, Z.; Alipanah, H.; Javanmardi, K. Alamandine Significantly Reduces Doxorubicin-Induced Cardiotoxicity in Rats. Hum. Exp. Toxicol. 2021, 40, 1781–1789. [Google Scholar] [CrossRef]
- Villela, D.C.; Passos-Silva, D.G.; Santos, R.A.S. Alamandine: A New Member of the Angiotensin Family: A New Member of the Angiotensin Family. Curr. Opin. Nephrol. Hypertens. 2014, 23, 130–134. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, J.G.; Xu, W.-T.; Ma, H.-X.; Jiang, K. Alamandine Protects against Renal Ischaemia-Reperfusion Injury in Rats via Inhibiting Oxidative Stress. J. Pharm. Pharmacol. 2021, 73, 1491–1502. [Google Scholar] [CrossRef]
- Angiotensin-(1-7) Cardiovascular Effects in Aging. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05301192?term=NCT05301192&draw=2&rank=1 (accessed on 3 March 2023).
- Angiotensin 1-7 in Obesity Hypertension. 2022. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03604289?term=NCT03604289&draw=2&rank=1 (accessed on 3 March 2023).
- Sampaio, W.; Nascimento, A.; Santos, R. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, 1985–1994. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Park, B.; Gao, S.; Kim, S. Stimulation of ANP by angiotensin-(1-9) via the angiotensin type 2 receptor. Life Sci. 2013, 93, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, M.; Xu, M.; Xu, Z.; Na, Y.; Zhang, N.; Geng, F. Simultaneous determination of nine trace concentration angiotensin peptides in human serum using ultra high performance liquid chromatography with tandem mass spectrometry with sephadex LH-20 gel solid-phase extraction. J. Sep. Sci. 2019, 42, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.; Lawrence, A.C.; Towrie, A.; Kladis, A.; Valentijn, A.J. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension 1991, 18, 763–773. [Google Scholar] [CrossRef] [PubMed]
- The Safety, Tolerability, PK and PD of GSK2586881 in Patients with Acute Lung Injury. 2014. Available online: https://ClinicalTrials.gov/show/NCT01597635 (accessed on 11 December 2023).
- Safety and Tolerability Study of APN01 (Recombinant Human Angiotensin Converting Enzyme 2). 2009. Available online: https://clinicaltrials.gov/study/NCT00886353?term=APN01&rank=1 (accessed on 11 December 2023).
- Effect of C21 on Forearm Blood Flow. 2022. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05277922 (accessed on 11 December 2023).
- Angiotensin-(1-7) in Peripheral Arterial Disease. 2 December 2022. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03240068?term=NCT03240068&draw=2&rank=1 (accessed on 3 March 2023).
- Wagener, G.; Goldklang, M.P.; Gerber, A.; Elisman, K.; Eiseman, K.A.; Fonseca, L.D.; D’Armiento, J.M. A Randomized, Placebo-Controlled, Double-Blinded Pilot Study of Angiotensin 1–7 (TXA-127) for the Treatment of Severe COVID-19. Crit. Care 2022, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- de Souza-Neto, F.P.; Silva, M.M.E.; Santuchi, M.C.; de Alcântara-Leonídio, T.C.; Motta-Santos, D.; Oliveira, A.C.; Melo, M.B.; Canta, G.N.; de Souza, L.E.; Irigoyen, M.C.C.; et al. Alamandine Attenuates Arterial Remodelling Induced by Transverse Aortic Constriction in Mice. Clin. Sci. 2019, 133, 629–643. [Google Scholar] [CrossRef]
- TXA127 in Non-Ambulant Patients with DMD Cardiomyopathy. 2023. Available online: https://clinicaltrials.gov/study/NCT06013839?intr=ang-(1-7)&rank=4#study-plan (accessed on 22 December 2023).
- Phase 2 Study of TXA127 in Post-Ischemic Stroke Patients. 2023. Available online: https://clinicaltrials.gov/study/NCT06135103?intr=ang-(1-7)&rank=7#study-plan (accessed on 21 December 2023).
- Marfella, R.; D’Onofrio, N.; Mansueto, G.; Grimaldi, V.; Trotta, M.C.; Sardu, C.; Sasso, F.C.; Scisciola, L.; Amarelli, C.; Esposito, S.; et al. Glycated ACE2 reduces anti-remodeling effects of renin-angiotensin system inhibition in human diabetic hearts. Cardiovasc. Diabetol. 2022, 21, 146. [Google Scholar] [CrossRef]
- Zhao, K.; Xu, T.; Mao, Y. Alamandine Alleviated Heart Failure and Fibrosis in Myocardial Infarction Mice. Biol. Direct 2022, 17, 25. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Kogan, M.J.; Lavandero, S.; Chiong, M. Angiotensin-(1-9) Analogue Based on D-Aminoacids, Pharmaceutical Compositions and Uses Thereof. Patent Application PCT/IB2021/054074, 13 May 2021. [Google Scholar]

| Peptide | Cardioprotective Effects |
|---|---|
| Angiotensin- (1-9) | Prevention of cardiac hypertrophy in myocardial infarcted rats [102]. |
| Attenuation of cardiac fibrosis in stroke-prone spontaneously hypertensive rats [96]. | |
| Cardioprotection against I/R injury via AT2R/Akt pathway in neonatal rat cardiomyocytes and isolated rat hearts [99]. | |
| Prevention of cardiomyocyte hypertrophy by regulating mitochondrial dynamics in neonatal rat cardiomyocytes [97]. | |
| Angiotensin- (1-7) | Promotion of vasodilation via the activation of the MasR and NO–soluble guanylyl cyclase pathway in spontaneously hypertensive rats [81]. |
| Decreasing fibrosis in resistant arterioles via DUSP1/MAP kinase/Smad/CTGF signaling in rats with Ang II-induced hypertension [82]. | |
| Reduction in cardiac fibrosis by inhibiting effect on KCa3.1 channels through ERK1/2 pathway, decreasing TGF-β1, and reducing collagen synthesis in mice treated with Ang II via osmotic mini-pumps [83]. | |
| Alamandine | Reduction in the accumulation of collagen and fibronectin in cardiac tissue of rats treated with isoproterenol [77,109]. |
| Reduction in the vascular expression of proinflammatory genes (CCL2, TNF-α, and IL-1β) in rats treated with doxorubicin [110]. | |
| Prevention of Ang II-induced hypertrophy through AMPK/NO via the MrgD in neonatal rat cardiomyocytes [75]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamiño-Gutiérrez, J.A.; Terán-Hernández, I.M.; Castellar-Lopez, J.; Villamizar-Villamizar, W.; Osorio-Llanes, E.; Palacios-Cruz, M.; Rosales, W.; Chang, A.Y.; Díaz-Ariza, L.A.; Ospino, M.C.; et al. Novel Insights into the Cardioprotective Effects of the Peptides of the Counter-Regulatory Renin–Angiotensin System. Biomedicines 2024, 12, 255. https://doi.org/10.3390/biomedicines12020255
Gamiño-Gutiérrez JA, Terán-Hernández IM, Castellar-Lopez J, Villamizar-Villamizar W, Osorio-Llanes E, Palacios-Cruz M, Rosales W, Chang AY, Díaz-Ariza LA, Ospino MC, et al. Novel Insights into the Cardioprotective Effects of the Peptides of the Counter-Regulatory Renin–Angiotensin System. Biomedicines. 2024; 12(2):255. https://doi.org/10.3390/biomedicines12020255
Chicago/Turabian StyleGamiño-Gutiérrez, Janette Alejandra, Ivana María Terán-Hernández, Jairo Castellar-Lopez, Wendy Villamizar-Villamizar, Estefanie Osorio-Llanes, Mariali Palacios-Cruz, Wendy Rosales, Aileen Y. Chang, Luis Antonio Díaz-Ariza, María Clara Ospino, and et al. 2024. "Novel Insights into the Cardioprotective Effects of the Peptides of the Counter-Regulatory Renin–Angiotensin System" Biomedicines 12, no. 2: 255. https://doi.org/10.3390/biomedicines12020255







