High Hopes for the Biofabrication of Articular Cartilage—What Lies beyond the Horizon of Tissue Engineering and 3D Bioprinting?
Abstract
1. Introduction—Complexity of Hyaline Cartilage and Unsatisfied Clinical Needs
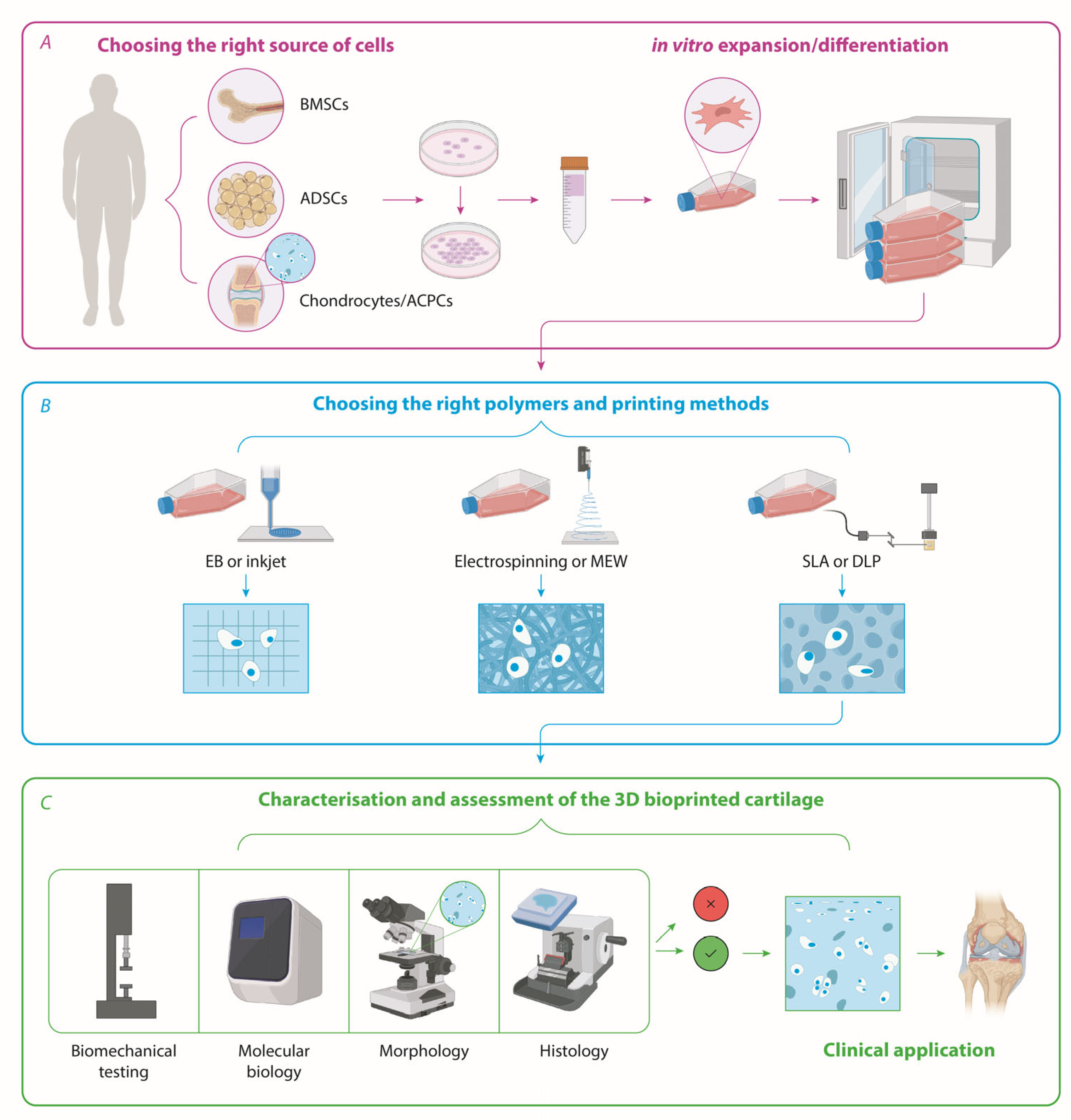
2. Sources of Patient-Derived Cells for Cartilage Tissue Engineering—Chondrocytes, iPSCs, Bone Marrow—Or Adipose-Derived Stem Cells?
2.1. Cartilage
2.2. Stem Cells—iPSCs, BMSCs, and ADSCs
3. The State of the Art in Bioprinting Technologies and Strategies for Cartilage Biofabrication
3.1. Have We Found the Best Natural and/or Synthetic Polymers for Cartilage Tissue Engineering?
3.2. Is It Possible to Engineer the Zonality, the Specific Orientation of Fibres, and the Mechanical Strength of Native Cartilage?
3.3. What Would the Best Type of Cells Be for Cartilage Reconstruction?
3.4. Are We Growing Biofabricated Implants in an Optimal Way?
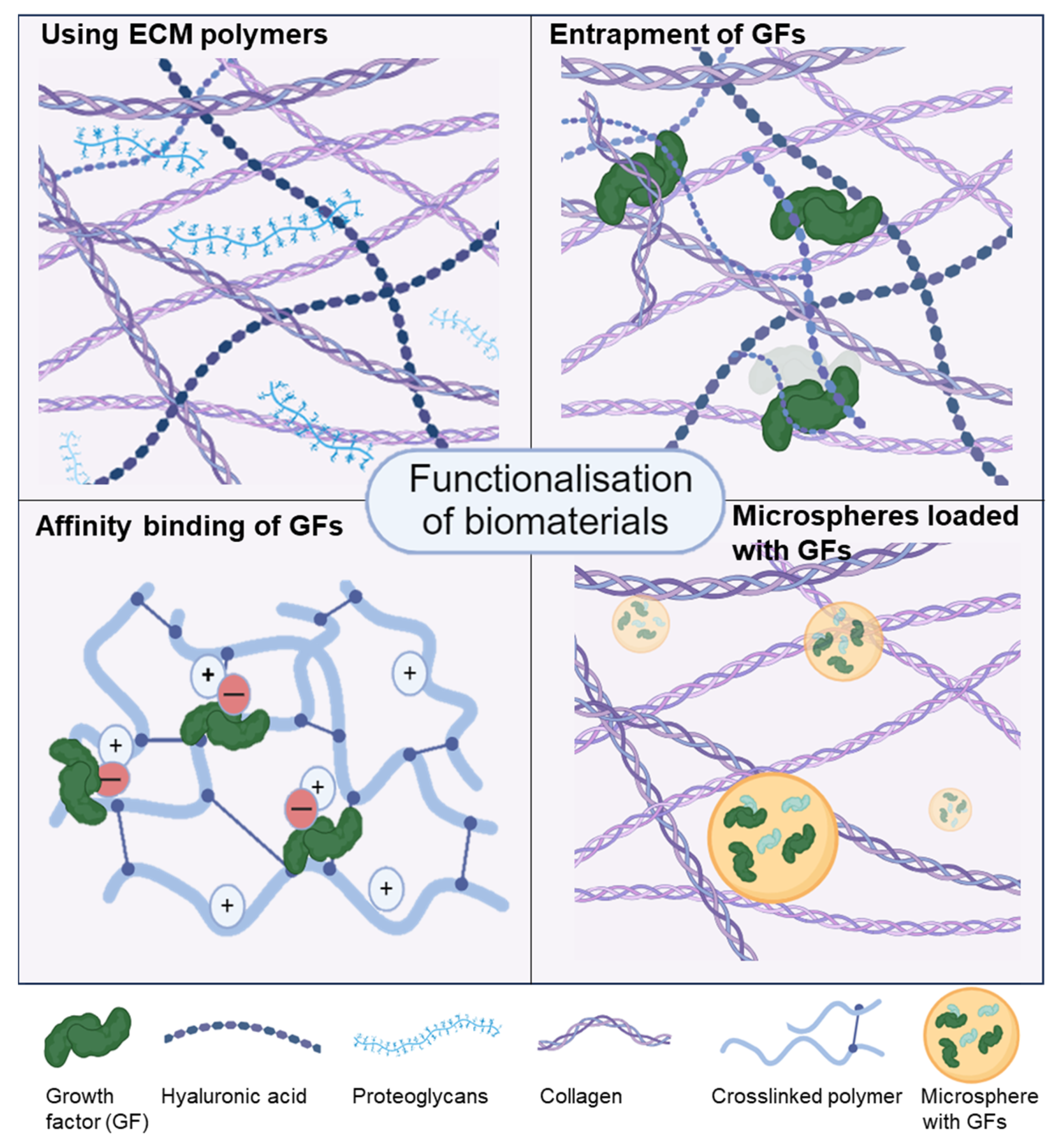
3.5. Functionalisation of Bioinks
4. Current Limitations in 3D Bioprinting of Cartilage and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Wang, G.; An, Y. Achieving Nasal Septal Cartilage In Situ Regeneration: Focus on Cartilage Progenitor Cells. Biomolecules 2023, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Gvaramia, D.; Kern, J.; Jakob, Y.; Zenobi-Wong, M.; Rotter, N. Regenerative Potential of Perichondrium: A Tissue Engineering Perspective. Tissue Eng. Part. B Rev. 2022, 28, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Acevedo Rua, L.; Mumme, M.; Manferdini, C.; Darwiche, S.; Khalil, A.; Hilpert, M.; Buchner, D.A.; Lisignoli, G.; Occhetta, P.; von Rechenberg, B.; et al. Engineered nasal cartilage for the repair of osteoarthritic knee cartilage defects. Sci. Transl. Med. 2021, 13, eaaz4499. [Google Scholar] [CrossRef]
- Seitz, A.M.; Dürselen, D.W.L. Chapter 9—Cartilage biomechanics. In Human Orthopaedic Biomechanics; Academic Press: Cambridge, MA, USA, 2022; pp. 151–176. [Google Scholar]
- Wang, W.; Ye, R.; Xie, W.; Zhang, Y.; An, S.; Li, Y.; Zhou, Y. Roles of the calcified cartilage layer and its tissue engineering reconstruction in osteoarthritis treatment. Front. Bioeng. Biotechnol. 2022, 10, 911281. [Google Scholar] [CrossRef] [PubMed]
- Volova, L.T.; Kotelnikov, G.P.; Shishkovsky, I.; Volov, D.B.; Ossina, N.; Ryabov, N.A.; Komyagin, A.V.; Kim, Y.H.; Alekseev, D.G. 3D Bioprinting of Hyaline Articular Cartilage: Biopolymers, Hydrogels, and Bioinks. Polymers 2023, 15, 2695. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Quinn, T.M.; Hauselmann, H.J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef]
- Duan, W.; Zhao, Y.; Ren, X.; Zhao, R.; Li, Q.; Sun, Z.; Song, W.; Yang, Y.; Li, P.; Wei, X. Combination of chondrocytes and chondrons improves extracellular matrix production to promote the repairs of defective knee cartilage in rabbits. J. Orthop. Transl. 2021, 28, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Lehoczky, G.; Wolf, F.; Mumme, M.; Gehmert, S.; Miot, S.; Haug, M.; Jakob, M.; Martin, I.; Barbero, A. Intra-individual comparison of human nasal chondrocytes and debrided knee chondrocytes: Relevance for engineering autologous cartilage grafts. Clin. Hemorheol. Microcirc. 2020, 74, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wixmerten, A.; Miot, S.; Bittorf, P.; Wolf, F.; Feliciano, S.; Hackenberg, S.; Hausner, S.; Krenger, W.; Haug, M.; Martin, I.; et al. Good Manufacturing Practice-compliant change of raw material in the manufacturing process of a clinically used advanced therapy medicinal product-a comparability study. Cytotherapy 2023, 25, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Bluguermann, C.; Kyupelyan, L.; Latour, B.; Gonzalez, S.; Shah, S.; Galic, Z.; Ge, S.; Zhu, Y.; Petrigliano, F.A.; et al. Human developmental chondrogenesis as a basis for engineering chondrocytes from pluripotent stem cells. Stem Cell Rep. 2013, 1, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lee, W.Y.W.; Feng, Q.; Xu, L.; Wang, B.; Man, G.C.W.; Chen, Y.; Jiang, X.; Bian, L.; Cui, L.; et al. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res. Ther. 2017, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A single day of TGF-beta1 exposure activates chondrogenic and hypertrophic differentiation pathways in bone marrow-derived stromal cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef]
- Yan, B.; Lv, S.; Tong, P.; Yan, L.; Chen, Z.; Zhou, L.; Yuan, Q.; Guo, L.; Shan, L. Intra-Articular Injection of Adipose-Derived Stem Cells Ameliorates Pain and Cartilage Anabolism/Catabolism in Osteoarthritis: Preclinical and Clinical Evidences. Front. Pharmacol. 2022, 13, 854025. [Google Scholar] [CrossRef]
- Meng, H.Y.; Lu, V.; Khan, W. Adipose Tissue-Derived Mesenchymal Stem Cells as a Potential Restorative Treatment for Cartilage Defects: A PRISMA Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1280. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Kartolo, W.A.; Lee, S.H. Cartilage Regeneration in Human with Adipose Tissue-Derived Stem Cells: Current Status in Clinical Implications. Biomed. Res. Int. 2016, 2016, 4702674. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, T.B.; Malda, J.; de Wijn, J.; Peters, F.; Riesle, J.; van Blitterswijk, C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Levato, R.; Bonassar, L.J.; D’Lima, D.D.; Grande, D.A.; Klein, T.J.; Saris, D.B.F.; Zenobi-Wong, M.; Gawlitta, D.; Malda, J. Three-Dimensional Bioprinting and Its Potential in the Field of Articular Cartilage Regeneration. Cartilage 2017, 8, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006, 12, 1325–1335. [Google Scholar] [CrossRef]
- Cui, X.; Gao, G.; Yonezawa, T.; Dai, G. Human cartilage tissue fabrication using three-dimensional inkjet printing technology. J. Vis. Exp. 2014, e51294. [Google Scholar] [CrossRef]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef]
- Kesti, M.; Muller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jimenez, G.; Chocarro, C.; Carrillo, E.; Montanez, E.; Galvez-Martin, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martinez Avila, H.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hagg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Go, C.S.; Ferreira, M.J.S.; Ligorio, C.; Kimber, S.J.; Dumanli, A.G.; Domingos, M.A.N. Nanocrystalline Cellulose as a Versatile Engineering Material for Extrusion-Based Bioprinting. Pharmaceutics 2023, 15, 2432. [Google Scholar] [CrossRef] [PubMed]
- Beketov, E.E.; Isaeva, E.V.; Yakovleva, N.D.; Demyashkin, G.A.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Kharlov, V.I.; Osidak, E.O.; et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021, 22, 11351. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, E.V.; Beketov, E.E.; Demyashkin, G.A.; Yakovleva, N.D.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Smirnova, A.N.; Petriev, V.M.; et al. Cartilage Formation In Vivo Using High Concentration Collagen-Based Bioink with MSC and Decellularized ECM Granules. Int. J. Mol. Sci. 2022, 23, 2703. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110578. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Goering, P.L.; Elespuru, R.K.; Sarkar Das, S.; Narayan, R.J. The Photoinitiator Lithium Phenyl (2,4,6-Trimethylbenzoyl) Phosphinate with Exposure to 405 nm Light Is Cytotoxic to Mammalian Cells but Not Mutagenic in Bacterial Reverse Mutation Assays. Polymers 2020, 12, 1489. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Rosenwasser, M.P.; Buckwalter, J.A.; Malinin, T.I.; Mow, V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef]
- Schinagl, R.M.; Gurskis, D.; Chen, A.C.; Sah, R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 1997, 15, 499–506. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; Francis, S.; O’Connell, C.D.; Caballero Aguilar, L.M.; Doyle, S.; Yue, Z.; Wallace, G.G.; Choong, P.F.; Di Bella, C. FLASH: Fluorescently LAbelled Sensitive Hydrogel to monitor bioscaffolds degradation during neocartilage generation. Biomaterials 2021, 264, 120383. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Abinzano, F.; Bernal, P.N.; Sanchez, A.A.; Atienza-Roca, P.; Otto, I.A.; Peiffer, Q.C.; Matsusaki, M.; Woodfield, T.B.F.; Malda, J.; et al. One-Step Photoactivation of a Dual-Functionalized Bioink as Cell Carrier and Cartilage-Binding Glue for Chondral Regeneration. Adv. Healthc. Mater. 2020, 9, 1901792. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, Y.G.; Fu, Q.W.; Hong, Y.P.; Li, L.X.; Cao, J.; Li, H.B.; Liu, Y.; Chen, Y.; Zhu, J.; et al. Application of tissue-derived bioink for articular cartilage lesion repair. Chem. Eng. J. 2022, 450, 138292. [Google Scholar] [CrossRef]
- Daly, A.C.; Kelly, D.J. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 2019, 197, 194–206. [Google Scholar] [CrossRef]
- Lam, T.; Dehne, T.; Kruger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Levato, R.; Mensinga, A.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect. Tissue Res. 2020, 61, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Shopperly, L.K.; Spinnen, J.; Kruger, J.P.; Endres, M.; Sittinger, M.; Lam, T.; Kloke, L.; Dehne, T. Blends of gelatin and hyaluronic acid stratified by stereolithographic bioprinting approximate cartilaginous matrix gradients. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2310–2322. [Google Scholar] [CrossRef]
- Dufour, A.; Gallostra, X.B.; O’Keeffe, C.; Eichholz, K.; Von Euw, S.; Garcia, O.; Kelly, D.J. Integrating melt electrowriting and inkjet bioprinting for engineering structurally organized articular cartilage. Biomaterials 2022, 283, 121405. [Google Scholar] [CrossRef]
- Castilho, M.; Mouser, V.; Chen, M.; Malda, J.; Ito, K. Bi-layered micro-fibre reinforced hydrogels for articular cartilage regeneration. Acta Biomater. 2019, 95, 297–306. [Google Scholar] [CrossRef]
- Mouser, V.H.; Abbadessa, A.; Levato, R.; Hennink, W.E.; Vermonden, T.; Gawlitta, D.; Malda, J. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication 2017, 9, 015026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, G.; Song, Y.; Xu, Y.; Zhao, S. 3D Bioprinted Integrated Osteochondral Scaffold-Mediated Repair of Articular Cartilage Defects in the Rabbit Knee. J. Med. Biol. Eng. 2020, 40, 71–81. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; You, Y.; Jiang, W.; Zhai, Z.; Dai, K. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics 2019, 9, 6949–6961. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef] [PubMed]
- Irmak, G.; Gumusderelioglu, M. Photo-activated platelet-rich plasma (PRP)-based patient-specific bio-ink for cartilage tissue engineering. Biomed. Mater. 2020, 15, 065010. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Shin, J.Y.; Shin, J.; Kim, B.S. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng. Part. A 2012, 18, 1949–1956. [Google Scholar] [CrossRef]
- Schmidt, O.; Mizrahi, J.; Elisseeff, J.; Seliktar, D. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnol. Bioeng. 2006, 95, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Muller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Diaz-Payno, P.J.; Browe, D.C.; Freeman, F.E.; Nulty, J.; Burdis, R.; Kelly, D.J. Affinity-bound growth factor within sulfated interpenetrating network bioinks for bioprinting cartilaginous tissues. Acta Biomater. 2021, 128, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Molander, D.; Sbirkov, Y.; Sarafian, V. 3D bioprinting as an emerging standard for cancer modeling and drug testing. Folia Med. 2022, 64, 559–565. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, C.; Forraz, N.; Milet, C.; Lacroix, M.; Sbirkov, Y.; Sarafian, V.; Ebel, C.; Spindler, A.; Koerper, V.; Balloul, J.M.; et al. World’s First Long-Term Colorectal Cancer Model by 3D Bioprinting as a Mechanism for Screening Oncolytic Viruses. Cancers 2023, 15, 4724. [Google Scholar] [CrossRef] [PubMed]
- Sbirkov, Y.; Molander, D.; Milet, C.; Bodurov, I.; Atanasov, B.; Penkov, R.; Belev, N.; Forraz, N.; McGuckin, C.; Sarafian, V. A Colorectal Cancer 3D Bioprinting Workflow as a Platform for Disease Modeling and Chemotherapeutic Screening. Front. Bioeng. Biotechnol. 2021, 9, 755563. [Google Scholar] [CrossRef] [PubMed]
- Barreiro Carpio, M.; Dabaghi, M.; Ungureanu, J.; Kolb, M.R.; Hirota, J.A.; Moran-Mirabal, J.M. 3D Bioprinting Strategies, Challenges, and Opportunities to Model the Lung Tissue Microenvironment and Its Function. Front. Bioeng. Biotechnol. 2021, 9, 773511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, W.; Kuss, M.; Mirza, S.; Qi, D.; Krasnoslobodtsev, A.; Zeng, J.; Band, H.; Band, V.; Duan, B. 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomater. Sci. Eng. 2018, 4, 4401–4411. [Google Scholar] [CrossRef]
- Gonzalez-Callejo, P.; Vazquez-Aristizabal, P.; Garcia-Astrain, C.; Jimenez de Aberasturi, D.; Henriksen-Lacey, M.; Izeta, A.; Liz-Marzan, L.M. 3D bioprinted breast tumor-stroma models for pre-clinical drug testing. Mater. Today Bio 2023, 23, 100826. [Google Scholar] [CrossRef]
- Neufeld, L.; Yeini, E.; Pozzi, S.; Satchi-Fainaro, R. 3D bioprinted cancer models: From basic biology to drug development. Nat. Rev. Cancer 2022, 22, 679–692. [Google Scholar] [CrossRef]
- Germain, N.; Dhayer, M.; Dekiouk, S.; Marchetti, P. Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine. Int. J. Mol. Sci. 2022, 23, 3432. [Google Scholar] [CrossRef]
- Shopova, D.; Yaneva, A.; Bakova, D.; Mihaylova, A.; Kasnakova, P.; Hristozova, M.; Sbirkov, Y.; Sarafian, V.; Semerdzhieva, M. (Bio)printing in Personalized Medicine-Opportunities and Potential Benefits. Bioengineering 2023, 10, 287. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Lanaro, M.; O’Loughlin, D.A.; D’Sa, R.A.; Woodruff, M.A.; Williams, R.L. Melt electro-written scaffolds with box-architecture support orthogonally oriented collagen. Biofabrication 2021, 14, 015015. [Google Scholar] [CrossRef]
- Chen, P.; Tao, J.; Zhu, S.; Cai, Y.; Mao, Q.; Yu, D.; Dai, J.; Ouyang, H. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials 2015, 39, 114–123. [Google Scholar] [CrossRef]
- Pattappa, G.; Zellner, J.; Johnstone, B.; Docheva, D.; Angele, P. Cells under pressure—The relationship between hydrostatic pressure and mesenchymal stem cell chondrogenesis. Eur. Cell Mater. 2019, 37, 360–381. [Google Scholar] [CrossRef]
- Kesti, M.; Fisch, P.; Pensalfini, M.; Mazza, E.; Zenobi-Wong, M. Guidelines for standardization of bioprinting: A systematic study of process parameters and their effect on bioprinted structures. BioNanoMaterials 2016, 17, 193–204. [Google Scholar] [CrossRef]
- Mladenovska, T.; Choong, P.F.; Wallace, G.G.; O’Connell, C.D. The regulatory challenge of 3D bioprinting. Regen. Med. 2023, 18, 659–674. [Google Scholar] [CrossRef] [PubMed]

| Type of 3DBP | Type of Cells | Type of Biomaterial Ink | Key Feature | Chondrocytes and Cartilage Assessment Histological Molecular Mechanical | Ref. | ||
|---|---|---|---|---|---|---|---|
| EB | Equine chondrocytes, ACPCs, BMSCs | 10% GelMA | Characterisation of combinations of three different cell types | Safranin O, IHC | Gene expression of chondrocyte and hypertrophy markers | Compression modulus of up to ~200 kPa at day 56 of culture | [11] |
| Inkjet | Human chondrocytes | PEGDA | Droplets of bioinks crosslinked by photopolymerisation | Yes (Safranin O) | no | no | [26] |
| EB | Human nasal septum chondrocytes | PCL/alginate | Functionalisation with TGFβ | Alcian blue, IHC | no | no | [28] |
| EB | Bovine chondrocytes | poly(N-isopropylacrylamide) hyaluronan (HA-pNIPAAM) and HAMA | Combination of thermosensitive and UV-crosslinkable polymers | no | no | no | [30] |
| EB | Primary human ADSCs (MSCs) | collagen type I (4%) and ECM granules (2.5%) | Rat dECM, human ADSCs cultured for 24 h before subcutaneous implantation in rats | Yes (Alcian blue, collagen IHC) | no | no | [36] |
| Inkjet and EB | Porcine chondrocytes and BMSCs | Droplets printed in PCL microchannels and GelMA | Guided spheroid formation; dynamic perfusion culture; parallel orientation of collagen fibres | Yes (Alcian blue, Safranin O, and collagen IHC) | Polarised light microscopy for collagen fibre orientation | in vitro tests (together with PCL scaffold) | [46] |
| EB | Equine chondrocytes, ACPCs, MSCs | 10% GelMA/gellan and GelMA/gellan/HAMA (0.5%) | Two zones with ACPCs and MSCs were printed; bioprinting interfered with chondrogenesis | Safranin O staining; IHC for ECM components | Gene expression of chondrocyte markers | no | [48] |
| EB | Equine chondrocytes | 0.5% HAMA—pHPMA-lac-PEG +/− PCL | Optimisation of concentrations according to matrix formation | Safranin O, GAG, and collagen IHC | no | in vitro Young’s modulus of 31 kPa or 4.6 MPa with PCL | [52] |
| EB | Rabbit BMSCs | 8% alginate, 5% gelatin with or without 4% HAP | Scoring of cartilage repair and mechanical properties | Wakitani scores | no | Maximum load and compressive strength (40% restoration) | [53] |
| EB | hUC-MSCs | 10% gelatin and 5% HAP | Knee joint lesion repair in pigs | Standard staining | Gene expression of chondrocyte markers | no | [54] |
| EB | Rabbit BMSCs | gelatin, fibrinogen, hyaluronic acid, and glycerol | Functionalisation with GDF5; printing together with PCL to make a scaffold | ICRS and Mankin scores | Gene expression of chondrocyte markers | in vitro tensile modulus and ultimate tensile strength (together with PCL) | [55] |
| EB | Rabit BMSCs | 4.5% gelatin, 3% fibrinogen, 0.3% HA, and 10% glycerol; TGFβ3 and BMP4 encapsulated in PLGA; PCL scaffold | PCL/hydrogel scaffold, functionalised with TGFβ3 and BMP4 microspheres, implanted in rabbit knee joints; achieving zonal distribution of ECM | ICRS and Mankin scores; Alcian blue, Safranin O staining; immunofluorescence for ECM and chondrocyte markers | Gene expression of chondrocyte markers | Compressive modulus of ~300 kPa 12 weeks post implantation (together with PCL) | [56] |
| EB | ATDC5 murine chondrogenic cell line | GelMA/PRP | Photoactivation of platelets; continuous release of growth factors | Safranin O, toluidine blue, IF for collagens | Gene expression of chondrocyte markers | Storage modulus (~40 kPa) | [57] |
| Light-based | Porcine chondrocytes | 5% GelMA or 1% HAMA | Optimisation of cell density 5–25 million/mL | Safranin O, toluidine blue, IF for collagens | Gene expression of chondrocyte markers | no | [47] |
| Light-based | Acellular; chondrocytes in vitro; local chondrocytes are attracted in vivo | 10% GelMa, 2% decellularised ECM, and BMSCs exosomes | Radial orientation of channels; exosome functionalisation | ICRS macroscopic and histological scores | Proteomics | no | [58] |
| Light-based | Human BMSCs | 10% GelMA, 5% PEGDA, TGF-β1 nanospheres | Mechanical strength with PEGDA and functionalisation with TGF-β1 | Safranin O and Alcian blue | Gene expression of chondrocyte markers | In vitro compressive modulus (~MPa at 5% PEGDA) | [59] |
| MEW | Equine chondrocytes | PCL scaffold; 10% GelMA | Tangential orientation of PCL fibres; growth under dynamic compression bioreactor | Safranin O, IHC | no | 12-fold increase in peak modulus under congruent loading when GelMA was reinforced (~500 kPa) | [51] |
| MEW and inkjet | Porcine BMSCs | PCL scaffold; droplets | Self-organisation of collagen fibres secreted by spheroids into zones | Alcian blue, Safranin O, IHC | no | Compressive modulus of ~400 kPa; equilibrium modulus of ~200 kPa, and dynamic modulus of ~2.6 MPa | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbirkov, Y.; Redzheb, M.; Forraz, N.; McGuckin, C.; Sarafian, V. High Hopes for the Biofabrication of Articular Cartilage—What Lies beyond the Horizon of Tissue Engineering and 3D Bioprinting? Biomedicines 2024, 12, 665. https://doi.org/10.3390/biomedicines12030665
Sbirkov Y, Redzheb M, Forraz N, McGuckin C, Sarafian V. High Hopes for the Biofabrication of Articular Cartilage—What Lies beyond the Horizon of Tissue Engineering and 3D Bioprinting? Biomedicines. 2024; 12(3):665. https://doi.org/10.3390/biomedicines12030665
Chicago/Turabian StyleSbirkov, Yordan, Murad Redzheb, Nico Forraz, Colin McGuckin, and Victoria Sarafian. 2024. "High Hopes for the Biofabrication of Articular Cartilage—What Lies beyond the Horizon of Tissue Engineering and 3D Bioprinting?" Biomedicines 12, no. 3: 665. https://doi.org/10.3390/biomedicines12030665
APA StyleSbirkov, Y., Redzheb, M., Forraz, N., McGuckin, C., & Sarafian, V. (2024). High Hopes for the Biofabrication of Articular Cartilage—What Lies beyond the Horizon of Tissue Engineering and 3D Bioprinting? Biomedicines, 12(3), 665. https://doi.org/10.3390/biomedicines12030665







