Performance of Radiological and Biochemical Biomarkers in Predicting Radio-Symptomatic Knee Osteoarthritis Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definition of Radiographic and Symptomatic Progression
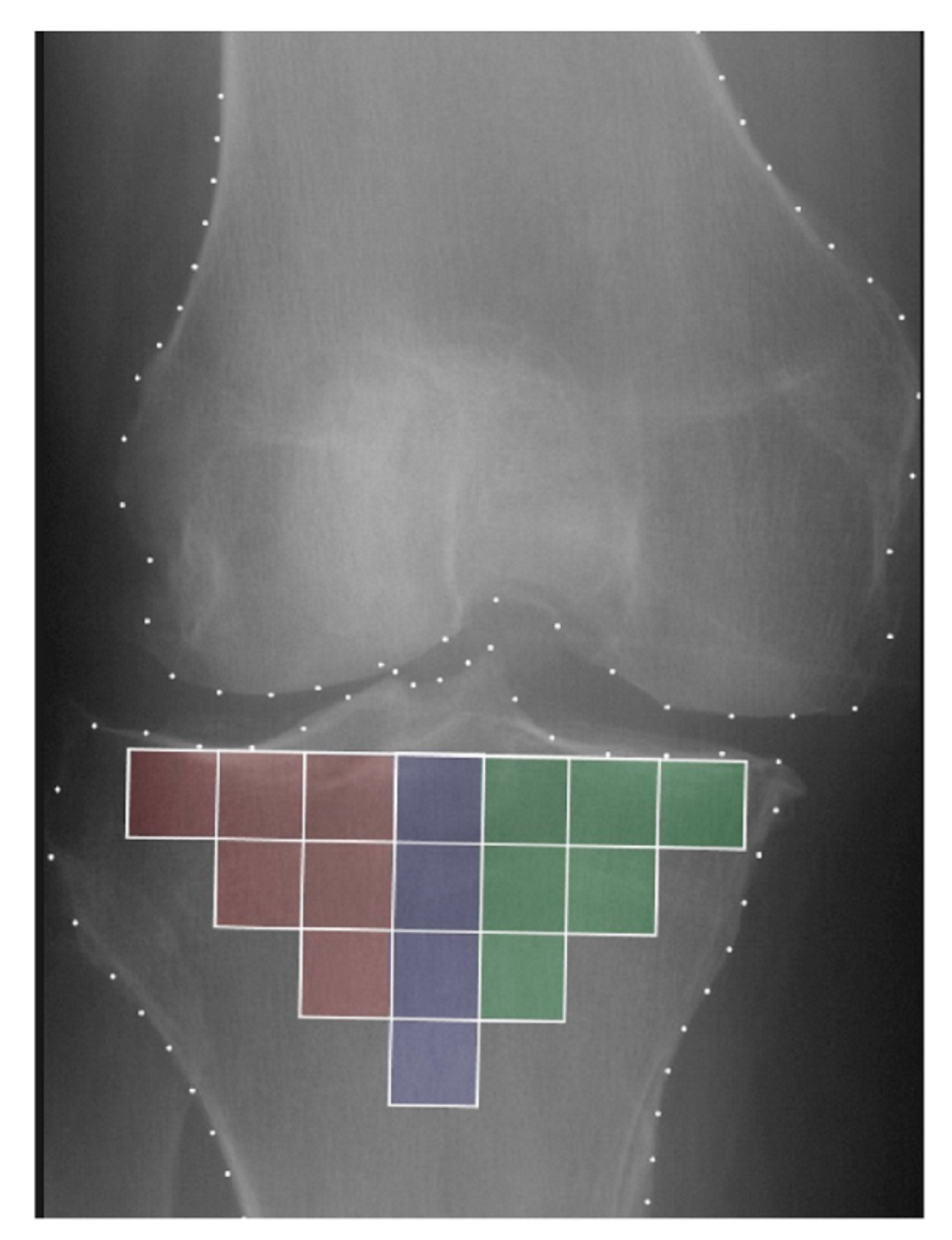
2.3. Trabecular Bone Texture Analysis
2.4. Biochemical Parameters
2.5. Prediction Models
- Model 1: TBT ← CLIN + KL + JSNM (Reference model)
- Model 2: ∆TBT ← CLIN + KL + JSNM
- Model 3: TBT + ∆TBT ← CLIN + KL + JSNM
- Model 4: TBT + ∆TBT
- Model 5: TBT + ∆TBT ← BIO + CLIN + KL + JSNM
- Model 6: TBTM + ∆TBTM ← CLIN + KL + JSNM
- Model 7: TBTL + ∆TBTL ← CLIN + KL + JSNM
- Model 8: TBTC + ∆TBTC ← CLIN + KL + JSNM
2.6. Statistical Analysis
- Scenario 1 evaluated the proposed models to predict any progression (knees with either radiographic or symptomatic progression, or both (Groups 1, 2, and 3; 397 progressors) compared to knees without any progression (Group 4; 200 controls).
- Scenario 2 evaluated the proposed models to predict all progression (knees with either radiographic or symptomatic progression (Groups 2 and 3; 205 progressors) compared to knees without any progression (Group 4; 200 controls).
- Scenario 3 evaluated the proposed models to predict radiographic progression (knees with radiographic-only progression (Group 2; 102 progressors) compared to knees without radiographic progression (Groups 3 and 4; 303 controls).
- Scenario 4 evaluated the proposed models to predict symptomatic progression (knees with symptomatic-only progression (Group 3; 102 progressors) compared to knees without radiographic progression (Groups 2 and 4; 303 controls).
3. Results
3.1. Primary Analysis: Radio-Symptomatic Progression
3.2. Secondary Analysis
3.2.1. Any Progression
3.2.2. All Progression
3.2.3. Radiographic Progression
3.2.4. Symptomatic Progression
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attur, M.; Krasnokutsky, S.; Zhou, H.; Samuels, J.; Chang, G.; Bencardino, J.; Rosenthal, P.; Rybak, L.; Huebner, J.L.; Kraus, V.B.; et al. The combination of an inflammatory peripheral blood gene expression and imaging biomarkers enhance prediction of radiographic progression in knee osteoarthritis. Arthritis Res. Ther. 2020, 22, 208. [Google Scholar] [CrossRef]
- Cai, G.; Otahal, P.; Cicuttini, F.; Wu, F.; Munugoda, I.P.; Jones, G.; Aitken, D. The association of subchondral and systemic bone mineral density with osteoarthritis-related joint replacements in older adults. Osteoarthr. Cartil. 2020, 28, 438–445. [Google Scholar] [CrossRef]
- Felson, D.T.; Gale, D.R.; Elon Gale, M.; Niu, J.; Hunter, D.J.; Goggins, J.; Lavalley, M.P. Osteophytes and progression of knee osteoarthritis. Rheumatol. Oxf. Engl. 2005, 44, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Almhdie-Imjabbar, A.; Podsiadlo, P.; Ljuhar, R.; Jennane, R.; Nguyen, K.-L.; Toumi, H.; Saarakkala, S.; Lespessailles, E. Trabecular bone texture analysis of conventional radiographs in the assessment of knee osteoarthritis: Review and viewpoint. Arthritis Res. Ther. 2021, 23, 208. [Google Scholar] [CrossRef]
- Woloszynski, T.; Podsiadlo, P.; Stachowiak, G.W.; Kurzynski, M.; Lohmander, L.S.; Englund, M. Prediction of progression of radiographic knee osteoarthritis using tibial trabecular bone texture. Arthritis Rheum. 2012, 64, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Almhdie-Imjabbar, A.; Toumi, H.; Harrar, K.; Pinti, A.; Lespessailles, E. Subchondral tibial bone texture of conventional X-rays predicts total knee arthroplasty. Sci. Rep. 2022, 12, 8327. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Collins, J.E.; Charles, H.C.; Pieper, C.F.; Whitley, L.; Losina, E.; Nevitt, M.; Hoffmann, S.; Roemer, F.; Guermazi, A.; et al. Predictive Validity of Radiographic Trabecular Bone Texture in Knee Osteoarthritis: The Osteoarthritis Research Society International/Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2018, 70, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Collins, J.E.; Hargrove, D.; Losina, E.; Nevitt, M.; Katz, J.N.; Wang, S.X.; Sandell, L.J.; Hoffmann, S.C.; Hunter, D.J.; et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: Data from the FNIH OA Biomarkers Consortium. Ann. Rheum. Dis. 2017, 76, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Janvier, T.; Jennane, R.; Toumi, H.; Lespessailles, E. Subchondral tibial bone texture predicts the incidence of radiographic knee osteoarthritis: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2017, 25, 2047–2054. [Google Scholar] [CrossRef]
- Janvier, T.; Jennane, R.; Valery, A.; Harrar, K.; Delplanque, M.; Lelong, C.; Loeuille, D.; Toumi, H.; Lespessailles, E. Subchondral tibial bone texture analysis predicts knee osteoarthritis progression: Data from the Osteoarthritis Initiative: Tibial bone texture & knee OA progression. Osteoarthr. Cartil. 2017, 25, 259–266. [Google Scholar]
- Almhdie-Imjabbar, A.; Nguyen, K.-L.; Toumi, H.; Jennane, R.; Lespessailles, E. Prediction of knee osteoarthritis progression using radiological descriptors obtained from bone texture analysis and Siamese neural networks: Data from OAI and MOST cohorts. Arthritis Res. Ther. 2022, 24, 66. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Manginelli, A.A.; Karsdal, M.; Luo, Y.; He, Y.; Michaelis, M.; Guehring, H.; Ladel, C. Low levels of type II collagen formation (PRO-C2) are associated with response to sprifermin: A pre-defined, exploratory biomarker analysis from the FORWARD study. Osteoarthr. Cartil. 2022, 30, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Bihlet, A.R.; Bjerre-Bastos, J.J.; Andersen, J.R.; Byrjalsen, I.; Karsdal, M.A.; Bay-Jensen, A.-C. Clinical and biochemical factors associated with risk of total joint replacement and radiographic progression in osteoarthritis: Data from two phase III clinical trials. Semin. Arthritis Rheum. 2020, 50, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Haque, N.; Huang, J.; Zhai, G. Osteoarthritis year in review 2023: Metabolite and protein biomarkers. Osteoarthr. Cartil. 2023, 31, 1437–1453. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.-L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef]
- Hosnijeh, F.S.; Runhaar, J.; van Meurs, J.B.J.; Bierma-Zeinstra, S.M. Biomarkers for osteoarthritis: Can they be used for risk assessment? A systematic review. Maturitas 2015, 82, 36–49. [Google Scholar] [CrossRef]
- Nelson, A.E.; Fang, F.; Arbeeva, L.; Cleveland, R.J.; Schwartz, T.A.; Callahan, L.F.; Marron, J.S.; Loeser, R.F. A machine learning approach to knee osteoarthritis phenotyping: Data from the FNIH Biomarkers Consortium. Osteoarthr. Cartil. 2019, 27, 994–1001. [Google Scholar] [CrossRef]
- Ying, G.-S.; Maguire, M.G.; Glynn, R.; Rosner, B. Tutorial on Biostatistics: Statistical Analysis for Correlated Binary Eye Data. Ophthalmic Epidemiol. 2018, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Collins, J.E.; Deveza, L.; Hoffmann, S.C.; Kraus, V.B. Biomarkers in osteoarthritis: Current status and outlook—The FNIH Biomarkers Consortium PROGRESS OA study. Skeletal Radiol. 2023, 52, 2323–2339. [Google Scholar] [CrossRef]
- Lynch, J.A.; Hawkes, D.J.; Buckland-Wright, J.C. A robust and accurate method for calculating the fractal signature of texture in macroradiographs of osteoarthritic knees. Med. Inform. Med. Inform. 1991, 16, 241–251. [Google Scholar] [CrossRef]
- Lee, C.Y. Nested logistic regression models and ΔAUC applications: Change-point analysis. Stat. Methods Med. Res. 2021, 30, 1654–1666. [Google Scholar] [CrossRef]
- Bradley, A.P. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Assessing the Fit of the Model. In Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Sharma, D.K.; Chatterjee, M.; Kaur, G.; Vavilala, S. 3-Deep learning applications for disease diagnosis. In Deep Learning for Medical Applications with Unique Data; Academic Press: New York, NY, USA, 2022; pp. 31–51. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Leyland, K.M.; Hart, D.J.; Javaid, M.K.; Judge, A.; Kiran, A.; Soni, A.; Goulston, L.M.; Cooper, C.; Spector, T.D.; Arden, N.K. The natural history of radiographic knee osteoarthritis: A fourteen-year population-based cohort study. Arthritis Rheum. 2012, 64, 2243–2251. [Google Scholar] [CrossRef]
- Hunter, D.J.; Deveza, L.A.; Collins, J.E.; Losina, E.; Nevitt, M.C.; Roemer, F.W.; Guermazi, A.; Bowes, M.A.; Dam, E.B.; Eckstein, F.; et al. Multivariable modeling of biomarker data from the phase 1 Foundation for the NIH Osteoarthritis Biomarkers Consortium. Arthritis Care Res. 2022, 74, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Almhdie-Imjabbar, A.; Toumi, H.; Lespessailles, E. Radiographic Biomarkers for Knee Osteoarthritis: A Narrative Review. Life 2023, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; van den Berg, W.B. Osteophytes: Relevance and biology. Osteoarthr. Cartil. 2007, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lourido, L.; Balboa-Barreiro, V.; Ruiz-Romero, C.; Rego-Pérez, I.; Camacho-Encina, M.; Paz-González, R.; Calamia, V.; Oreiro, N.; Nilsson, P.; Blanco, F.J. A clinical model including protein biomarkers predicts radiographic knee osteoarthritis: A prospective study using data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2021, 29, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Akalin, A. Computational Genomics with R: Logistic Regression and Regularization; CRC Press: New York, NY, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2023. [Google Scholar]
- Hirvasniemi, J.; Thevenot, J.; Immonen, V.; Liikavainio, T.; Pulkkinen, P.; Jämsä, T.; Arokoski, J.; Saarakkala, S. Quantification of differences in bone texture from plain radiographs in knees with and without osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- MacKay, J.; Kapoor, G.; Driban, J.; Lo, G.; McAlindon, T.; Toms, A.; McCaskie, A.; Gilbert, F. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: Data from the Osteoarthritis Initiative Bone Ancillary Study. Eur. Radiol. 2018, 28, 4687–4695. [Google Scholar] [CrossRef]
- Chang, G.H.; Park, L.K.; Le, N.A.; Jhun, R.S.; Surendran, T.; Lai, J.; Seo, H.; Promchotichai, N.; Yoon, G.; Scalera, J.; et al. Subchondral bone length in knee osteoarthritis: A deep learning derived imaging measure and its association with radiographic and clinical outcomes. Arthritis Rheumatol. 2021, 73, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; McLaughlin, S.; Goggins, J.; LaValley, M.P.; Gale, M.E.; Totterman, S.; Li, W.; Hill, C.; Gale, D. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann. Intern. Med. 2003, 139, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Widera, P.; Welsing, P.; Ladel, C.; Loughlin, J.; Lafeber, F.; Petit Dop, F.; Larkin, J.; Weinans, H.; Mobasheri, A.; Bacardit, J. Multi-classifier prediction of knee osteoarthritis progression from incomplete imbalanced longitudinal data. Sci. Rep. 2020, 10, 8427. [Google Scholar] [CrossRef] [PubMed]

| N° of Knees | Females (%) | Left Knee (%) | KL 1 = 1 (%) | KL = 2 (%) | KL = 3 (%) | |
|---|---|---|---|---|---|---|
| Complete dataset | 597 | 59.0 | 46.2 | 12.6 | 51.1 | 36.3 |
| Radio-symptomatic progressors | 192 | 56.8 | 48.4 | 12.5 | 43.2 | 44.3 |
| Radiographic progressors | 102 | 45.1 | 47.1 | 13.7 | 46.1 | 40.2 |
| Symptomatic progressors | 103 | 65.0 | 42.7 | 12.6 | 59.2 | 28.2 |
| Non-progressors | 200 | 65.0 | 45.5 | 12.0 | 57.0 | 31.0 |
| N° | Model | BACC | PPV | NPV | AUC (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Model 1 | TBT ← CLIN + KL + JSNM * | 0.55 | 0.50 | 0.70 | 0.613 (0.565–0.662) | - |
| Model 2 | ∆TBT ← CLIN + KL + JSNM | 0.53 | 0.45 | 0.70 | 0.606 (0.558–0.653) | 0.777 |
| Model 3 | TBT + ∆TBT ← CLIN + KL + JSNM | 0.59 | 0.52 | 0.73 | 0.650 (0.603–0.697) | 0.044 |
| Model 4 | TBT + ∆TBT | 0.58 | 0.51 | 0.72 | 0.658 (0.612–0.705) | 0.030 |
| Model 5 | TBT + ∆TBT ← BIO + CLIN + KL + JSNM | 0.59 | 0.52 | 0.73 | 0.649 (0.601–0.696) | 0.054 |
| Model 6 | TBTM + ∆TBTM ← CLIN + KL + JSNM | 0.53 | 0.52 | 0.69 | 0.594 (0.545–0.643) | 0.456 |
| Model 7 | TBTL + ∆TBTL ← CLIN + KL + JSNM | 0.51 | 0.46 | 0.68 | 0.577 (0.527–0.627) | 0.173 |
| Model 8 | TBTC + ∆TBTC ← CLIN + KL + JSNM | 0.51 | 0.46 | 0.68 | 0.572 (0.524–0.620) | 0.131 |
| Progression | Model | BACC | PPV | NPV | AUC (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Any (Scenario 1) | TBT ← CLIN + KL + JSNM * | 0.55 | 0.69 | 0.46 | 0.628 (0.582–0.675) | - |
| TBT + ∆TBT ← CLIN + KL + JSNM | 0.60 | 0.72 | 0.52 | 0.679 (0.634–0.724) | 0.009 | |
| TBT + ∆TBT ← BIO + CLIN + KL + JSNM | 0.60 | 0.72 | 0.52 | 0.678 (0.633–0.723) | 0.011 | |
| All (Scenario 2) | TBT ← CLIN + KL + JSNM * | 0.59 | 0.59 | 0.58 | 0.628 (0.574–0.682) | - |
| TBT + ∆TBT ← CLIN + KL + JSNM | 0.63 | 0.64 | 0.63 | 0.684 (0.632–0.736) | 0.022 | |
| TBT + ∆TBT ← BIO + CLIN + KL + JSNM | 0.63 | 0.64 | 0.62 | 0.684 (0.632–0.735) | 0.023 | |
| Radiographic (Scenario 3) | TBT ← CLIN + KL + JSNM * | 0.57 | 0.45 | 0.78 | 0.709 (0.653–0.765) | - |
| TBT + ∆TBT ← CLIN + KL + JSNM | 0.65 | 0.51 | 0.82 | 0.779 (0.731–0.827) | 0.012 | |
| TBT + ∆TBT ← BIO + CLIN + KL + JSNM | 0.65 | 0.51 | 0.82 | 0.779 (0.731–0.828) | 0.011 | |
| Symptomatic (Scenario 4) | TBT ← CLIN + KL + JSNM * | 0.53 | 0.40 | 0.76 | 0.643 (0.583–0.703) | - |
| TBT + ∆TBT ← CLIN + KL + JSNM | 0.60 | 0.49 | 0.79 | 0.710 (0.654–0.766) | 0.027 | |
| TBT + ∆TBT ← BIO + CLIN + KL + JSNM | 0.60 | 0.49 | 0.79 | 0.710 (0.654–0.766) | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almhdie-Imjabbar, A.; Toumi, H.; Lespessailles, E. Performance of Radiological and Biochemical Biomarkers in Predicting Radio-Symptomatic Knee Osteoarthritis Progression. Biomedicines 2024, 12, 666. https://doi.org/10.3390/biomedicines12030666
Almhdie-Imjabbar A, Toumi H, Lespessailles E. Performance of Radiological and Biochemical Biomarkers in Predicting Radio-Symptomatic Knee Osteoarthritis Progression. Biomedicines. 2024; 12(3):666. https://doi.org/10.3390/biomedicines12030666
Chicago/Turabian StyleAlmhdie-Imjabbar, Ahmad, Hechmi Toumi, and Eric Lespessailles. 2024. "Performance of Radiological and Biochemical Biomarkers in Predicting Radio-Symptomatic Knee Osteoarthritis Progression" Biomedicines 12, no. 3: 666. https://doi.org/10.3390/biomedicines12030666
APA StyleAlmhdie-Imjabbar, A., Toumi, H., & Lespessailles, E. (2024). Performance of Radiological and Biochemical Biomarkers in Predicting Radio-Symptomatic Knee Osteoarthritis Progression. Biomedicines, 12(3), 666. https://doi.org/10.3390/biomedicines12030666




