Antiviral Drug Candidate Repositioning for Streptococcus suis Infection in Non-Tumorigenic Cell Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Inhibitor Solutions for IPEC-J2, Microsomal, and Hepatocyte Assays
2.3. MIC Measurements
2.4. IPEC-J2 Cell Culture and Cytotoxicity Assay
2.5. Cytotoxicity Assays in Hepatocytes
2.6. CYP Enzyme Fluorometric Activity Measurements
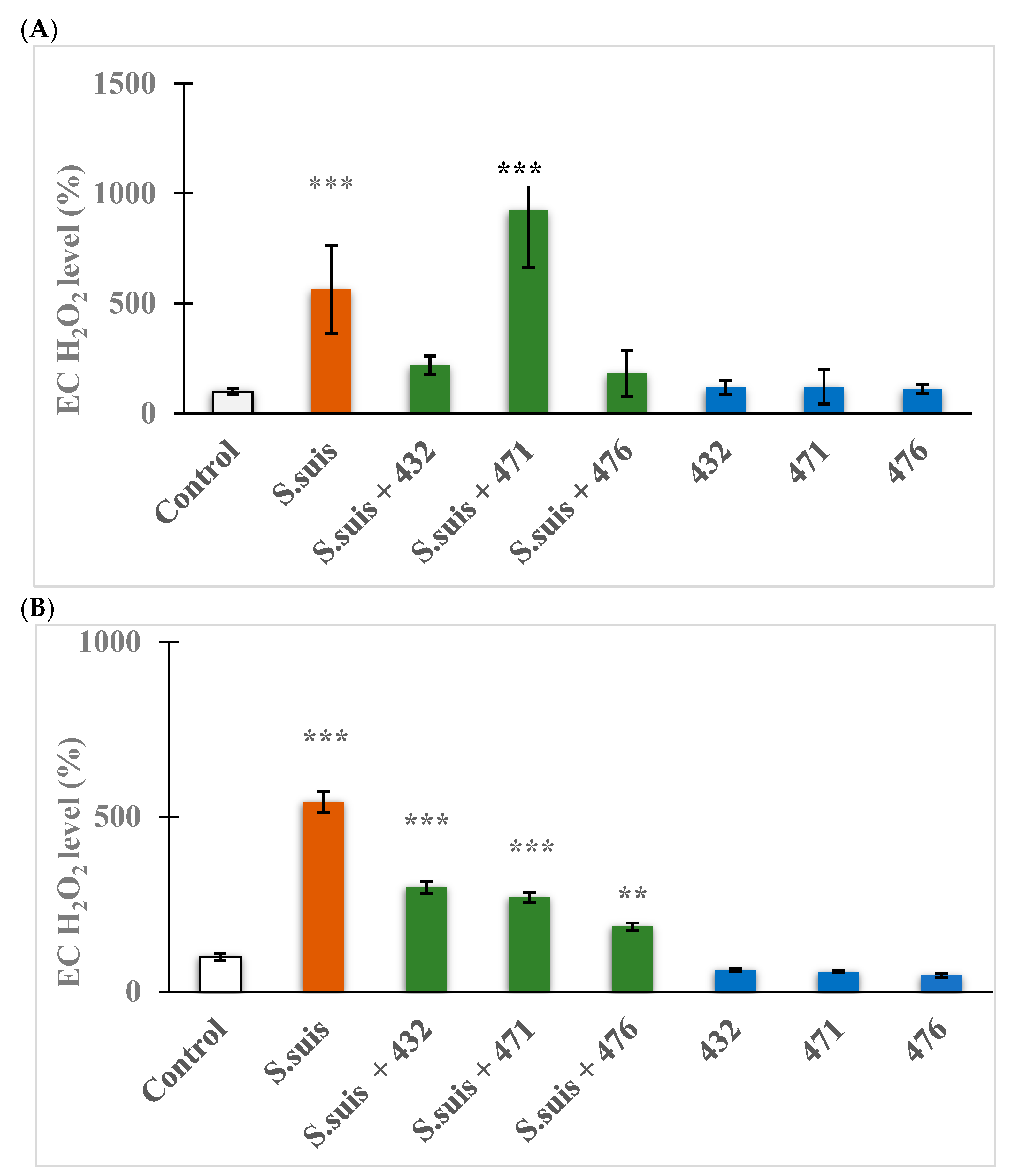
2.7. Examination of EC H₂O₂ Status
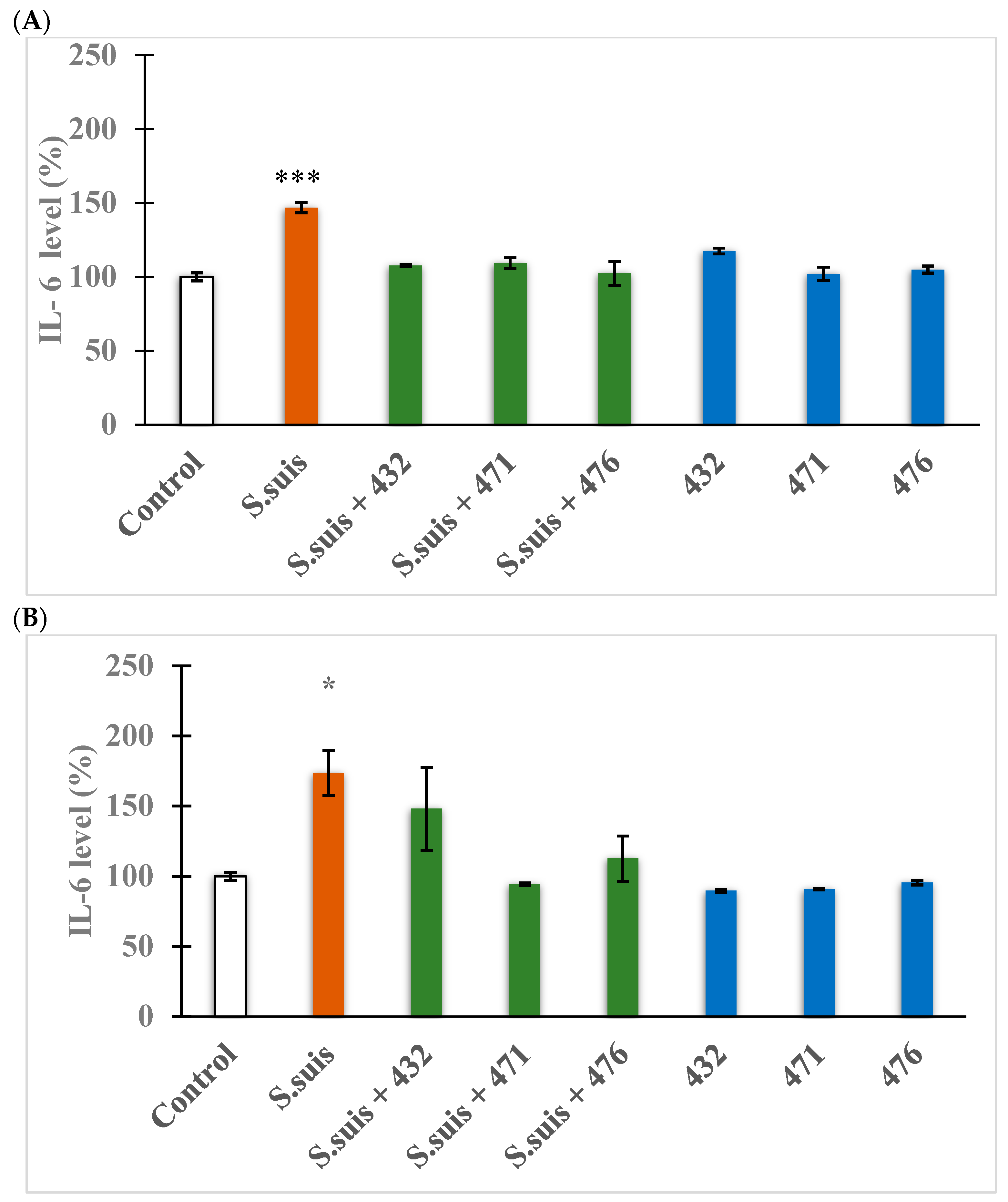
2.8. Evaluation of Proinflammatory Cytokine IL-6 Expression
2.9. Statistical Analysis
3. Results
3.1. Determination of MIC Values of Inhibitors
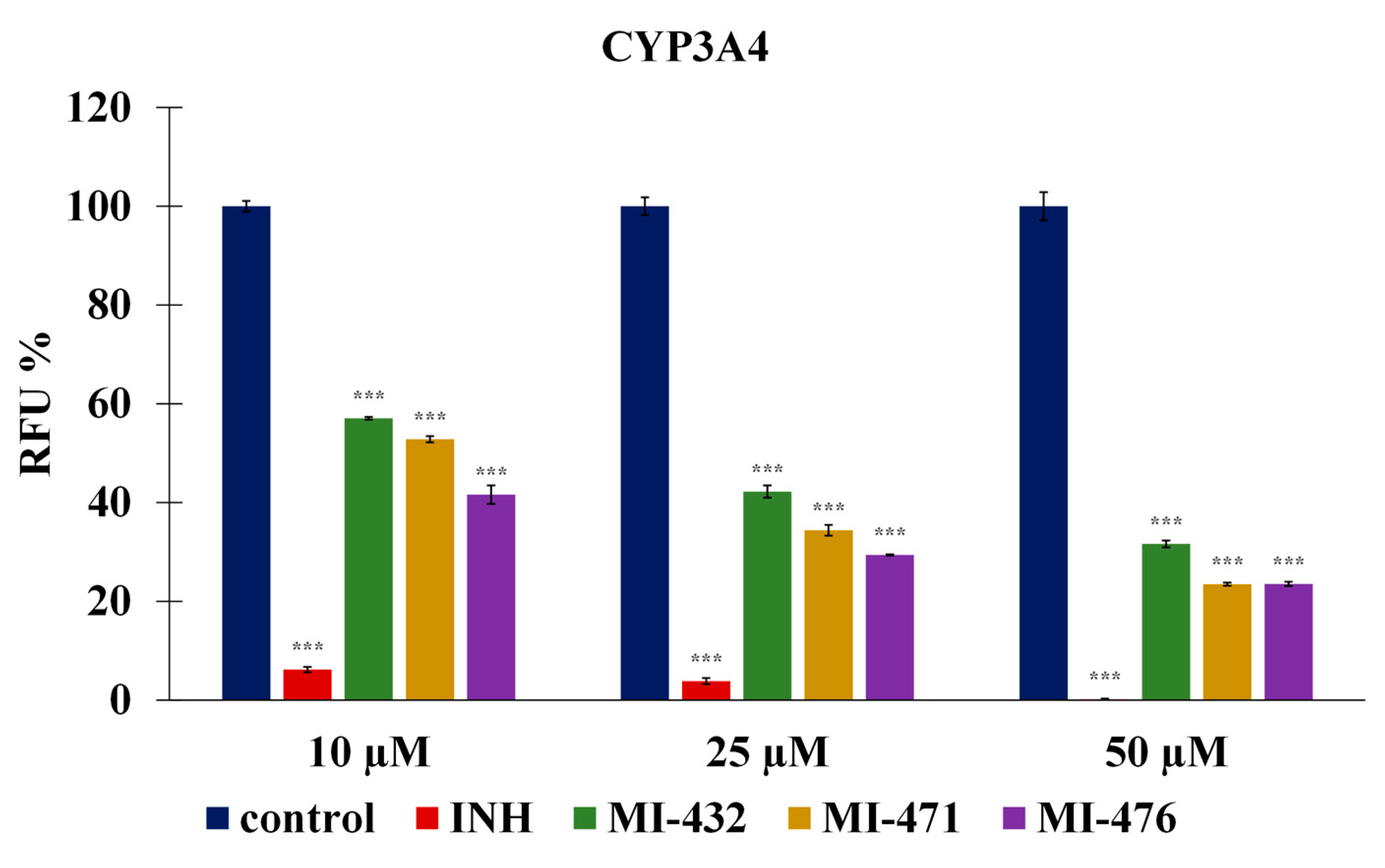
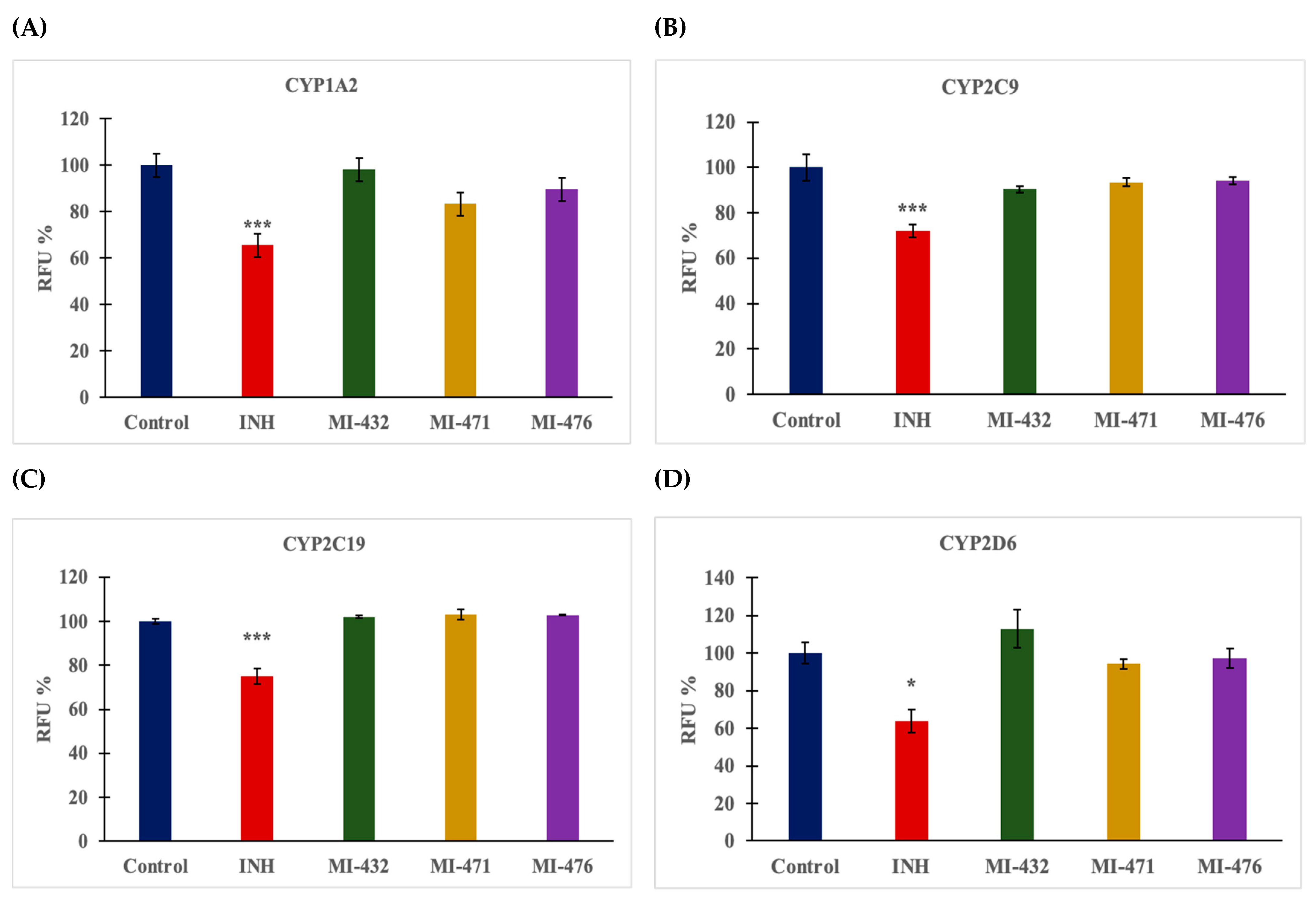
3.2. Influence on Human CYP3A4, 1A2, 2D6, 2C9, and 2C19 Activities
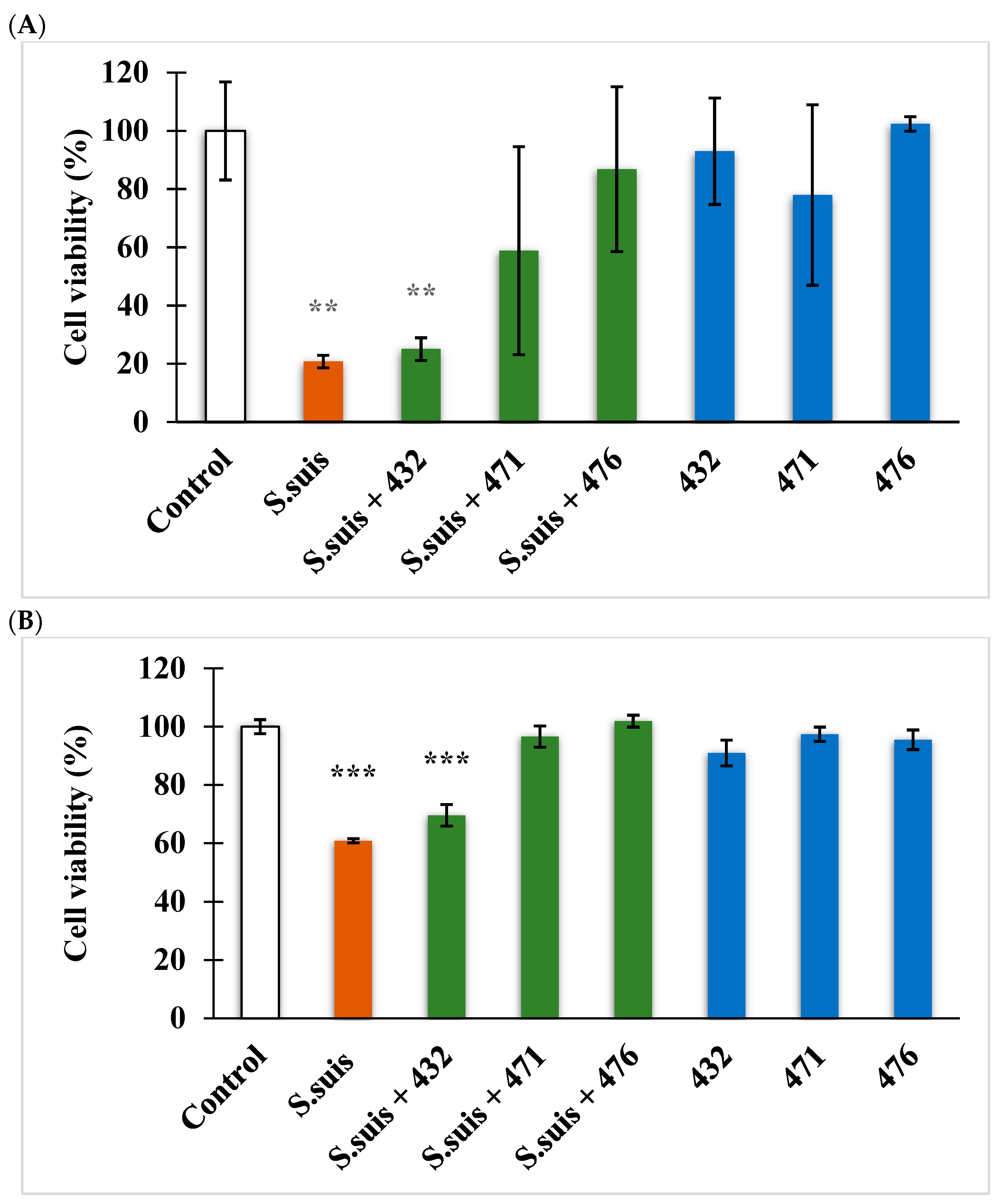
3.3. Cell Viability Assay
3.4. Determination of EC ROS Status
3.5. Changes in Proinflammatory Cytokine IL-6 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus Plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Q.; Yang, S.; Yang, B.; Khaliq, H.; Li, K.; Ahmed, S.; Sajid, A.; Zhang, B.; Chen, P.; et al. PK-PD Integration Modeling and Cutoff Value of Florfenicol against Streptococcus suis in Pigs. Front. Pharmacol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Lunha, K.; Chumpol, W.; Samngamnim, S.; Jiemsup, S.; Assavacheep, P.; Yongkiettrakul, S. Antimicrobial Susceptibility of Streptococcus suis Isolated from Diseased Pigs in Thailand, 2018–2020. Antibiotics 2022, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.-P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an Important Pig Pathogen and Emerging Zoonotic Agent—An Update on the Worldwide Distribution Based on Serotyping and Sequence Typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ning, Y.; Zhang, Z.; Song, L.; Qiu, H.; Gao, H. In Vitro Antimicrobial Susceptibility of Streptococcus suis Strains Isolated from Clinically Healthy Sows in China. Vet. Microbiol. 2008, 131, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Osaki, M.; Nomoto, R.; Arai, S.; Osawa, R.; Sekizaki, T.; Takamatsu, D. Current Taxonomical Situation of Streptococcus suis. Pathogens 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Tohya, M.; Sekizaki, T.; Miyoshi-Akiyama, T. Complete Genome Sequence of Streptococcus ruminantium sp. Nov. GUT-187T (=DSM 104980T =JCM 31869T), the Type Strain of S. ruminantium, and Comparison with Genome Sequences of Streptococcus suis Strains. Genome Biol. Evol. 2018, 10, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, Z.; Mag, P.; Simon, R.; Kerek, Á.; Makrai, L.; Biksi, I.; Jerzsele, Á. Susceptibility of Actinobacillus Pleuropneumoniae, Pasteurella Multocida and Streptococcus Suis Isolated from Pigs in Hungary between 2018 and 2021. Antibiotics 2023, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Calzas, C.; Grenier, D.; Gottschalk, M. Initial Steps of the Pathogenesis of the Infection Caused By Streptococcus suis: Fighting against Nonspecific Defenses. FEBS Lett. 2016, 590, 3772–3799. [Google Scholar] [CrossRef]
- Obradovic, M.R.; Segura, M.; Segalés, J.; Gottschalk, M. Review of the Speculative Role of Co-Infections in Streptococcus suis-Associated Diseases in Pigs. Vet. Res. 2021, 52, 49. [Google Scholar] [CrossRef]
- Gottschalk, M.; Xu, J.; Lecours, M.-P.; Grenier, D.; Fittipaldi, N.; Segura, M. Streptococcus suis Infections in Humans: What Is the Prognosis for Western Countries? (Part II). Clin. Microbiol. Newsl. 2010, 32, 97–102. [Google Scholar] [CrossRef]
- Takeuchi, D.; Kerdsin, A.; Pienpringam, A.; Loetthong, P.; Samerchea, S.; Luangsuk, P.; Khamisara, K.; Wongwan, N.; Areeratana, P.; Chiranairadul, P.; et al. Population-Based Study of Streptococcus suis Infection in Humans in Phayao Province in Northern Thailand. PLoS ONE 2012, 7, e31265. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.L.; Schultsz, C. A Hypothetical Model of Host-Pathogen Interaction of Streptococcus suis in the Gastro-Intestinal Tract. Gut Microbes 2016, 7, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Nghia, H.; Taylor, W.; Schultsz, C. Streptococcus suis: An Emerging Human Pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chao, Y.; Zhou, Y.; Zhou, R.; Zhang, W.; Fischetti, V.A.; Wang, X.; Feng, Y.; Li, J. The Global Emergence of a Novel Streptococcus suis Clade Associated with Human Infections. EMBO Mol. Med. 2021, 13, e13810. [Google Scholar] [CrossRef] [PubMed]
- Kerdsin, A.; Hatrongjit, R.; Gottschalk, M.; Takeuchi, D.; Hamada, S.; Akeda, Y.; Oishi, K. Emergence of Streptococcus suis Serotype 9 Infection in Humans. J. Microbiol. Immunol. Infect. 2017, 50, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.L.; De Greeff, A.; Van Rooijen, W.J.M.; Stockhofe-Zurwieden, N.; Nielsen, J.; Schreur, P.J.W.; Pannekoek, Y.; Heuvelink, A.; Van Der Ende, A.; Smith, H.; et al. Host-Pathogen Interaction at the Intestinal Mucosa Correlates with Zoonotic Potential of Streptococcus suis. J. Infect. Dis. 2014, 212, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Neila-Ibáñez, C.; Brogaard, L.; Pailler-García, L.; Martínez, J.; Segalés, J.; Segura, M.; Heegaard, P.M.H.; Aragon, V. Piglet Innate Immune Response to Streptococcus suis Colonization Is Modulated by the Virulence of the Strain. Vet. Res. 2021, 52, 145. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Gottschalk, M. Streptococcus suis Interactions with the Murine Macrophage Cell Line J774: Adhesion and Cytotoxicity. Infect. Immun. 2002, 70, 4312–4322. [Google Scholar] [CrossRef]
- de Jong, A.; Thomas, V.; Simjee, S.; Moyaert, H.; El Garch, F.; Maher, K.; Morrissey, I.; Butty, P.; Klein, U.; Marion, H.; et al. Antimicrobial Susceptibility Monitoring of Respiratory Tract Pathogens Isolated from Diseased Cattle and Pigs across Europe: The VetPath Study. Vet. Microbiol. 2014, 172, 202–215. [Google Scholar] [CrossRef]
- El Garch, F.; de Jong, A.; Simjee, S.; Moyaert, H.; Klein, U.; Ludwig, C.; Marion, H.; Haag-Diergarten, S.; Richard-Mazet, A.; Thomas, V.; et al. Monitoring of Antimicrobial Susceptibility of Respiratory Tract Pathogens Isolated from Diseased Cattle and Pigs across Europe, 2009–2012: VetPath Results. Vet. Microbiol. 2016, 194, 11–22. [Google Scholar] [CrossRef] [PubMed]
- van Hout, J.; Heuvelink, A.; Gonggrijp, M. Monitoring of Antimicrobial Susceptibility of Streptococcus suis in The Netherlands, 2013–2015. Vet. Microbiol. 2016, 194, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Cucco, L.; Paniccià, M.; Massacci, F.R.; Morelli, A.; Ancora, M.; Mangone, I.; Pasquale, A.D.; Luppi, A.; Vio, D.; Cammà, C.; et al. New Sequence Types and Antimicrobial Drug–Resistant Strains of Streptococcus suis in Diseased Pigs, Italy, 2017–2019. Emerg. Infect. Dis. 2022, 28, 139–147. [Google Scholar] [CrossRef]
- Tan, M.; Tan, J.; Zeng, Y.; Li, H.; Yang, Q.; Zhou, R. Antimicrobial Resistance Phenotypes and Genotypes of Streptococcus suis Isolated from Clinically Healthy Pigs from 2017 to 2019 in Jiangxi Province, China. J. Appl. Microbiol. 2020, 130, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Yongkiettrakul, S.; Maneerat, K.; Arechanajan, B.; Malila, Y.; Srimanote, P.; Gottschalk, M.; Visessanguan, W. Antimicrobial Susceptibility of Streptococcus suis Isolated from Diseased Pigs, Asymptomatic Pigs, and Human Patients in Thailand. BMC Vet. Res. 2019, 15, 5. [Google Scholar] [CrossRef]
- Tarnow, C.; Engels, G.; Arendt, A.; Schwalm, F.; Sediri, H.; Preuss, A.; Nelson, P.S.; Garten, W.; Klenk, H.D.; Gabriel, G.; et al. TMPRSS2 Is a Host Factor That Is Essential for Pneumotropism and Pathogenicity of H7N9 Influenza a Virus in Mice. J. Virol. 2014, 88, 4744–4751. [Google Scholar] [CrossRef]
- Garten, W.; Braden, C.; Arendt, A.; Peitsch, C.; Baron, J.; Lu, Y.; Pawletko, K.; Hardes, K.; Steinmetzer, T.; Böttcher-Friebertshäuser, E. Influenza Virus Activating Host Proteases: Identification, Localization and Inhibitors as Potential Therapeutics. Eur. J. Cell Biol. 2015, 94, 375–383. [Google Scholar] [CrossRef]
- Böttcher-Friebertshäuser, E.; Garten, W.; Klenk, H.D. Activation of Viruses by Host Proteases; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Mossel, E.C.; Wang, J.; Jeffers, S.; Edeen, K.E.; Wang, S.; Cosgrove, G.P.; Funk, C.J.; Manzer, R.; Miura, T.A.; Pearson, L.D.; et al. SARS-CoV Replicates in Primary Human Alveolar Type II Cell Cultures but Not in Type I-like Cells. Virology 2008, 372, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam Van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Pilgram, O.; Keils, A.; Benary, G.E.; Müller, J.; Merkl, S.; Ngaha, S.; Huber, S.; Chevillard, F.; Harbig, A.; Magdolen, V.; et al. Improving the Selectivity of 3-Amidinophenylalanine-Derived Matriptase Inhibitors. Eur. J. Med. Chem. 2022, 238, 114437. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A.; Clare, B.W. Bacterial Protease Inhibitors. Med. Res. Rev. 2002, 22, 329–372. [Google Scholar] [CrossRef]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D.A. Proteases and Protease Inhibitors in Infectious Diseases. Med. Res. Rev. 2017, 38, 1295–1331. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.M.d.S.; Segundo, V.H.d.O.; Aguiar, A.J.F.C.; Piuvezam, G.; Passos, T.S.; Damasceno, K.S.F.d.S.F.d.S.C.; Morais, A.H.d.A. Antibacterial Action Mechanisms and Mode of Trypsin Inhibitors: A Systematic Review. J. Enzym. Inhib. Med. Chem. 2022, 37, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Fedor, Z.; Szentkirályi-Tóth, A.; Nagy, G.; Szimrók, Z.; Varga, E.; Pászti, A.; Pászti, Z.; Jerzsele, Á.; Pilgram, O.; Steinmetzer, T.; et al. Interspecies Comparisons of the Effects of Potential Antiviral 3-Amidinophenylalanine Derivatives on Cytochrome P450 1A2 Isoenzyme. Vet. Sci. 2022, 9, 156. [Google Scholar] [CrossRef]
- Pászti-Gere, E.; Szentkirályi, A.; Fedor, Z.; Nagy, G.; Szimrók, Z.; Pászti, Z.; Pászti, A.; Pilgram, O.; Steinmetzer, T.; Bodnárová, S.; et al. In Vitro Interaction of Potential Antiviral TMPRSS2 Inhibitors with Human Serum Albumin and Cytochrome P 450 Isoenzymes. Biomed. Pharmacother. 2022, 146, 112513. [Google Scholar] [CrossRef] [PubMed]
- Schierack, P.; Nordhoff, M.; Pollmann, M.; Weyrauch, K.D.; Amasheh, S.; Lodemann, U.; Jores, J.; Tachu, B.; Kleta, S.; Blikslager, A.; et al. Characterization of a Porcine Intestinal Epithelial Cell Line for in Vitro Studies of Microbial Pathogenesis in Swine. Histochem. Cell Biol. 2006, 125, 293–305. [Google Scholar] [CrossRef]
- Ayuso, M.; Van Cruchten, S.; Van Ginneken, C. A Medium-Throughput system for in vitro oxidative stress assessment in IPEC-J2 cells. Int. J. Mol. Sci. 2020, 21, 7263. [Google Scholar] [CrossRef]
- Donato, M.T.; Jover, R.; Gomez-Lechon, M.J. Hepatic cell lines for drug hepatotoxicity testing: Limitations and strategies to upgrade their metabolic competence by gene engineering. Curr. Drug Metab. 2013, 14, 946–968. [Google Scholar] [CrossRef]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Skjolaas, K.A.; Burkey, T.E.; Dritz, S.S.; Minton, J.E. Effects of Salmonella enterica Serovar Typhimurium, or Serovar Choleraesuis, Lactobacillus Reuteri and Bacillus Licheniformis on Chemokine and Cytokine Expression in the Swine Jejunal Epithelial Cell Line, IPEC-J2. Vet. Immunol. Immunopathol. 2007, 115, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Devriendt, B.; Stuyven, E.; Verdonck, F.; Goddeeris, B.M.; Cox, E. Enterotoxigenic Escherichia coli (K88) Induce Proinflammatory Responses in Porcine Intestinal Epithelial Cells. Dev. Comp. Immunol. 2010, 34, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.L.; Vasquez, E.; Gebhart, C.J.; Isaacson, R.E. The Effects of Lawsonia intracellularis, Salmonella enterica Serovar Typhimurium and Co-Infection on IL-8 and TNFα Expression in IPEC-J2 Cells. Vet. Microbiol. 2019, 231, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Pézsa, N.P.; Kovács, D.; Rácz, B.; Farkas, O. Effects of Bacillus licheniformis and Bacillus subtilis on Gut Barrier Function, Proinflammatory Response, ROS Production and Pathogen Inhibition Properties in IPEC-J2—Escherichia coli/Salmonella Typhimurium Co-Culture. Microorganisms 2022, 10, 936. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.P.; Pavlovic, I.; Polijsak, B.; Suput, D.; Milisav, I. Beneficial role of ROS in cell survival: Moderate increases in H2O2 production induced by hepatocyte isolation mediate stress adaptation and enhances survival. Antioxidants 2019, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Jobin, M.C.; Grenier, D. Identification and Characterization of Four Proteases Produced by Streptococcus suis. FEMS Microbiol. Lett. 2003, 220, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zan, Y.; Zhang, Y.; Zheng, N.; Yan, Q.; Zhang, W.; Zhang, H.; Jin, M.; Chen, F.; Zhang, X.; et al. The Cysteine Protease ApdS from Streptococcus suis Promotes Evasion of Innate Immune Defenses by Cleaving the Antimicrobial Peptide Cathelicidin LL-37. J. Biol. Chem. 2019, 294, 17962–17977. [Google Scholar] [CrossRef]
- Seele, J.; Singpiel, A.; Spoerry, C.; von Pawel-Rammingen, U.; Valentin-Weigand, P.; Baums, C.G. Identification of a Novel Host-Specific IgM Protease in Streptococcus suis. J. Bacteriol. 2013, 195, 930–940. [Google Scholar] [CrossRef]
- Eick, S.; Pfister, W.; Stürzebecher, U.; Jarema, S.; Stürzebecher, J. Inhibitors of Benzamidine Type Influence the Virulence Properties of Porphyromonas Gingivalis Strains. Acta Biochim. Pol. 2003, 50, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.; Rühmann, E.; Maurer, E.; Heine, A.; Gütschow, M.; Klebe, G.; Steinmetzer, T. New 3-amidinophenylalanine-derived inhibitors of matriptase. MedChemComm 2012, 3, 807–813. [Google Scholar] [CrossRef]
| Strains | Sources | MIC (µM) of Phe(3-Am)-Derived Protease Inhibitors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 432 | 463 | 471 | 472 | 476 | 477 | 485 | 490 | 1903 | 1904 | ||
| P. aeruginosa | ATCC | . | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 |
| E. coli | ATCC | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 |
| S. enterica | 204/22 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 |
| P. multocida | 380/22 | 50 | >800 | 50 | 400 | 50 | 400 | >800 | 800 | 800 | 800 |
| S. aureus | ATCC | 50 | >800 | 100 | 800 | 100 | 800 | 200 | 800 | 800 | 800 |
| S. suis | 672/22 | <1.5625 | >800 | 3.125 | 50 | 6.25 | 50 | 50 | 400 | 200 | 200 |
| B. cepacia | 20-10299 | 800 | >800 | 400 | 800 | 400 | 800 | 800 | 800 | 800 | 400 |
| E. fecalis | 442 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 | >800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Niekerk, A.A.; Maluck, S.; Mag, P.; Kővágó, C.; Kerek, Á.; Jerzsele, Á.; Steinmetzer, T.; Pászti-Gere, E. Antiviral Drug Candidate Repositioning for Streptococcus suis Infection in Non-Tumorigenic Cell Models. Biomedicines 2024, 12, 783. https://doi.org/10.3390/biomedicines12040783
van Niekerk AA, Maluck S, Mag P, Kővágó C, Kerek Á, Jerzsele Á, Steinmetzer T, Pászti-Gere E. Antiviral Drug Candidate Repositioning for Streptococcus suis Infection in Non-Tumorigenic Cell Models. Biomedicines. 2024; 12(4):783. https://doi.org/10.3390/biomedicines12040783
Chicago/Turabian Stylevan Niekerk, Ashley Anzet, Sara Maluck, Patrik Mag, Csaba Kővágó, Ádám Kerek, Ákos Jerzsele, Torsten Steinmetzer, and Erzsébet Pászti-Gere. 2024. "Antiviral Drug Candidate Repositioning for Streptococcus suis Infection in Non-Tumorigenic Cell Models" Biomedicines 12, no. 4: 783. https://doi.org/10.3390/biomedicines12040783
APA Stylevan Niekerk, A. A., Maluck, S., Mag, P., Kővágó, C., Kerek, Á., Jerzsele, Á., Steinmetzer, T., & Pászti-Gere, E. (2024). Antiviral Drug Candidate Repositioning for Streptococcus suis Infection in Non-Tumorigenic Cell Models. Biomedicines, 12(4), 783. https://doi.org/10.3390/biomedicines12040783







