Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights
Abstract
1. Introduction
2. Methods
3. Osteoarthritis
3.1. Epidemiology
3.2. Current Topics of Lower-Extremity Osteoarthritis Treatment
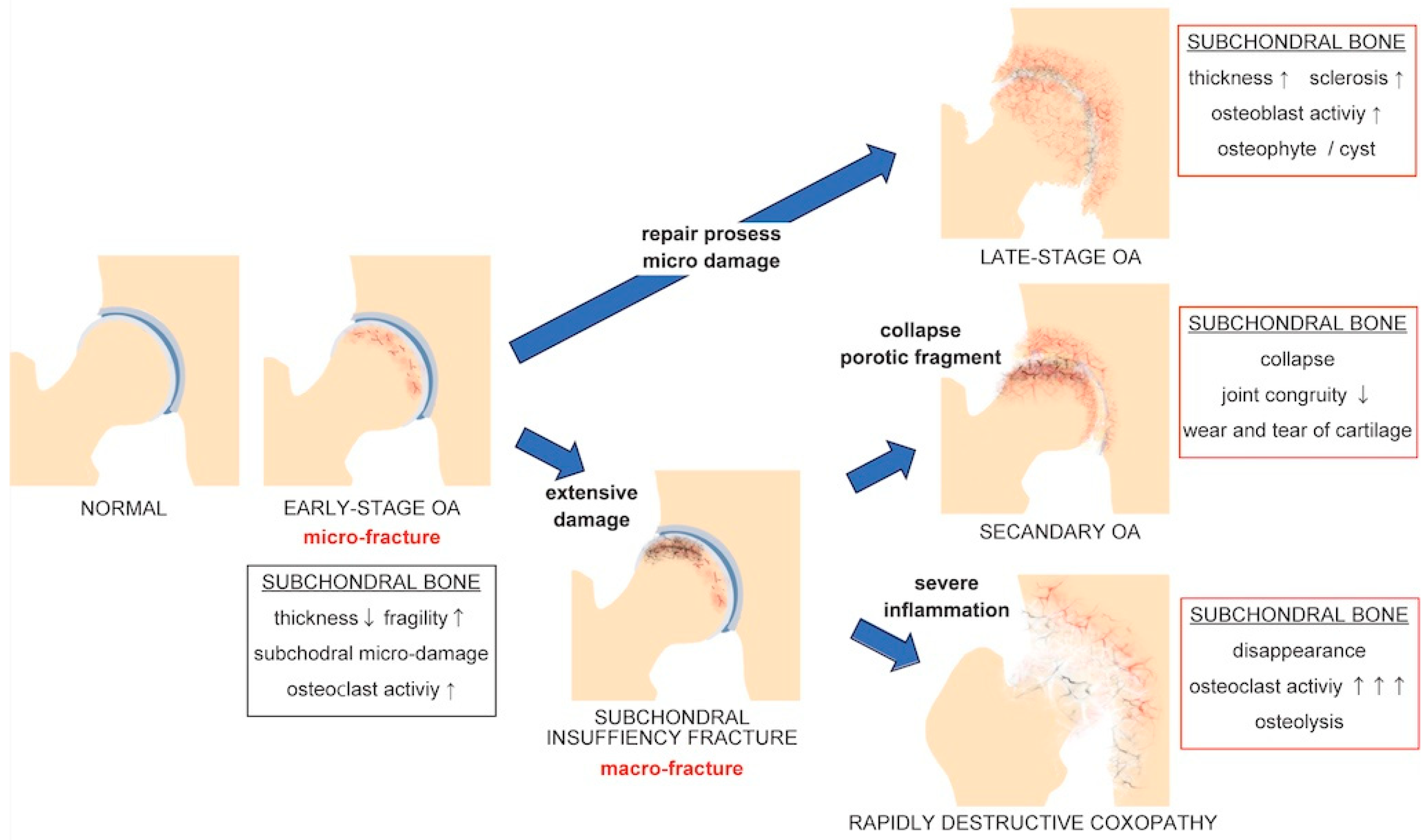
3.3. Subchondral Microenvironment of Osteoarthritis
4. Osteoporosis
4.1. Epidemiology
4.2. Diagnosis
4.3. Treatment
4.4. Pathogenesis
5. Relationship between Osteoarthritis and Osteoporosis
5.1. High Bone Mineral Density and Osteoarthritis
5.2. Low Bone Mineral Density and Osteoarthritis
5.3. Osteoporotic Fracture and Osteoarthritis
5.4. Osteoporosis Treatments and Osteoarthritis
6. Subchondral Insufficiency Fracture of the Knee
6.1. Epidemiology
6.2. Diagnosis and Progression
6.3. Treatment
6.4. Risk Factors and Pathogenesis
7. Subchondral Insufficiency Fracture of Femoral Head
7.1. Epidemiology
7.2. Diagnosis and Progression
7.3. Treatment
7.4. Risk Factor and Pathogenesis
8. Discussion
9. Conclusions
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kinter, M.; Hudson, J.; Humphries, K.M.; Lane, R.S.; White, J.R.; Hakim, M.; Pan, Y.; Verdin, E.; Griffin, T.M. Aging Promotes Sirtuin 3-Dependent Cartilage Superoxide Dismutase 2 Acetylation and Osteoarthritis. Arthritis Rheumatol. 2016, 68, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Katzir, I.; Adler, M.; Karin, O.; Mendelsohn-Cohen, N.; Mayo, A.; Alon, U. Senescent cells and the incidence of age-related diseases. Aging Cell 2021, 20, e13314. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wong, P.; Guo, C.; Tam, L.S.; Gu, J. Pattern and trend of five major musculoskeletal disorders in China from 1990 to 2017: Findings from the Global Burden of Disease Study 2017. BMC Med. 2021, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, D.; Zhang, H.; Liang, J.; Feng, X.; Zhao, J.; Sun, L. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: Results from the global burden of disease study 2017. Ann. Rheum. Dis. 2020, 79, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Hannon, C.P.; Fillingham, Y.A.; Gililland, J.M.; Sporer, S.M.; Casambre, F.D.; Verity, T.J.; Woznica, A.; Nelson, N.; Hamilton, W.G.; Della Valle, C.J. A Systematic Review of the Efficacy and Safety of Ketamine in Total Joint Arthroplasty. J. Arthroplast. 2023, 38, 763–768.e2. [Google Scholar] [CrossRef]
- Nogalo, C.; Meena, A.; Abermann, E.; Fink, C. Complications and downsides of the robotic total knee arthroplasty: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.A.; Abudalou, A.; Khatkar, H.; Ahmed, G.; Dakin, S.G.; Kendrick, B.; Murray, D.W. Impact of age on unicompartmental knee arthroplasty outcomes: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Vera-Garcia, D.V.; Nugen, F.; Padash, S.; Khosravi, B.; Mickley, J.P.; Erickson, B.J.; Wyles, C.C.; Taunton, M.J. Educational Overview of the Concept and Application of Computer Vision in Arthroplasty. J. Arthroplast. 2023, 38, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.B.T.; Somerville, L.; Vasarhelyi, E.M.; Howard, J.L.; Naudie, D.D.R.; McCalden, R.W. Minimum 5-Year Clinical Outcomes and Survivorship for a Single Revision Total Knee Arthroplasty System Using Hybrid Fixation and Press-Fit Stems. J. Arthroplast. 2023, 38, S297–S301. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Hwang, K.L.; Amanatullah, D.F.; Huddleston, J.I., 3rd; Maloney, W.J.; Goodman, S.B. Revision Hip Arthroplasty Using a Modular, Cementless Femoral Stem: Long-Term Follow-Up. J. Arthroplast. 2023, 38, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.W.; Huizinga, M.R.; Duivenvoorden, T.; van Raaij, T.M.; Verhagen, A.P.; Bierma-Zeinstra, S.M.; Verhaar, J.A. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst. Rev. 2014, 2014, Cd004019. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Dandu, N.; Sonnier, J.H.; Rao, S.; Holston, K.; Liu, J.; Freedman, K.; Tjoumakaris, F. The Influence of Psychosocial Factors on Hip Surgical Disorders and Outcomes After Hip Arthroscopy: A Systematic Review. Arthroscopy 2022, 38, 3194–3206. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Yamada, K.; Takahashi, K.; Enomoto, H.; Osaki, M.; Shindo, H. Joint congruency in abduction before surgery as an indication for rotational acetabular osteotomy in early hip osteoarthritis. Int. Orthop. 2010, 34, 27–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasunaga, Y.; Yamasaki, T.; Ochi, M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin. Orthop. Relat. Res. 2012, 470, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Miltenberg, B.; Puzzitiello, R.N.; Ruelos, V.C.B.; Masood, R.; Pagani, N.R.; Moverman, M.A.; Menendez, M.E.; Ryan, S.P.; Salzler, M.J.; Drager, J. Incidence of Complications and Revision Surgery After High Tibial Osteotomy: A Systematic Review. Am. J. Sports Med. 2024, 52, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, T.; Nethander, M.; Moverare-Skrtic, S.; Richette, P.; Ohlsson, C. Causal Factors for Knee, Hip, and Hand Osteoarthritis: A Mendelian Randomization Study in the UK Biobank. Arthritis Rheumatol. 2019, 71, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Cronin, C.; Daniels, M.; Worthy, T.; Doyle, D.V.; Spector, T.D. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: The Chingford Study. Arthritis Rheum. 2002, 46, 92–99. [Google Scholar] [CrossRef]
- Nevitt, M.C.; Felson, D.T. High bone density and radiographic osteoarthritis: Questions answered and unanswered. Osteoarthr. Cartil. 2020, 28, 1151–1153. [Google Scholar] [CrossRef]
- Goerres, G.W.; Hauselmann, H.J.; Seifert, B.; Michel, B.A.; Uebelhart, D. Patients with knee osteoarthritis have lower total hip bone mineral density in the symptomatic leg than in the contralateral hip. J. Clin. Densitom. 2005, 8, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Kostev, K. Osteoarthritis and the incidence of fracture in the United Kingdom: A retrospective cohort study of 258,696 patients. Osteoarthr. Cartil. 2021, 29, 215–221. [Google Scholar] [CrossRef]
- Stamenkovic, B.N.; Rancic, N.K.; Bojanovic, M.R.; Stojanovic, S.K.; Zivkovic, V.G.; Djordjevic, D.B.; Stankovic, A.M. Is Osteoarthritis Always Associated with Low Bone Mineral Density in Elderly Patients? Medicina 2022, 58, 1207. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.O. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Noh, J.H.; Kim, D.J. The prevalence of and demographic factors associated with radiographic knee osteoarthritis in Korean adults aged >/=50 years: The 2010–2013 Korea National Health and Nutrition Examination Survey. PLoS ONE 2020, 15, e0230613. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, S.; Chen, Q.; Xie, X. The Prevalence of Symptomatic Knee Osteoarthritis in Relation to Age, Sex, Area, Region, and Body Mass Index in China: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 304. [Google Scholar] [CrossRef]
- Kim, C.; Linsenmeyer, K.D.; Vlad, S.C.; Guermazi, A.; Clancy, M.M.; Niu, J.; Felson, D.T. Prevalence of radiographic and symptomatic hip osteoarthritis in an urban United States community: The Framingham osteoarthritis study. Arthritis Rheumatol. 2014, 66, 3013–3017. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, L.; Liu, H.; Li, X.; Fan, K.; Liu, Q.; Li, J.J.; Wang, B. The prevalence of hip osteoarthritis: A systematic review and meta-analysis. Arthritis Res. Ther. 2023, 25, 51. [Google Scholar] [CrossRef]
- Iidaka, T.; Muraki, S.; Oka, H.; Horii, C.; Kawaguchi, H.; Nakamura, K.; Akune, T.; Tanaka, S.; Yoshimura, N. Incidence rate and risk factors for radiographic hip osteoarthritis in Japanese men and women: A 10-year follow-up of the ROAD study. Osteoarthr. Cartil. 2020, 28, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.F.; Cleveland, R.J.; Allen, K.D.; Golightly, Y. Racial/Ethnic, Socioeconomic, and Geographic Disparities in the Epidemiology of Knee and Hip Osteoarthritis. Rheum. Dis. Clin. N. Am. 2021, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Canetti, E.F.D.; Schram, B.; Orr, R.M.; Knapik, J.; Pope, R. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: A systematic review and meta-analysis. Appl. Ergon. 2020, 86, 103097. [Google Scholar] [CrossRef] [PubMed]
- Schram, B.; Orr, R.; Pope, R.; Canetti, E.; Knapik, J. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: A narrative umbrella review. J. Occup. Health 2020, 62, e12103. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.; Barker, K.; Shamley, D.; Newman, M.; Oskrochi, G.R.; Sandall, S. The role of foot and ankle assessment of patients with lower limb osteoarthritis. Physiotherapy 2009, 95, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Abourazzak, F.E.; Kadi, N.; Azzouzi, H.; Lazrak, F.; Najdi, A.; Nejjari, C.; Harzy, T. A positive association between foot posture index and medial compartment knee osteoarthritis in moroccan people. Open Rheumatol. J. 2014, 8, 96–99. [Google Scholar] [CrossRef]
- Kubo, T.; Uritani, D.; Ogaya, S.; Kita, S.; Fukumoto, T.; Fujii, T.; Inagaki, Y.; Tanaka, Y.; Imagita, H. Association between foot posture and tibiofemoral contact forces during barefoot walking in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2022, 23, 660. [Google Scholar] [CrossRef] [PubMed]
- Tateuchi, H. Gait- and postural-alignment-related prognostic factors for hip and knee osteoarthritis: Toward the prevention of osteoarthritis progression. Phys. Ther. Res. 2019, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, R.; Imagama, S.; Muramoto, A.; Tsuboi, M.; Ishiguro, N.; Hasegawa, Y. Influence of spinal imbalance on knee osteoarthritis in community-living elderly adults. Nagoya J. Med. Sci. 2015, 77, 329–337. [Google Scholar] [PubMed]
- Wang, W.J.; Liu, F.; Zhu, Y.W.; Sun, M.H.; Qiu, Y.; Weng, W.J. Sagittal alignment of the spine-pelvis-lower extremity axis in patients with severe knee osteoarthritis: A radiographic study. Bone Jt. Res. 2016, 5, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.J.; Wang, W.J.; Wu, M.D.; Xu, Z.H.; Xu, L.L.; Qiu, Y. Characteristics of sagittal spine-pelvis-leg alignment in patients with severe hip osteoarthritis. Eur. Spine J. 2015, 24, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Egerton, T.; Martin, J.; Abbott, J.H.; Metcalf, B.; McManus, F.; Sims, K.; Pua, Y.H.; Wrigley, T.V.; Forbes, A.; et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: A randomized clinical trial. JAMA 2014, 311, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Krauss, I.; Steinhilber, B.; Haupt, G.; Miller, R.; Martus, P.; Janssen, P. Exercise therapy in hip osteoarthritis--a randomized controlled trial. Dtsch. Arztebl. Int. 2014, 111, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Overton, C.; Nelson, A.E.; Neogi, T. Osteoarthritis Treatment Guidelines from Six Professional Societies: Similarities and Differences. Rheum. Dis. Clin. N. Am. 2022, 48, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Simic, M.; Harmer, A.R.; Agaliotis, M.; Nairn, L.; Bridgett, L.; March, L.; Votrubec, M.; Edmonds, J.; Woodward, M.; Day, R.; et al. Clinical risk factors associated with radiographic osteoarthritis progression among people with knee pain: A longitudinal study. Arthritis Res. Ther. 2021, 23, 160. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.L.; Allen, K.D.; Golightly, Y.M.; Arbeeva, L.S.; Goode, A.; Huffman, K.M.; Schwartz, T.A.; Hill, C.H. Fall Risk and Utilization of Balance Training for Adults with Symptomatic Knee Osteoarthritis: Secondary Analysis from a Randomized Clinical Trial. J. Geriatr. Phys. Ther. 2019, 42, E39–E44. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Schwartz, S.; Teo, P.L.; Hawkins, S.; Mackenzie, D.; McManus, F.; Lamb, K.E.; Kimp, A.J.; Metcalf, B.; Hunter, D.J.; et al. Effectiveness of an Unsupervised Online Yoga Program on Pain and Function in People with Knee Osteoarthritis: A Randomized Clinical Trial. Ann. Intern. Med. 2022, 175, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Egerton, T.; Bennell, K.L.; McManus, F.; Lamb, K.E.; Hinman, R.S. Comparative effect of two educational videos on self-efficacy and kinesiophobia in people with knee osteoarthritis: An online randomised controlled trial. Osteoarthr. Cartil. 2022, 30, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Nelligan, R.K.; Hinman, R.S.; Kasza, J.; Crofts, S.J.C.; Bennell, K.L. Effects of a Self-directed Web-Based Strengthening Exercise and Physical Activity Program Supported by Automated Text Messages for People with Knee Osteoarthritis: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 776–785. [Google Scholar] [CrossRef]
- Walrabenstein, W.; Wagenaar, C.A.; van de Put, M.; van der Leeden, M.; Gerritsen, M.; Twisk, J.W.R.; van der Esch, M.; van Middendorp, H.; Weijs, P.J.M.; Roorda, L.D.; et al. A multidisciplinary lifestyle program for metabolic syndrome-associated osteoarthritis: The “Plants for Joints” randomized controlled trial. Osteoarthr. Cartil. 2023, 31, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Ziola-Frankowska, A.; Frankowski, M.; Daroszewski, P.; Szymankiewicz-Szukala, A.; Kubaszewski, L. Sex- and Age-Related Dynamic Changes of the Macroelements Content in the Femoral Bone with Hip Osteoarthritis. Biology 2022, 11, 344. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Luo, P.; Li, S.; Zhu, J.; Xue, S.; Cao, P.; Zhu, Z.; Li, J.; Wang, X.; et al. Associations of Dietary Macroelements with Knee Joint Structures, Symptoms, Quality of Life, and Comorbid Conditions in People with Symptomatic Knee Osteoarthritis. Nutrients 2022, 14, 3576. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.B.; McCulloch, C.E.; Nevitt, M.C.; Neumann, J.; Lynch, J.A.; Lane, N.E.; Link, T.M. Associations between Vitamins C and D Intake and Cartilage Composition and Knee Joint Morphology over 4 Years: Data from the Osteoarthritis Initiative. Arthritis Care Res. 2020, 72, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; La Tegola, L.; Mattera, M.; Maggi, S.; Guglielmi, G. Vitamin D Intake and Magnetic Resonance Parameters for Knee Osteoarthritis: Data from the Osteoarthritis Initiative. Calcif. Tissue Int. 2018, 103, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jin, X.; Cicuttini, F.; Wang, X.; Zhu, Z.; Wluka, A.; Han, W.; Winzenberg, T.; Antony, B.; Aitken, D.; et al. Maintaining Vitamin D Sufficiency Is Associated with Improved Structural and Symptomatic Outcomes in Knee Osteoarthritis. Am. J. Med. 2017, 130, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, L.; Cicuttini, F.; Han, W.; Zhu, Z.; Antony, B.; Wluka, A.; Winzenberg, T.; Meng, T.; Aitken, D.; et al. Effect of Vitamin D Supplementation on Depressive Symptoms in Patients with Knee Osteoarthritis. J. Am. Med. Dir. Assoc. 2019, 20, 1634–1640.e1. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jones, G.; Cicuttini, F.; Wluka, A.; Zhu, Z.; Han, W.; Antony, B.; Wang, X.; Winzenberg, T.; Blizzard, L.; et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients with Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2016, 315, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.; LaValley, M.; Schneider, E.; Nuite, M.; Lee, J.Y.; Price, L.L.; Lo, G.; Dawson-Hughes, B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA 2013, 309, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Vitamin D Effect on Bone Mineral Density and Fractures. Endocrinol. Metab. Clin. N. Am. 2017, 46, 935–945. [Google Scholar] [CrossRef]
- Oláh, T.; Cucchiarini, M.; Madry, H. Subchondral bone remodeling patterns in larger animal models of meniscal injuries inducing knee osteoarthritis—A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5346–5364. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yu, Y.E.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 33, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Day, J.S.; Ding, M.; van der Linden, J.C.; Hvid, I.; Sumner, D.R.; Weinans, H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J. Orthop. Res. 2001, 19, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Iwata, K.; Mashiba, T.; Miki, T.; Yamamoto, T. Accumulation of microdamage in subchondral bone at the femoral head in patients with end-stage osteoarthritis of the hip. J. Bone Miner. Metab. 2019, 37, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Zarka, M.; Hay, E.; Ostertag, A.; Marty, C.; Chappard, C.; Oudet, F.; Engelke, K.; Laredo, J.D.; Cohen-Solal, M. Microcracks in subchondral bone plate is linked to less cartilage damage. Bone 2019, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Fassio, A.; Rossini, M.; Caimmi, C.; Giollo, A.; Orsolini, G.; Viapiana, O.; Gatti, D. Osteoporosis in Rheumatic Diseases. Int. J. Mol. Sci. 2019, 20, 5867. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Maslen, C.; Vindlacheruvu, M.; Abel, R.L.; Bhattacharya, P.; Bromiley, P.A.; Clark, E.M.; Compston, J.E.; Crabtree, N.; Gregory, J.S.; et al. Digital health interventions for osteoporosis and post-fragility fracture care. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720x221083523. [Google Scholar] [CrossRef]
- Keen, R. Osteoporosis: Strategies for prevention and management. Best. Pract. Res. Clin. Rheumatol. 2007, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Kocijan, R.; Klaushofer, K.; Misof, B.M. Osteoporosis Therapeutics 2020. Handb. Exp. Pharmacol. 2020, 262, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Alito, A.; Bellone, F.; Portaro, S.; Leonardi, G.; Cannavò, V.; Coppini, F.; Leonetti, D.; Catalano, A.; Squadrito, G.; Fenga, D. Haemophilia and Fragility Fractures: From Pathogenesis to Multidisciplinary Approach. Int. J. Mol. Sci. 2023, 24, 9395. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, A.; Högler, W. Mechanisms of Bone Fragility: From Osteogenesis Imperfecta to Secondary Osteoporosis. Int. J. Mol. Sci. 2021, 22, 625. [Google Scholar] [CrossRef] [PubMed]
- Formosa, M.M.; Christou, M.A.; Mäkitie, O. Bone fragility and osteoporosis in children and young adults. J. Endocrinol. Investig. 2024, 47, 285–298. [Google Scholar] [CrossRef]
- Hong, A.R.; Kang, H.C. Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association. Endocrinol. Metab. 2023, 38, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fu, Z.; Wang, X.; Zhou, P.; Yang, Q.; Jiang, Y.; Zhu, D. A narrative review of diabetic bone disease: Characteristics, pathogenesis, and treatment. Front. Endocrinol. 2022, 13, 1052592. [Google Scholar] [CrossRef]
- Wysham, K.D.; Baker, J.F.; Narla, R. Osteoporosis and fractures in rheumatoid arthritis—Risk factors. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101757. [Google Scholar] [CrossRef] [PubMed]
- Zavatta, G.; Clarke, B.L. Glucocorticoid- and Transplantation-Induced Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2021, 50, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Liu, C.; Liang, T.; Zhang, Z.; Qin, Z.; Zhan, X. Predictors of osteoporotic fracture in postmenopausal women: A meta-analysis. J. Orthop. Surg. Res. 2023, 18, 574. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhu, J.; Wang, Y.; Zhang, N.; Gober, H.J.; Qiu, X.; Li, D.; Wang, L. Chinese single herbs and active ingredients for postmenopausal osteoporosis: From preclinical evidence to action mechanism. Biosci. Trends 2017, 11, 496–506. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shin, D.W.; Han, K.; Kim, D.; Yoon, J.W.; Lee, D.Y. Association of Female Reproductive Factors with Incidence of Fracture Among Postmenopausal Women in Korea. JAMA Netw. Open 2021, 4, e2030405. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Zamani, M.; Zamani, V.; Heidari, B.; Parsian, H.; Esmaeilnejad-Ganji, S.M. Prevalence of osteoporosis with the World Health Organization diagnostic criteria in the Eastern Mediterranean Region: A systematic review and meta-analysis. Arch. Osteoporos. 2018, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef]
- Wehren, L.E.; Hawkes, W.G.; Orwig, D.L.; Hebel, J.R.; Zimmerman, S.I.; Magaziner, J. Gender differences in mortality after hip fracture: The role of infection. J. Bone Miner. Res. 2003, 18, 2231–2237. [Google Scholar] [CrossRef]
- Kannegaard, P.N.; van der Mark, S.; Eiken, P.; Abrahamsen, B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 2010, 39, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.J.; Chih-Hsing Wu, P.; Bergin, D. Risk assessment tools for osteoporosis and fractures in 2022. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101775. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Bazarjani, S.; Zuckier, L.S. Artifacts and Incidental Findings Encountered on Dual-Energy X-Ray Absorptiometry: Atlas and Analysis. Semin. Nucl. Med. 2015, 45, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.L.; Prater, G.L. Quality in dual-energy X-ray absorptiometry scans. Bone 2017, 104, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Horiuchi, T.; Hosoi, T.; Orimo, H.; Nakamura, K. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos. Int. 2004, 15, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Conversano, F.; Franchini, R.; Greco, A.; Soloperto, G.; Chiriacò, F.; Casciaro, E.; Aventaggiato, M.; Renna, M.D.; Pisani, P.; Di Paola, M.; et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med. Biol. 2015, 41, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, A.; Brandi, M.L.; Al-Daghri, N.; Branco, J.C.; Bruyère, O.; Cavalli, L.; Cooper, C.; Cortet, B.; Dawson-Hughes, B.; Dimai, H.P.; et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: State of the art-outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin. Exp. Res. 2019, 31, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Tomai Pitinca, M.D.; Al Refaie, A.; De Vita, M.; Catapano, S.; Gonnelli, S. Could radiofrequency echographic multispectrometry (REMS) overcome the overestimation in BMD by dual-energy X-ray absorptiometry (DXA) at the lumbar spine? BMC Musculoskelet. Disord. 2022, 23, 469. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, H.; Shimizu, T.; Sakamoto, Y.; Toyama, F.; Kitahara, K.; Takayama, H.; Miyamoto, M.; Iwasaki, N. Radiofrequency Echographic Multispectrometry (REMS) can Overcome the Effects of Structural Internal Artifacts and Evaluate Bone Fragility Accurately. Calcif. Tissue Int. 2024, 114, 246–254. [Google Scholar] [CrossRef]
- Cosman, F. Long-term treatment strategies for postmenopausal osteoporosis. Curr. Opin. Rheumatol. 2018, 30, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, H.; Arita, K.; Terkawi, M.A.; Shimizu, T.; Iwasaki, N. Risks vs. benefits of switching therapy in patients with postmenopausal osteoporosis. Expert Rev. Endocrinol. Metab. 2021, 16, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef] [PubMed]
- Bergink, A.P.; Rivadeneira, F.; Bierma-Zeinstra, S.M.; Zillikens, M.C.; Ikram, M.A.; Uitterlinden, A.G.; van Meurs, J.B.J. Are Bone Mineral Density and Fractures Related to the Incidence and Progression of Radiographic Osteoarthritis of the Knee, Hip, and Hand in Elderly Men and Women? The Rotterdam Study. Arthritis Rheumatol. 2019, 71, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zucker, B.E.; Ebsim, R.; Lindner, C.; Hardcastle, S.; Cootes, T.; Tobias, J.H.; Whitehouse, M.R.; Gregson, C.L.; Faber, B.G.; Hartley, A.E. High bone mass and cam morphology are independently related to hip osteoarthritis: Findings from the High Bone Mass cohort. BMC Musculoskelet. Disord. 2022, 23, 757. [Google Scholar] [CrossRef]
- Zamzam, M.; Alamri, M.S.; Aldarsouni, F.G.; Al Zaid, H.; Al Ofair, A.A. Impact of Osteoporosis in Postmenopausal Women with Primary Knee Osteoarthritis. Cureus 2023, 15, e40645. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Otahal, P.; Cicuttini, F.; Wu, F.; Munugoda, I.P.; Jones, G.; Aitken, D. The association of subchondral and systemic bone mineral density with osteoarthritis-related joint replacements in older adults. Osteoarthr. Cartil. 2020, 28, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Hardcastle, S.A.; Paternoster, L.; McCloskey, E.; Poole, K.E.S.; Javaid, M.K.; Aye, M.; Moss, K.; Granell, R.; Gregory, J.; et al. Individuals with high bone mass have increased progression of radiographic and clinical features of knee osteoarthritis. Osteoarthr. Cartil. 2020, 28, 1180–1190. [Google Scholar] [CrossRef]
- Choi, Y.S.; Jeong, J.U.; Lee, S.H. Comparison of both lower leg bone mineral density in single limb knee osteoarthritis patients. Arch. Orthop. Trauma. Surg. 2023, 143, 7147–7151. [Google Scholar] [CrossRef] [PubMed]
- Heiss, R.; Laredo, J.D.; Wirth, W.; Jansen, M.P.; Marijnissen, A.C.A.; Lafeber, F.; Lalande, A.; Weinans, H.H.; Blanco, F.J.; Berenbaum, F.; et al. Quantitative CT of the knee in the IMI-APPROACH osteoarthritis cohort: Association of bone mineral density with radiographic disease severity, meniscal coverage and meniscal extrusion. Bone 2023, 168, 116673. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Jeong, H.W.; Park, S.B.; Shim, S.J.; Nam, H.S.; Lee, Y.S. Do Individualized Patient-Specific Situations Predict the Progression Rate and Fate of Knee Osteoarthritis? Prediction of Knee Osteoarthritis. J. Clin. Med. 2023, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- de Matos, O.; Ruthes, E.M.P.; Lenardt, B.C.C.; Beira de Andrade, A.; Petroski, C.A.; de Mello, M.F.; Biagini, G.; Lass, A.D.; Castelo-Branco, C. Relationship between postural changes, osteoarthritis and bone mineral density in postmenopausal women. Gynecol. Endocrinol. 2022, 38, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Delsmann, M.M.; Schmidt, C.; Muhlenfeld, M.; Jandl, N.M.; Boese, C.K.; Beil, F.T.; Rolvien, T.; Ries, C. Prevalence of osteoporosis and osteopenia in elderly patients scheduled for total knee arthroplasty. Arch. Orthop. Trauma Surg. 2022, 142, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Drees, P.; Decking, J.; Ghezel-Ahmadi, V.; Delank, K.S.; Wilhelm, B.; Eckardt, A. The common occurrence of osteoarthritis and osteoporosis and the value of markers of bone turnover. Z. Rheumatol. 2005, 64, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Gupta, A.; Sethi, S.; Kumar, S. Study of Relationship between Bone Mineral Density in Ipsilateral Proximal Femur and Severity of Osteoarthritis of Knee. J. Family Med. Prim. Care 2022, 11, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Germosen, C.; Kil, N.; Bucovsky, M.; Colon, I.; Williams, J.; Shane, E.; Walker, M.D. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone 2020, 140, 115552. [Google Scholar] [CrossRef]
- Karp, J.F.; Zhang, J.; Wahed, A.S.; Anderson, S.; Dew, M.A.; Fitzgerald, G.K.; Weiner, D.K.; Albert, S.; Gildengers, A.; Butters, M.; et al. Improving Patient Reported Outcomes and Preventing Depression and Anxiety in Older Adults with Knee Osteoarthritis: Results of a Sequenced Multiple Assignment Randomized Trial (SMART) Study. Am. J. Geriatr. Psychiatry 2019, 27, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Marin, F.; Geusens, P.; Lopez-Romero, P.; Lespessailles, E.; Body, J.J.; Minisola, S. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone 2020, 130, 115113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, L.; Cicuttini, F.; Zhu, Z.; Han, W.; Antony, B.; Wluka, A.E.; Winzenberg, T.; Aitken, D.; Blizzard, L.; et al. Depression in patients with knee osteoarthritis: Risk factors and associations with joint symptoms. BMC Musculoskelet. Disord. 2021, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, S.; Chen, Y.; Xu, H.; Qi, T.; Xiong, A.; Wang, D.; Yu, F.; Weng, J.; Zeng, H. Teriparatide ameliorates articular cartilage degradation and aberrant subchondral bone remodeling in DMM mice. J. Orthop. Translat 2023, 38, 241–255. [Google Scholar] [CrossRef]
- Corciulo, C.; Scheffler, J.M.; Humeniuk, P.; Del Carpio Pons, A.; Stubelius, A.; Von Mentzer, U.; Drevinge, C.; Barrett, A.; Wustenhagen, S.; Poutanen, M.; et al. Physiological levels of estradiol limit murine osteoarthritis progression. J. Endocrinol. 2022, 255, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Sapundzhiev, L.; Sapundzhieva, T.; Mitev, M.; Simitchiev, K.; Batalov, A. Correlation between Bone Mineral Density and Progression of Hip Osteoarthritis in Adult Men and Women in Bulgaria-Results from a 7-Year Study. Life 2023, 13, 421. [Google Scholar] [CrossRef]
- Cai, G.; Aitken, D.; Laslett, L.L.; Pelletier, J.P.; Martel-Pelletier, J.; Hill, C.; March, L.; Wluka, A.E.; Wang, Y.; Antony, B.; et al. Effect of Intravenous Zoledronic Acid on Tibiofemoral Cartilage Volume among Patients with Knee Osteoarthritis with Bone Marrow Lesions: A Randomized Clinical Trial. JAMA 2020, 323, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Baciut, M.; Baciut, G.; Bran, S.; Onisor, F.; Piciu, A.; Pasca, R.D.; et al. Systemic drugs with impact on osteoarthritis. Drug Metab. Rev. 2019, 51, 498–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, Y.; Yang, X.; He, J.; Zhang, F.; Zhong, Q.; Guo, X. Strontium ranelate promotes chondrogenesis through inhibition of the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2021, 12, 296. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Badurski, J.; Bellamy, N.; Bensen, W.; Chapurlat, R.; Chevalier, X.; Christiansen, C.; Genant, H.; Navarro, F.; Nasonov, E.; et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: Results of a double-blind, randomised placebo-controlled trial. Ann. Rheum. Dis. 2013, 72, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.A.; de Oliveira Freire, A.; Carvalho, H.C.O.; Silva, G.E.B.; Vasconcelos, J.W.; Guerra, R.N.M.; de Sousa Cartágenes, M.D.S.; Garcia, J.B.S. Prophylactic and Therapeutic Use of Strontium Ranelate Reduces the Progression of Experimental Osteoarthritis. Front. Pharmacol. 2018, 9, 975. [Google Scholar] [CrossRef]
- Han, W.; Fan, S.; Bai, X.; Ding, C. Strontium ranelate, a promising disease modifying osteoarthritis drug. Expert. Opin. Investig. Drugs 2017, 26, 375–380. [Google Scholar] [CrossRef]
- Ahlbäck, S.; Bauer, G.C.; Bohne, W.H. Spontaneous osteonecrosis of the knee. Arthritis Rheum. 1968, 11, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Bullough, P.G. Spontaneous osteonecrosis of the knee: The result of subchondral insufficiency fracture. J. Bone Jt. Surg. Am. 2000, 82, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Saifuddin, A. Magnetic resonance imaging of subchondral insufficiency fractures of the lower limb. Skeletal Radiol. 2019, 48, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.L.; Shah, N.; Smith, S.E.; Notino, A.; Kluczynski, M.A.; Jordan, K.; Bisson, L.J.; Chen, A.F.; Selzer, F.; Losina, E.; et al. Prevalence of Undiagnosed Subchondral Insufficiency Fractures of the Knee in Middle Age Adults with Knee Pain and Suspected Meniscal Tear. Osteoarthr. Cartil. Open 2020, 2, 100089. [Google Scholar] [CrossRef]
- Mont, M.A.; Marker, D.R.; Zywiel, M.G.; Carrino, J.A. Osteonecrosis of the knee and related conditions. J. Am. Acad. Orthop. Surg. 2011, 19, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Pape, D.; Seil, R.; Fritsch, E.; Rupp, S.; Kohn, D. Prevalence of spontaneous osteonecrosis of the medial femoral condyle in elderly patients. Knee Surg. Sports Traumatol. Arthrosc. 2002, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, A.S.; Ruutiainen, A.T.; Bakhru, P.T.; Schweitzer, M.E.; Shabshin, N. Subchondral insufficiency fracture of the knee: A recognizable associated soft tissue edema pattern and a similar distribution among men and women. Eur. J. Radiol. 2016, 85, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.V.; Saseendar, S.; Shanmugasundaram, S.; Bidwai, R.; Gómez, D.; D’Ambrosi, R. Spontaneous Osteonecrosis of the Knee: State of the Art. J. Clin. Med. 2022, 11, 6943. [Google Scholar] [CrossRef] [PubMed]
- Ochi, J.; Nozaki, T.; Nimura, A.; Yamaguchi, T.; Kitamura, N. Subchondral insufficiency fracture of the knee: Review of current concepts and radiological differential diagnoses. Jpn. J. Radiol. 2022, 40, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Husain, R.; Nesbitt, J.; Tank, D.; Verastegui, M.O.; Gould, E.S.; Huang, M. Spontaneous osteonecrosis of the knee (SONK): The role of MR imaging in predicting clinical outcome. J. Orthop. 2020, 22, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Plett, S.K.; Hackney, L.A.; Heilmeier, U.; Nardo, L.; Yu, A.; Zhang, C.A.; Link, T.M. Femoral condyle insufficiency fractures: Associated clinical and morphological findings and impact on outcome. Skeletal Radiol. 2015, 44, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Zaremski, J.L.; Vincent, K.R. Spontaneous Osteonecrosis of the Knee. Curr. Sports Med. Rep. 2016, 15, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Narváez, J.A.; Narváez, J.; De Lama, E.; Sánchez, A. Spontaneous osteonecrosis of the knee associated with tibial plateau and femoral condyle insufficiency stress fracture. Eur. Radiol. 2003, 13, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Sayyid, S.; Younan, Y.; Sharma, G.; Singer, A.; Morrison, W.; Zoga, A.; Gonzalez, F.M. Subchondral insufficiency fracture of the knee: Grading, risk factors, and outcome. Skeletal Radiol. 2019, 48, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Mitsugi, N.; Hayashi, T.; Kobayashi, H.; Saito, T. Low bone mineral density is associated with the onset of spontaneous osteonecrosis of the knee. Acta Orthop. 2012, 83, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Young, J.R.; Shamrock, A.G.; Rosenbaum, A.J. Spontaneous Osteonecrosis of the Knee. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Musbahi, O.; Waddell, L.; Shah, N.; Smith, S.E.; Chen, A.F.; Bisson, L.; Katz, J.N. Subchondral Insufficiency Fractures of the Knee: A Clinical Narrative Review. JBJS Rev. 2023, 11, e23.00084. [Google Scholar] [CrossRef] [PubMed]
- Bittner, J.; Hartstein, A. Spontaneous Osteonecrosis of the Knee. J. Orthop. Sports Phys. Ther. 2018, 48, 824. [Google Scholar] [CrossRef] [PubMed]
- Saccone, L.; Franceschetti, E.; Campi, S.; Za, P.; Zampogna, B.; Esposito, C.; Papalia, G.F.; Papapietro, N.; Papalia, R. Unicompartmental knee arthroplasty (UKA) for primary spontaneous osteonecrosis of the knee (SONK): A systematic review. Orthop. Rev. 2023, 15, 73916. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.J.; Lin, T.Y.; Lu, Y.C. A Retrospective Study of Unicompartmental Knee Arthroplasty Functional Outcome and the Incidence of Medial Meniscus Posterior Root Tear in Spontaneous Osteonecrosis of the Knee. Biomed. Res. Int. 2021, 2021, 6614122. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Huang, Z.; Zhang, W.; Lin, J.; Li, W. Analysis of medial unicompartmental knee arthroplasty for patients with spontaneous osteonecrosis of the knee. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.K.; Park, J.G.; Kim, J.; Suh, D.W.; Han, S.B. Functional improvement of unicompartmental knee arthroplasty compared with total knee arthroplasty for subchondral insufficiency fracture of the knee. Sci. Rep. 2023, 13, 20041. [Google Scholar] [CrossRef] [PubMed]
- Flury, A.; Weigelt, L.; Camenzind, R.S.; Fritz, B.; Hasler, J.; Baumgaertner, B.; Helmy, N.; Fucentese, S.F. Total and unicondylar knee arthroplasty are equivalent treatment options in end-stage spontaneous osteonecrosis of the knee, and the size of the lesion has no influence on the results. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3254–3261. [Google Scholar] [CrossRef]
- Choi, H.G.; Kim, J.S.; Yoo, H.J.; Jung, Y.S.; Lee, Y.S. The Fate of Bone Marrow Lesions after Open Wedge High Tibial Osteotomy: A Comparison between Knees with Primary Osteoarthritis and Subchondral Insufficiency Fractures. Am. J. Sports Med. 2021, 49, 1551–1560. [Google Scholar] [CrossRef]
- Yabuuchi, K.; Kondo, E.; Onodera, J.; Onodera, T.; Yagi, T.; Iwasaki, N.; Yasuda, K. Clinical Outcomes and Complications During and After Medial Open-Wedge High Tibial Osteotomy Using a Locking Plate: A 3- to 7-Year Follow-up Study. Orthop. J. Sports Med. 2020, 8, 2325967120922535. [Google Scholar] [CrossRef] [PubMed]
- Deie, M.; Ochi, M.; Adachi, N.; Nishimori, M.; Yokota, K. Artificial bone grafting [calcium hydroxyapatite ceramic with an interconnected porous structure (IP-CHA)] and core decompression for spontaneous osteonecrosis of the femoral condyle in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lu, Z.K.; Huang, C.; Wang, F.; Miao, S.; Zeng, L.; Dai, S.J.; Li, L.; Li, C.Z. Comparison of curative effect between osteochondral mosaic transplantation and micro-fracture in the treatment of knee joint articular cartilage injury. Zhongguo Gu Shang 2019, 32, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mima, H.; Yonetani, Y.; Shiozaki, Y.; Nakamura, N.; Horibe, S. Histological evaluation of spontaneous osteonecrosis of the medial femoral condyle and short-term clinical results of osteochondral autografting: A case series. Knee 2009, 16, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Fokter, S.K.; Kuhta, M.; Hojnik, M.; Ledinek, Ž.; Kostanjšek, R. Tissue Integration of Calcium Phosphate Compound after Subchondroplasty: 4-Year Follow-Up in a 76-Year-Old Female Patient. Bioengineering 2023, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.; Kang, J.Y.B.; Ng, F.D.J.; Pang, H.N.; Lie, D.T.T.; Silva, A.; Chang, P.C.C. Subchondroplasty for Bone Marrow Lesions in the Arthritic Knee Results in Pain Relief and Improvement in Function. J. Knee Surg. 2021, 34, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.R.; Cherian, J.J.; Jauregui, J.J.; Pierce, T.; Mont, M.A. Osteonecrosis of the knee: Review. Ann. Transl. Med. 2015, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Chang, M.J.; Chang, C.B.; Choi, J.H.; Lee, S.A.; Kang, S.B. Does unicompartmental knee arthroplasty have worse outcomes in spontaneous osteonecrosis of the knee than in medial compartment osteoarthritis? A systematic review and meta-analysis. Arch. Orthop. Trauma. Surg. 2019, 139, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.B.; Chahla, J.; Mandelbaum, B.R.; Gomoll, A.H.; LaPrade, R.F. The Role of Meniscal Tears in Spontaneous Osteonecrosis of the Knee: A Systematic Review of Suspected Etiology and a Call to Revisit Nomenclature. Am. J. Sports Med. 2019, 47, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.I.; Strauss, M.; LaPrade, R.F. Injury of the Meniscus Root. Clin. Sports Med. 2020, 39, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Reardon, P.J.; Johnson, N.R.; Mohan, R.; Peter, L.; Levy, B.A.; Stuart, M.J. Non-operative management of medial meniscus posterior horn root tears is associated with worsening arthritis and poor clinical outcome at 5-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 383–389. [Google Scholar] [CrossRef]
- Juréus, J.; Lindstrand, A.; Geijer, M.; Robertsson, O.; Tägil, M. The natural course of spontaneous osteonecrosis of the knee (SPONK): A 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013, 84, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Lin, Y.C.; Liu, Y.H.; Weng, P.W.; Chen, K.H.; Chiang, C.J.; Wong, C.C. Correlation between Subchondral Insufficiency Fracture of the Knee and Osteoarthritis Progression in Patients with Medial Meniscus Posterior Root Tear. Diagnostics 2023, 13, 3532. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Kobayashi, H.; Kusayama, Y.; Aratake, M.; Kumagai, K.; Saito, T. Predictive factors for the progression of spontaneous osteonecrosis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bangil, M.; Soubrier, M.; Dubost, J.J.; Rami, S.; Carcanagues, Y.; Ristori, J.M.; Bussiere, J.L. Subchondral insufficiency fracture of the femoral head. Rev. Rhum. Engl. Ed. 1996, 63, 859–861. [Google Scholar] [PubMed]
- Song, W.S.; Yoo, J.J.; Koo, K.H.; Yoon, K.S.; Kim, Y.M.; Kim, H.J. Subchondral fatigue fracture of the femoral head in military recruits. J. Bone Jt. Surg. Am. 2004, 86, 1917–1924. [Google Scholar] [CrossRef]
- Ikemura, S.; Yamamoto, T.; Nakashima, Y.; Shuto, T.; Jingushi, S.; Iwamoto, Y. Bilateral subchondral insufficiency fracture of the femoral head after renal transplantation: A case report. Arthritis Rheum. 2005, 52, 1293–1296. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Takahashi, E.; Kaneuji, A.; Zhou, Y.; Kawahara, N. Current Research on Subchondral Insufficiency Fracture of the Femoral Head. Clin. Orthop. Surg. 2022, 14, 477–485. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwamoto, Y.; Schneider, R.; Bullough, P.G. Histopathological prevalence of subchondral insufficiency fracture of the femoral head. Ann. Rheum. Dis. 2008, 67, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Karasuyama, K.; Iwasaki, K.; Doi, T.; Iwamoto, Y. Subchondral insufficiency fracture of the femoral head in males. Arch. Orthop. Trauma Surg. 2014, 134, 1199–1203. [Google Scholar] [CrossRef]
- Shimizu, T.; Yokota, S.; Kimura, Y.; Asano, T.; Shimizu, H.; Ishizu, H.; Iwasaki, N.; Takahashi, D. Predictors of cartilage degeneration in patients with subchondral insufficiency fracture of the femoral head: A retrospective study. Arthritis Res. Ther. 2020, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Subchondral insufficiency fractures of the femoral head. Clin. Orthop. Surg. 2012, 4, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Schneider, R.; Bullough, P.G. Subchondral insufficiency fracture of the femoral head: Histopathologic correlation with MRI. Skeletal Radiol. 2001, 30, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Miyanishi, K.; Ishihara, K.; Jingushi, S.; Torisu, T. Risk factors leading to total hip arthroplasty in patients with subchondral insufficiency fractures of the femoral head. J. Orthop. Surg. 2010, 18, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takabatake, K.; Iwamoto, Y. Subchondral insufficiency fracture of the femoral head resulting in rapid destruction of the hip joint: A sequential radiographic study. AJR Am. J. Roentgenol. 2002, 178, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Niimi, R.; Hasegawa, M.; Sudo, A.; Uchida, A. Rapidly destructive coxopathy after subchondral insufficiency fracture of the femoral head. Arch. Orthop. Trauma Surg. 2005, 125, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Shimizu, T.; Matsumae, G.; Ebata, T.; Alhasan, H.; Takahashi, D.; Terkawi, M.A.; Iwasaki, N. Inflammasome Activation in the Hip Synovium of Rapidly Destructive Coxopathy Patients and Its Relationship with the Development of Synovitis and Bone Loss. Am. J. Pathol. 2022, 192, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Won, S.H.; Park, J.W.; Im, J.W.; Ha, Y.C.; Koo, K.H. Cementless Hip Arthroplasty in Patients with Subchondral Insufficiency Fracture of the Femoral Head. J. Bone Jt. Surg. Am. 2022, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Solasz, S.; Konda, S.R.; Schwarzkopf, R.; Slover, J.; Chang, G.; Egol, K.A. Total Hip Arthroplasty is the Most Effective Treatment for Atraumatic Subchondral Insufficiency Fractures of the Femoral Head. Bull. Hosp. Jt. Dis. 2023, 81, 173–178. [Google Scholar]
- Yamamoto, T.; Iwasaki, K.; Iwamoto, Y. Transtrochanteric rotational osteotomy for a subchondral insufficiency fracture of the femoral head in young adults. Clin. Orthop. Relat. Res. 2010, 468, 3181–3185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonoda, K.; Motomura, G.; Ikemura, S.; Kubo, Y.; Yamamoto, T.; Nakashima, Y. Favorable Clinical and Radiographic Results of Transtrochanteric Anterior Rotational Osteotomy for Collapsed Subchondral Insufficiency Fracture of the Femoral Head in Young Adults. JB JS Open Access 2017, 2, e0013. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Kamath, A.F. Subchondral Insufficiency Fracture of the Femoral Head treated with Core Decompression and Bone Void Filler Support. Arch. Bone Jt. Surg. 2016, 4, 264–268. [Google Scholar]
- Uchida, S.; Noguchi, M.; Utsunomiya, H.; Kanezaki, S.; Mori, T.; Matsuda, D.K.; Sakai, A. Hip arthroscopy enables classification and treatment of precollapse subchondral insufficiency fracture of the femoral head associated intra-articular pathology. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Kapil, N.; Samuel, L.T.; Kamath, A.F. Management of Bone Marrow Lesions of the Hip with Subchondral Calcium Phosphate Injection: Surgical Technique and Tips. Arthrosc. Tech. 2020, 9, e863–e875. [Google Scholar] [CrossRef]
- Hamada, T.; Yamamoto, T.; Shida, J.; Inokuchi, A.; Arizono, T. Subchondral insufficiency fracture of the femoral head in a patient with alkaptonuria. Skeletal Radiol. 2014, 43, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Yamamoto, T.; Nakashima, Y.; Mawatari, T.; Motomura, G.; Ikemura, S.; Iwamoto, Y. Subchondral insufficiency fracture of the femoral head after liver transplantation. Skeletal Radiol. 2009, 38, 925–928. [Google Scholar] [CrossRef]
- Baba, S.; Motomura, G.; Ikemura, S.; Sonoda, K.; Kubo, Y.; Utsunomiya, T.; Hatanaka, H.; Nakashima, Y. Femoral head fracture similar to slipped capital femoral epiphysis in an elderly woman with antecedent hip osteoarthritis after subchondral insufficiency fracture: A case report. J. Orthop. Sci. 2020, 25, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Schneider, R.; Iwamoto, Y.; Bullough, P.G. Subchondral insufficiency fracture of the femoral head in a patient with systemic lupus erythematosus. Ann. Rheum. Dis. 2006, 65, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Miyanishi, K.; Ihara, H.; Jingushi, S.; Torisu, T. Subchondral insufficiency fracture of the femoral head may be associated with hip dysplasia: A pilot study. Clin. Orthop. Relat. Res. 2010, 468, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Motomura, G.; Utsunomiya, T.; Fujii, M.; Ikemura, S.; Sonoda, K.; Nakashima, Y. Distribution of Femoral Head Subchondral Fracture Site Relates to Contact Pressures, Age, and Acetabular Structure. AJR Am. J. Roentgenol. 2020, 215, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Kaneuji, A.; Fukushima, M.; Matsumoto, T. Inversion of the acetabular labrum triggers rapidly destructive osteoarthritis of the hip: Representative case report and proposed etiology. J. Arthroplast. 2014, 29, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fukui, K.; Kaneuji, A.; Hirosaki, K.; Miyakawa, H.; Kawahara, N. Inversion of the acetabular labrum causes increased localized contact pressure on the femoral head: A biomechanical study. Int. Orthop. 2019, 43, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Yoon, P.W.; Kwak, H.S.; Yoo, J.J.; Yoon, K.S.; Kim, H.J. Subchondral insufficiency fracture of the femoral head in elderly people. J. Korean Med. Sci. 2014, 29, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Yamamoto, T.; Motomura, G.; Karasuyama, K.; Sonoda, K.; Kubo, Y.; Iwamoto, Y. Common site of subchondral insufficiency fractures of the femoral head based on three-dimensional magnetic resonance imaging. Skelet. Radiol. 2016, 45, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Yamamoto, T.; Motomura, G.; Ikemura, S.; Mawatari, T.; Nakashima, Y.; Iwamoto, Y. Prognostic factors associated with a subchondral insufficiency fracture of the femoral head. Br. J. Radiol. 2012, 85, 214–218. [Google Scholar] [CrossRef]
- Onishi, E.; Ota, S.; Fujita, S.; Tsukamoto, Y.; Yamashita, S.; Hashimura, T.; Matsunaga, K.; Yasuda, T. Association between sagittal spinopelvic alignment and femoral head destruction in the early stage of rapidly destructive coxopathy. Bone Jt. Open 2022, 3, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Kitajima, M.; Tsukamoto, M.; Yoshihara, T.; Sonohata, M.; Mawatari, M. Sagittal spino-pelvic alignment in rapidly destructive coxarthrosis. Eur. Spine J. 2018, 27, 475–481. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in subchondral bone: Predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 607764. [Google Scholar] [CrossRef] [PubMed]
- Sibilska, A.; Góralczyk, A.; Hermanowicz, K.; Malinowski, K. Spontaneous osteonecrosis of the knee: What do we know so far? A literature review. Int. Orthop. 2020, 44, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Walsh, D.A. Osteochondral alterations in osteoarthritis. Bone 2012, 51, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cabahug-Zuckerman, P.; Frikha-Benayed, D.; Majeska, R.J.; Tuthill, A.; Yakar, S.; Judex, S.; Schaffler, M.B. Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J. Bone Miner. Res. 2016, 31, 1356–1365. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Gortazar, A.R.; Davis, H.M.; Condon, K.W.; Gabilondo, H.; Maycas, M.; Allen, M.R.; Bellido, T. Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor κB ligand (RANKL) but does not stop bone resorption or the loss of bone induced by unloading. J. Biol. Chem. 2015, 290, 18934–18942. [Google Scholar] [CrossRef] [PubMed]
- Lacourt, M.; Gao, C.; Li, A.; Girard, C.; Beauchamp, G.; Henderson, J.E.; Laverty, S. Relationship between cartilage and subchondral bone lesions in repetitive impact trauma-induced equine osteoarthritis. Osteoarthr. Cartil. 2012, 20, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Hügle, T.; Geurts, J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology 2017, 56, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Klose-Jensen, R.; Hartlev, L.B.; Boel, L.W.T.; Laursen, M.B.; Stengaard-Pedersen, K.; Keller, K.K.; Hauge, E.M. Subchondral bone turnover, but not bone volume, is increased in early stage osteoarthritic lesions in the human hip joint. Osteoarthr. Cartil. 2015, 23, 2167–2173. [Google Scholar] [CrossRef]
- Dai, G.; Xiao, H.; Liao, J.; Zhou, N.; Zhao, C.; Xu, W.; Xu, W.; Liang, X.; Huang, W. Osteocyte TGFβ1-Smad2/3 is positively associated with bone turnover parameters in subchondral bone of advanced osteoarthritis. Int. J. Mol. Med. 2020, 46, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Pollintine, P.; Powell, D.E.; Davie, M.W.; Sharp, C.A. Regional differences in mechanical and material properties of femoral head cancellous bone in health and osteoarthritis. Calcif. Tissue Int. 2002, 71, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, A.; Miyakoshi, N.; Hongo, M.; Kasukawa, Y.; Shimada, Y.; Kodama, H.; Sano, A. Treatment of spontaneous osteonecrosis of the knee by daily teriparatide: A report of 3 cases. Medicine 2020, 99, e18989. [Google Scholar] [CrossRef] [PubMed]
- Yates, P.J.; Calder, J.D.; Stranks, G.J.; Conn, K.S.; Peppercorn, D.; Thomas, N.P. Early MRI diagnosis and non-surgical management of spontaneous osteonecrosis of the knee. Knee 2007, 14, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Sharoff, L.; Jagani, N. Effect of Zoledronic Acid and Alendronate on Bone Edema and Pain in Spontaneous Osteonecrosis of the Knee: A New Paradigm in the Medical Management. Rev. Bras. Ortop. 2020, 55, 543–550. [Google Scholar] [CrossRef]
- Tat, S.K.; Pelletier, J.P.; Mineau, F.; Caron, J.; Martel-Pelletier, J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone 2011, 49, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Ding, H.F.; Mao, Y.Q.; Liu, M.; Yu, B.; Zhao, X.; Wang, X.Q.; Li, Y.; Liu, G.W.; Nie, S.B.; et al. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol. Sin. 2013, 34, 393–402. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | n | Sex | Instruments for BMD Measurement | KOA or HOA | Onset Factors | Progressive Factors |
|---|---|---|---|---|---|---|
| Bergink, 2019 [106] | 4154 | Both | NR | Both | High FN BMD | NR |
| Funck-Brentano, 2022 [18] | 384,838 | Both | QUS (calcaneus) MR analyses | Both | High FN BMD (According to MR Analyses) BMI, Low Systolic BP | NR |
| Zamzam, 2023 [108] | 487 | Female | DXA | KOA | High FN BMD Age, Weight | High FN BMD |
| Cai, 2020 [109] | 1095 | Both | DXA | KOA (TKA) | High Medial Tibial Subchondral BMD | NR |
| HOA (THA) | High Systemic BMD, High LS BMD | NR | ||||
| Hartley, 2020 [110] | 169 | Both | DXA | KOA | High Bone Mass (TH, LS, or Systemic), Age | NR |
| Author, Year | n | Sex | Instruments for BMD Measurement | KOA or HOA | Onset Factors | Progressive Factors |
|---|---|---|---|---|---|---|
| Choi, 2023 [111] | 149 | Both | DXA | KOA | Low FN BMD | NR |
| Heiss, 2023 [112] | 275 | Both | Quantitative CT | KOA | Low Medial Femoral Condyle BMD | NR |
| Yoo, 2023 [113] | 2492 | Both | NR | KOA | Age, Sex, BMI, Occupation | Low BMD Age, Sex, BMI |
| Stamenkovic, 2022 [23] | 96 | Female | DXA | KOA | Low LS BMD, Low TH BMD | NR |
| Delsmann, 2022 [115] | 109 | Both | DXA | KOA | Low BMD | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokota, S.; Ishizu, H.; Miyazaki, T.; Takahashi, D.; Iwasaki, N.; Shimizu, T. Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines 2024, 12, 843. https://doi.org/10.3390/biomedicines12040843
Yokota S, Ishizu H, Miyazaki T, Takahashi D, Iwasaki N, Shimizu T. Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines. 2024; 12(4):843. https://doi.org/10.3390/biomedicines12040843
Chicago/Turabian StyleYokota, Shunichi, Hotaka Ishizu, Takuji Miyazaki, Daisuke Takahashi, Norimasa Iwasaki, and Tomohiro Shimizu. 2024. "Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights" Biomedicines 12, no. 4: 843. https://doi.org/10.3390/biomedicines12040843
APA StyleYokota, S., Ishizu, H., Miyazaki, T., Takahashi, D., Iwasaki, N., & Shimizu, T. (2024). Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines, 12(4), 843. https://doi.org/10.3390/biomedicines12040843







